The Origin of the Size Effect in the Oxidation of CO on Supported Palladium Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Study
3.3. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boudart, M.; Aldag, A.; Benson, J.E.; Dougharty, N.A.; Girvin Harkins, C. On the specific activity of platinum catalysts. J. Catal. 1966, 6, 92–99. [Google Scholar] [CrossRef]
- Kozuch, S.; Martin, J.M.L. “Turning over” definitions in catalytic cycles. ACS Catal. 2012, 2, 2787–2794. [Google Scholar] [CrossRef]
- Boudart, M. Catalysis by supported metals. Adv. Catal. 1969, 20, 153–166. [Google Scholar]
- Che, M.; Bennett, C.O. The influence of particle size on the catalytic properties of supported metals. Adv. Catal. 1989, 36, 55–172. [Google Scholar]
- Haruta, M.; Koboyashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Haruta, M.; Tsubota, S.; Kobayashi, T.; Kageyama, H.; Genet, M.J.; Delmon, B. Low-temperature oxidation of CO over gold supported on TiO2, α-Fe2O3, and Co3O4. J. Catal. 1993, 144, 175–192. [Google Scholar] [CrossRef]
- Emmanuel, J.; Hayden, B.E.; Saleh-Subaie, J. The particle size dependence of CO oxidation on model planar titania supported gold catalysts measured by parallel thermographic imaging. J. Catal. 2019, 369, 175–180. [Google Scholar] [CrossRef]
- Lopez, N.; Janssens, T.V.W.; Clausen, B.S.; Xu, Y.; Mavrikakis, M.; Bligaard, T.; Nørskov, J.K. On the origin of the catalytic activity of gold nanoparticles for low-temperature CO oxidation. J. Catal. 2004, 223, 232–235. [Google Scholar] [CrossRef]
- Janssens, T.V.W.; Clausen, B.S.; Hvolbæk, B.; Falsig, H.; Christensen, C.H.; Bligaard, T.; Nørskov, J.K. Insights into the reactivity of supported Au nanoparticles: Combining theory and experiments. Top. Catal. 2007, 44, 15–26. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, J.; Wu, Y.; Hu, W.; Qu, P.; Xiao, X.; Zhang, G.; Liu, X.; Jiao, Y.; Zhong, L.; et al. Particle size effects in stoichiometric methane combustion: Structure–activity relationship of Pd catalyst supported on gamma-alumina. ACS Catal. 2020, 10, 10339–10349. [Google Scholar] [CrossRef]
- Van Hardeveld, R.; Hartog, F. The statistics of surface atoms and surface sites on metal crystals. Surf. Sci. 1969, 15, 189–230. [Google Scholar] [CrossRef]
- Engel, T.; Ertl, G. Elementary steps in the catalytic oxidation of carbon monoxide on platinum metals. Adv. Catal. 1979, 28, 3214–3217. [Google Scholar]
- Ladas, S.; Poppa, H.; Boudart, M. The adsorption and catalytic oxidation of carbon monoxide on evaporated palladium particles. Surf. Sci. 1981, 102, 151–171. [Google Scholar] [CrossRef]
- Meusel, I.; Hoffmann, J.; Hartmann, J.; Heemeier, M.; Bäumer, M.; Libuda, J.; Freund, H.-J. The interaction of oxygen with alumina-supported palladium particles. Catal. Lett. 2001, 71, 5–13. [Google Scholar] [CrossRef]
- Van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Surface science under reaction conditions: CO oxidation on Pt and Pd model catalysts. Chem. Soc. Rev. 2017, 46, 4347–4374. [Google Scholar] [CrossRef]
- Mars, P.; Van Krevelen, D.W. Oxidations carried out by the means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Nilsson, J.; Carlsson, P.-A.; Grönbeck, H.; Skoglundh, M. First principles calculations of palladium nanoparticle XANES spectra. Top. Catal. 2017, 60, 283–288. [Google Scholar] [CrossRef]
- Muller, J.E.; Jepsen, O.; Andersen, O.K.; Wilkins, J.W. Systematic structure in the K-edge photoabsorption spectra of the 4d transition metals: Theory. Phys. Rev. Lett. 1978, 40, 720–722. [Google Scholar] [CrossRef]
- McCaulley, J.A. Temperature dependence of the Pd K-edge extended x-ray-absorption fine structure of PdCx (x~0.13). Phys. Rev. B 1993, 47, 4873–4879. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lemaux, S.; Demazeau, G.; Kim, J.-Y.; Choy, J.-H. X-Ray absorption spectroscopic study on LaPdO3. J. Mater. Chem. 2002, 12, 995–1000. [Google Scholar] [CrossRef]
- Hendriksen, B.L.M.; Ackermann, M.D.; Van Rijn, R.; Stoltz, D.; Popa, I.; Balmes, O.; Resta, A.; Wermeille, D.; Felici, R.; Ferrer, S.; et al. The role of steps in surface catalysis and reaction oscillations. Nature Chem. 2010, 2, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Baylet, A.; Marecot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In situ Raman and in situ XRD analysis of PdO reduction and Pd0 oxidation supported on γ-Al2O3 catalyst under different atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607–4613. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Chen, Q.-Y.; Luo, L.-F.; Huang, W.-X.; Luo, M.-F.; Hu, G.-S.; Lu, J.-Q. Kinetic study and the effect of particle size on low temperature CO oxidation over Pt/TiO2 catalysts. Appl. Catal. B 2013, 142–143, 523–532. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Vinokurov, Z.S.; Saraev, A.A. Self-sustained oscillations in oxidation of methane over palladium: The nature of “low-active” and “highly active” states. Catal. Sci. Technol. 2021, 11, 4392–4397. [Google Scholar] [CrossRef]
- Joo, S.H.; Park, J.Y.; Renzas, J.R.; Butcher, D.R.; Huang, W.; Somorjai, G.A. Size effect of ruthenium nanoparticles in catalytic carbon monoxide oxidation. Nano Lett. 2010, 10, 2709–2713. [Google Scholar] [CrossRef]
- Maksimov, G.M.; Gerasimov, E.Y.; Kenzhin, R.M.; Saraev, A.A.; Kaichev, V.V.; Vedyagin, A.A. CO oxidation over titania-supported gold catalysts obtained using polyoxometalate. Reac. Kinet. Mech. Catal. 2021, 132, 171–185. [Google Scholar] [CrossRef]
- Keita, B.; Liu, T.; Nadjo, L. Synthesis of remarkably stabilized metal nanostructures using polyoxometalates. J. Mater. Chem. 2009, 19, 19–33. [Google Scholar] [CrossRef]
- Wang, Y.; Weinstock, I.A. Polyoxometalate-decorated nanoparticles. Chem. Soc. Rev. 2012, 41, 7479–7496. [Google Scholar] [CrossRef]
- Andrushkevich, T.V.; Kaichev, V.V.; Chesalov, Y.A.; Saraev, A.A.; Buktiyarov, V.I. Selective oxidation of ethanol over vanadia-based catalysts: The influence of support material and reaction mechanism. Catal. Today 2017, 279, 95–106. [Google Scholar] [CrossRef]
- Caliebe, W.A.; Murzin, V.; Kalinko, A.; Gorlitz, M. High-flux XAFS-Beamline P64 at PETRA III. AIP Conf. Proc. 2019, 2054, 060031. [Google Scholar]
- Bearden, J.A.; Burr, A.F. Reevaluation of X-ray atomic energy levels. Rev. Mod. Phys. 1967, 39, 125–142. [Google Scholar] [CrossRef]
- Clausen, B.S.; Steffensen, G.; Fabius, B.; Villadsen, J.; Feidenhans’l, R.; Topsøe, H. In situ cell for combined XRD and on-line catalysis tests: Studies of Cu-based water gas shift and methanol catalysts. J. Catal. 1991, 132, 524–535. [Google Scholar] [CrossRef]
- Grunwaldt, J.-D.; Caravati, M.; Hannemann, S.; Baiker, A. X-ray absorption spectroscopy under reaction conditions: Suitability of different reaction cells for combined catalyst characterization and time-resolved studies. Phys. Chem. Chem. Phys. 2004, 6, 3037–3047. [Google Scholar] [CrossRef]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Rad. 2001, 8, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Ravel, B.; Newville, M. ATHEAN, ARTERMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron. Rad. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Fedorov, A.; Saraev, A.; Kremneva, A.; Selivanova, A.; Vorokhta, M.; Šmíd, B.; Bulavchenko, O.; Yakovlev, V.; Kaichev, V. Kinetic and mechanistic study of CO oxidation over nanocomposite Cu–Fe–Al oxide catalysts. ChemCatChem 2020, 12, 4911–4921. [Google Scholar] [CrossRef]



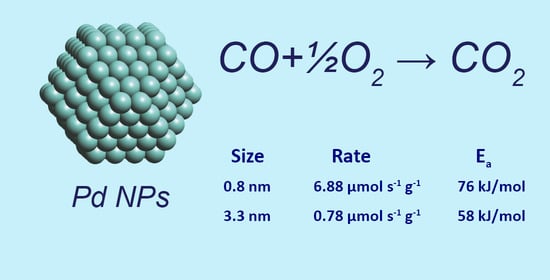
| Catalyst | d, nm | Ns, μmol/g | Ea, kJ/mol | TOF, s−1 | |
|---|---|---|---|---|---|
| Pd-1 | 0.8 | 6.88 | 49.5 | 76 ± 4 | 0.14 |
| Pd-3 | 3.3 | 0.78 | 34.8 | 58 ± 4 | 0.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaichev, V.V.; Saraev, A.A.; Fedorov, A.V.; Gerasimov, E.Y. The Origin of the Size Effect in the Oxidation of CO on Supported Palladium Nanoparticles. Catalysts 2023, 13, 1435. https://doi.org/10.3390/catal13111435
Kaichev VV, Saraev AA, Fedorov AV, Gerasimov EY. The Origin of the Size Effect in the Oxidation of CO on Supported Palladium Nanoparticles. Catalysts. 2023; 13(11):1435. https://doi.org/10.3390/catal13111435
Chicago/Turabian StyleKaichev, Vasily V., Andrey A. Saraev, Aleksandr V. Fedorov, and Evgeny Yu. Gerasimov. 2023. "The Origin of the Size Effect in the Oxidation of CO on Supported Palladium Nanoparticles" Catalysts 13, no. 11: 1435. https://doi.org/10.3390/catal13111435