Boosted Catalytic Performance of Ni2Co2@T-PMo@ZIF-67 for Glucose Oxidation in a Direct-Glucose Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electrochemical Performance Characterization of Anodic Catalysts
2.1.1. Linear Sweep Voltammetry (LSV) Measurement
2.1.2. Electrochemical Impedance Testing (EIS) of Anodes
2.1.3. The Tafel Curve of Anodes
2.1.4. Power Density (PD) and Polarization Curves (PC) of Different Anodes in Fuel Cells
2.2. Characterization of T-PMo@ZIF-67
2.2.1. Scanning Electron Microscope Analysis
2.2.2. X-ray Diffraction Analysis
2.2.3. X-ray Photoelectron Spectroscopy Analysis
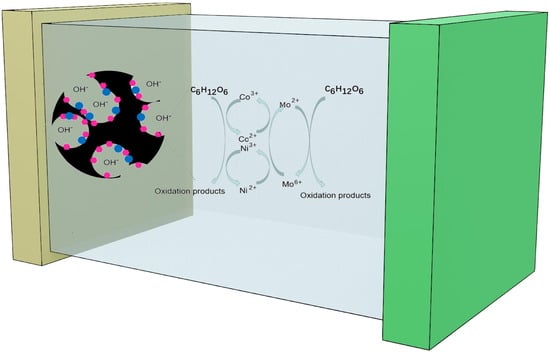
2.3. Catalytic Mechanism
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Preparation of Electrodes
3.3.1. Preparation of XPMo@ZIF-67 and NixCoy@PZ
3.3.2. Preparation of T-XPMo@ZIF-67 and NixCoy@T-PZ
3.3.3. Preparation of T-XPMo@ZIF-67 and NixCoy@PZ Modified Anode
- (1)
- An amount of 0.5 g of activated carbon was mixed with T-XPMo@ZIF-67/NixCoy@PZ in a ratio of 1:0.01~1:0.05 by using anhydrous ethanol as solvent. The obtained mixture was placed in an ultrasonic cleaner with ultrasonic shaking and constant stirring for 40 min;
- (2)
- An amount of 0.25 g PTFE emulsion (60 wt%) was slowly added, and stirred continuously for 40 min;
- (3)
- The mixture was placed in a thermostatic water bath at 70 °C, and stirred continuously;
- (4)
- The catalyst modified anode with a thickness of 2~4 mm was obtained by rolling the mixture onto a Ni foam disc (diameter: 36 mm).
3.4. Experimental Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, B.; Wen, H.-M.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal-Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wan, J.; Pang, G.; Tang, Z. Hollow Metal-Organic- Framework Micro/Nanostructures and their Derivatives: Emerging Multifunctional Materials. Adv. Mater. 2019, 31, 1803291. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, X.-Q.; Jiang, H.-L.; Sun, L.-B. Metal-Organic Frameworks for Heterogeneous Basic Catalysis. Chem. Rev. 2017, 117, 8129–8176. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Singh, G.; George, N.; Singh, G.; Gupta, S.; Singh, H.; Kaur, G.; Singh, J. Copper-Based Metal-Organic Frameworks (MOFs) as an Emerging Catalytic Framework for Click Chemistry. Catalysts 2023, 13, 130. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.-Y. Metal-organic frameworks with catalytic centers: From synthesis to catalytic application. Coordin. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Zhang, H.; Osgood, H.; Xie, X.; Shao, Y.; Wu, G. Engineering nanostructures of PGM-free oxygen-reduction catalysts using metal-organic frameworks. Nano Energy 2017, 31, 331–350. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, P.; Duan, C.; He, C. Three-Dimensional Neodymium Metal-Organic Framework for Catalyzing the Cyanosilylation of Aldehyde and the Synthesis of 2,3-Dihydroquinazolin-4(1H)-one Derivatives. Inorg. Chem. 2023, 62, 10359–10368. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Li, G.; Wei, Y. Recent Recent advances in alkoxylation chemistry of polyoxometalates: From synthetic strategies, structural overviews to functional applications. Coordin. Chem. Rev. 2019, 378, 395–414. [Google Scholar] [CrossRef]
- Liu, J.-X.; Zhang, X.-B.; Li, Y.-L.; Huang, S.-L.; Yang, G.-Y. Polyoxometalate functionalized architectures. Coordin. Chem. Rev. 2020, 414, 213260. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Synthesis, structures and applications of electron-rich polyoxometalates. Nat. Rev. Chem. 2018, 2, 0112. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, X.; Han, X.; Cui, H.; Lu, Y.; Liu, S.; Liu, Y. Straightforward construction of hollow polyoxometalate-based metal-organic framework via pseudo-homoepitaxial growth. Sci. China Chem. 2022, 65, 1921–1928. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Zhang, H.; Lv, Y.; Gao, S. Facile synthesis of POM@MOF embedded in SBA-15 as a steady catalyst for the hydroxylation of benzene. Micropor. Mesopor. Mat. 2014, 195, 87–91. [Google Scholar] [CrossRef]
- Du, D.-Y.; Qin, J.-S.; Li, S.-L.; Su, Z.-M.; Lan, Y.-Q. Recent advances in porous polyoxometalate-based metal-organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Mialane, P.; Mellot-Draznieks, C.; Gairola, P.; Duguet, M.; Benseghir, Y.; Oms, O.; Dolbecq, A. Heterogenisation of polyoxometalates and other metal-based complexes in metal-organic frameworks: From synthesis to characterisation and applications in catalysis. Chem. Soc. Rev. 2021, 50, 6152–6220. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Abednatanzi, S.; Van Der Voort, P.; Liu, Y.-Y.; Leus, K. POM@MOF Hybrids: Synthesis and Applications. Catalysts 2020, 10, 578. [Google Scholar] [CrossRef]
- Freire, C.; Fernandes, D.M.; Nunes, M.; Abdelkader, V.K. POM & MOF-based Electrocatalysts for Energy-related Reactions. Chemcatchem 2018, 10, 1703–1730. [Google Scholar]
- Liu, J.; Huang, M.; Hua, Z.; Dong, Y.; Feng, Z.; Sun, T.; Chen, C. Polyoxometalate-Based Metal Organic Frameworks: Recent Advances and Challenges. ChemistrySelect 2022, 7, e202200546. [Google Scholar] [CrossRef]
- Tiba, S.; Omri, A. Literature survey on the relationships between energy, environment and economic growth. Renew Sust. Energy Rev. 2017, 69, 1129–1146. [Google Scholar] [CrossRef]
- Nathaniel, S.P.; Adeleye, N. Environmental preservation amidst carbon emissions, energy consumption, and urbanization in selected african countries: Implication for sustainability. J. Clean. Prod. 2021, 285, 125409. [Google Scholar] [CrossRef]
- Ehsanullah, S.; Quyen Ha, T.; Sadiq, M.; Bashir, S.; Mohsin, M.; Iram, R. How energy insecurity leads to energy poverty? Do environmental consideration and climate change concerns matters. Environ. Sci. Pollut. Res. 2021, 28, 55041–55052. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Li, Y.; Liu, P.; Chen, X.; Zhang, P.; Wang, Z.; Liu, X. Sweet Drinks as Fuels for an Alkaline Fuel Cell with Nonprecious Catalysts. Energies 2021, 14, 206. [Google Scholar] [CrossRef]
- Liu, T. Glucose Fuel Cells and Membranes: A Brief Overview and Literature Analysis. Sustainability 2022, 14, 8376. [Google Scholar] [CrossRef]
- Kerzenmacher, S.; Ducree, J.; Zengerle, R.; von Stetten, F. Energy harvesting by implantable abiotically catalyzed glucose fuel cells. J. Power Sources 2008, 182, 1–17. [Google Scholar] [CrossRef]
- Antolini, E. External abiotic glucose fuel cells. Sustain. Energy Fuels. 2021, 5, 5038–5060. [Google Scholar] [CrossRef]
- Do, U.P.; Seland, F.; Johannessen, E.A. A micro fuel cell for abiotical catalysis of glucose. J. Power Sources. 2020, 478, 229032. [Google Scholar] [CrossRef]
- Ho, J.; Li, Y.; Dai, Y.; Kim, T.; Wang, J.; Ren, J.; Yun, H.; Liu, X. Ionothermal synthesis of N-doped carbon supported CoMn2O4 nanoparticles as ORR catalyst in direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2021, 46, 20503–20515. [Google Scholar] [CrossRef]
- Huang, J.; Simons, P.; Sunada, Y.; Rupp, J.L.M.; Yagi, S. Pt-Catalyzed D-Glucose Oxidation Reactions for Glucose Fuel Cells. J. Electrochem. Soc. 2021, 168, 064511. [Google Scholar] [CrossRef]
- Dong, F.; Liu, X.; Irfan, M.; Yang, L.; Li, S.; Ding, J.; Li, Y.; Khan, I.U.; Zhang, P. Macaroon-like FeCo2O4 modified activated carbon anode for enhancing power generation in direct glucose fuel cell. Int. J. Hydrogen Energy 2019, 44, 8178–8187. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, X.; Zhang, P.; Shi, J. Peony petal-like 3D graphene-nickel oxide nanocomposite decorated nickel foam as high-performance electrocatalyst for direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2017, 42, 29863–29873. [Google Scholar] [CrossRef]
- Irfan, M.; Liu, X.; Li, S.; Khan, I.U.; Li, Y.; Wang, J.; Wang, X.; Du, X.; Wang, G.; Zhang, P. High-performance glucose fuel cell with bimetallic Ni-Co composite anchored on reduced graphene oxide as anode catalyst. Renew Energy 2020, 155, 1118–1126. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Ding, J.; Li, S.; Dong, F.; Irfan, M.; Li, Y.; Wang, G.; Du, X.; Zhang, P. Chlorella-derived porous heteroatom-doped carbons as robust catalysts for oxygen reduction reaction in direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2019, 44, 2823–2831. [Google Scholar] [CrossRef]
- Li, Y.; Dong, F.; Jiao, S.; Wang, J.; Dai, Y.; Irfan, M.; Liu, X. Co2+-P(W3O10)(4)(3-) modified activated carbon as an efficient anode catalyst for direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2022, 47, 22952–22962. [Google Scholar] [CrossRef]
- Akhairi, M.A.F.; Kamarudin, S.K. Catalysts in direct ethanol fuel cell (DEFC): An overview. Int. J. Hydrogen Energy 2016, 41, 4214–4228. [Google Scholar] [CrossRef]
- Din, M.A.U.; Idrees, M.; Jamil, S.; Irfan, S.; Nazir, G.; Mudassir, M.A.; Saleem, M.S.; Batool, S.; Cheng, N.; Saidur, R. Advances and challenges of methanol-tolerant oxygen reduction reaction electrocatalysts for the direct methanol fuel cell. J. Energy Chem. 2023, 77, 499–513. [Google Scholar] [CrossRef]
- Gong, L.; Yang, Z.; Li, K.; Xing, W.; Liu, C.; Ge, J. Recent development of methanol electrooxidation catalysts for direct methanol fuel cell. J. Energy Chem. 2018, 27, 1618–1628. [Google Scholar] [CrossRef]
- Oncescu, V.; Erickson, D. High volumetric power density, non-enzymatic, glucose fuel cells. Sci. Rep. 2013, 3, 1226. [Google Scholar] [CrossRef] [PubMed]
- Simons, P.; Schenk, S.A.; Gysel, M.A.; Olbrich, L.F.; Rupp, J.L.M. A Ceramic-Electrolyte Glucose Fuel Cell for Implantable Electronics. Adv. Mater. 2022, 34, 2109075. [Google Scholar] [CrossRef]
- Su, C.-H.; Sun, C.-L.; Peng, S.-Y.; Wu, J.-J.; Huang, Y.-H.; Liao, Y.-C. High performance non-enzymatic graphene-based glucose fuel cell operated under moderate temperatures and a neutral solution. J. Taiwan Inst. Chem. E 2019, 95, 48–54. [Google Scholar] [CrossRef]
- Guo, H.; Yin, H.; Yan, X.; Shi, S.; Yu, Q.; Cao, Z.; Li, J. Pt-Bi decorated nanoporous gold for high performance direct glucose fuel cell. Sci. Rep. 2016, 6, 39162. [Google Scholar] [CrossRef]
- Lv, Z.; Zhong, Q.; Bu, Y. Controllable synthesis of Ni-Co nanosheets covered hollow box via altering the concentration of nitrate for high performance supercapacitor. Electrochim. Acta 2016, 215, 500–505. [Google Scholar] [CrossRef]
- Liu, H.; Jin, M.; Zhan, D.; Wang, J.; Cai, X.; Qiu, Y.; Lai, L. Stacking faults triggered strain engineering of ZIF-67 derived Ni-Co bimetal phosphide for enhanced overall water splitting. Appl. Catal. B-Environ. 2020, 272, 118951. [Google Scholar] [CrossRef]
- Guo, G.; Li, W.; Ahmed, T.; Hu, D.; Cui, R.; Zhang, B.; Zhang, X. Production of liquid fuels from Kraft lignin over bimetallic Ni-Mo supported on ZIF-derived porous carbon catalyst. RSC Adv. 2021, 11, 37932–37941. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Ye, Y.-S.; Wang, Z.-M.; Zhang, Z.-H.; Zhao, Y.-L.; Yang, F.; Zhu, Z.-Y.; Wei, T. POM-Based MOF-Derived Co3O4/CoMoO4Nanohybrids as Anodes for High-Performance Lithium-Ion Batteries. ACS Omega 2020, 5, 26230–26236. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, X.; Irfan, M.; Shi, J.; Wang, X.; Zhang, P. Nickle-cobalt composite catalyst-modified activated carbon anode for direct glucose alkaline fuel cell. Int. J. Hydrogen Energy 2018, 43, 1805–1815. [Google Scholar] [CrossRef]
- Irfan, M.; Khan, I.U.; Wang, J.; Li, Y.; Liu, X. 3D porous nanostructured Ni3N-Co3N as a robust electrode material for glucose fuel cell. RSC Adv. 2020, 10, 6444–6451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Zhang, L.; Liu, C.-S.; Pang, H. Core-shell-type ZIF-8@ZIF-67@POM hybrids as efficient electrocatalysts for the oxygen evolution reaction. Inorg. Chem. Front. 2019, 6, 2514–2520. [Google Scholar] [CrossRef]
- Abdelkader-Fernandez, V.K.; Fernandes, D.M.; Balula, S.S.; Cunha-Silva, L.; Freire, C. Oxygen Evolution Reaction Electrocatalytic Improvement in POM@ZIF Nanocomposites: A Bidirectional Synergistic Effect. ACS Appl. Energy Mater. 2020, 3, 2925–2934. [Google Scholar] [CrossRef]
- Tang, Y.; Zou, Z.; Wu, X.; Zuo, P.; Wang, L.; Huang, G.; Zhu, J.; Zhong, S. ZIF-67@POM hybrid-derived unique willow-shaped two-dimensional Mo-CoP nanostructures as efficient electrocatalysts for the oxygen evolution reaction. New J. Chem. 2023, 47, 9887–9893. [Google Scholar] [CrossRef]
- Abdelkader-Fernandez, V.K.; Fernandes, D.M.; Balula, S.S.; Cunha-Silva, L.; Freire, C. Advanced framework-modified POM@ZIF-67 nanocomposites as enhanced oxygen evolution reaction electrocatalysts. J. Mater. Chem. A 2020, 8, 13509–13521. [Google Scholar] [CrossRef]









| AC | T-CuPMo@ZIF-67 | T-FePMo@ZIF-67 | T-NiPMo@ZIF-67 | T-ZnPMo@ZIF-67 | T-PMo@ZIF-67 | |
|---|---|---|---|---|---|---|
| Rs (Ω) | 1.518 | 1.002 | 1.162 | 0.686 | 1.68 | 0.7869 |
| Rct (Ω) | 1.151 | 0.4188 | 0.6594 | 0.1755 | 2.643 | 0.3063 |
| Rd (Ω) | 1.957 | 0.7333 | 0.7416 | 0.201 | 2.403 | 0.3212 |
| Rt (Ω) | 4.626 | 2.1541 | 2.563 | 1.0625 | 6.726 | 1.4144 |
| Ni@T-PZ | Ni1Co3@T-PZ | Ni3Co1@T-PZ | Ni2Co2@T-PZ | T-PMo@ZIF-67 | |
|---|---|---|---|---|---|
| Rs (Ω) | 1.006 | 0.887 | 0.738 | 0.686 | 0.7869 |
| Rct (Ω) | 0.7582 | 0.4577 | 0.202 | 0.1755 | 0.3063 |
| Rd (Ω) | 0.967 | 0.8711 | 0.2467 | 0.201 | 0.3212 |
| Rt (Ω) | 2.7312 | 2.2158 | 1.1867 | 1.0625 | 1.4144 |
| Fitting Equation | R2 | Tafel (mV dec−1) | 10−4I0 (A cm−2) | |
|---|---|---|---|---|
| AC | y = 5.37143 × −3.41673 | 0.9983 | 186.1702 | 3.831 |
| Ni@T-PZ | y = 5.41948 × −3.08181 | 0.99818 | 184.5195 | 8.283 |
| Ni3Co1@T-PZ | y = 5.05714 × −2.45716 | 0.99876 | 197.7402 | 34.901 |
| Ni2Co2@T-PZ | y = 5.14286 × −2.42154 | 0.99758 | 194.4443 | 37.884 |
| Ni1Co3@T-PZ | y = 5.21818 × −2.99496 | 0.99807 | 191.6377 | 10.117 |
| T-PMo@ZIF-67 | y = 4.82208 × −2.61037 | 0.99766 | 207.3794 | 24.526 |
| Anode | Cathode | Catalyst | Power Density | Reference |
|---|---|---|---|---|
| Ni | Al | Pt | 2 μW cm−2; | [36] |
| Pt | Pt | Pt | 43 μW cm−2; | [37] |
| PtPd/graphene | N-doped graphene oxide nanoribbons | Pt | 24.9 µW cm−2; | [38] |
| Nanoporous gold | Pt/C | Pt/Bi | 8 mW cm−2; | [39] |
| Activated carbon/Ni-foam | Activated carbon/Ni-foam | Ni2Co2@T-PMo@ZIF-67 | 3.4 mW cm−2; | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, S.; Kang, N.; Liu, M.; Zhang, Y.; Li, Y.; Maryam, B.; Zhang, X.; Zhang, P.; Liu, X. Boosted Catalytic Performance of Ni2Co2@T-PMo@ZIF-67 for Glucose Oxidation in a Direct-Glucose Fuel Cell. Catalysts 2024, 14, 19. https://doi.org/10.3390/catal14010019
Jiao S, Kang N, Liu M, Zhang Y, Li Y, Maryam B, Zhang X, Zhang P, Liu X. Boosted Catalytic Performance of Ni2Co2@T-PMo@ZIF-67 for Glucose Oxidation in a Direct-Glucose Fuel Cell. Catalysts. 2024; 14(1):19. https://doi.org/10.3390/catal14010019
Chicago/Turabian StyleJiao, Shipu, Ning Kang, Miao Liu, Yihao Zhang, Yang Li, Bushra Maryam, Xu Zhang, Pingping Zhang, and Xianhua Liu. 2024. "Boosted Catalytic Performance of Ni2Co2@T-PMo@ZIF-67 for Glucose Oxidation in a Direct-Glucose Fuel Cell" Catalysts 14, no. 1: 19. https://doi.org/10.3390/catal14010019