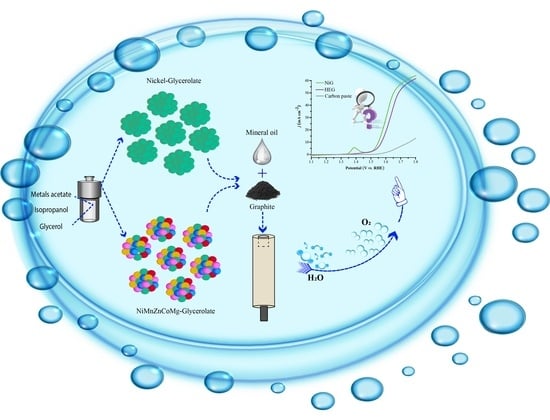
Nickel Glycerolate Overcoming a High-Entropy Configuration for High-Performance Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization
2.2. Electrochemical Characterization
| Electrocatalyst | Substrate | η10 (mV @ ten mA cm–2) | Tafel Slope (mV dec−1) | Stability (Hr) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|
| IrO2 | GCE | 380 | 83 | - | 1.0 M KOH | [35] |
| RuO2 | GCE | 350 | 106 | - | 1.0 M KOH | [35] |
| Ni6Co11Mn-LDH | GCE | 248 | 72.2 | 22 (j = 100 mA cm–2) | 1.0 M KOH | [36] |
| CrMnFeCoNiTiZn-glycerolate | Ni foam | 251 η50 (50 mA cm–2) | 42.3 | 60 | 1.0 M KOH | [1] |
| Mn-Co-P | GCE | 330 | 59 | 8 | 1.0 M KOH | [37] |
| Ni2Zn0.5Fe-LDH | CPE | 370 | 16 | 13 | Buffer pH = 7 | [32] |
| NiMn-DLH | GCE | 296 | 102 | 24 | 1.0 M KOH | [31] |
| NiCo-Fe LDH | GCE | 285 | 62 | 12 | 1.0 M KOH | [38] |
| CoCuFe-Gly | GCE | 317 | 69 | 69 | 1.0 M KOH | [39] |
| NiMnZnMgCoG | CPE | 342 | 83.2 | 24 | 1.0 M KOH | This work |
| NiG | CPE | 310 | 109.2 | 24 | 1.0 M KOH | This work |
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis
3.3. Preparation of Modified Carbon Paste Electrodes
3.4. Characterization
3.5. Scanning Electron Microscope (SEM)
3.6. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ting, N.-H.; Nguyen, T.X.; Lee, C.-H.; Chen, Y.-C.; Yeh, C.-H.; Chen, H.-Y.T.; Ting, J.-M. Composition-controlled high entropy metal glycerate as high-performance electrocatalyst for oxygen evolution reaction. Appl. Mater. Today 2022, 27, 101398. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Martins, P.R.; Angnes, L.; Araki, K. Recent advances in ternary layered double hydroxide electrocatalysts for the oxygen evolution reaction. New J. Chem. 2020, 44, 9981–9997. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Q.; Yan, B.; You, B.; Zheng, J.; Feng, L.; Zhang, C.; Jiang, S.; Chen, W.; He, S. Facet Engineering of Advanced Electrocatalysts Toward Hydrogen/Oxygen Evolution Reactions. Nanomicro Lett. 2023, 15, 52. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, X.; Li, J.; Qian, J.; Liu, Y.; Yang, S.; Wang, Y.; Gao, D.; Xue, D. Interfacial Engineering of NiO/NiCo2O4 Porous Nanofibers as Efficient Bifunctional Catalysts for Rechargeable Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2020, 12, 21661–21669. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, S.; Lin, H.; Wang, G.; Zhao, K.; Cai, R.; Tao, K.; Zhang, C.; Sun, M.; Hu, J.; et al. Atomically targeting NiFe LDH to create multivacancies for OER catalysis with a small organic anchor. Nano Energy 2021, 81, 105606. [Google Scholar] [CrossRef]
- Liu, Y.; Ran, N.; Ge, R.; Liu, J.; Li, W.; Chen, Y.; Feng, L.; Che, R. Porous Mn-doped cobalt phosphide nanosheets as highly active electrocatalysts for oxygen evolution reaction. Chem. Eng. J. 2021, 425, 131642. [Google Scholar] [CrossRef]
- Du, X.; Ding, Y.; Zhang, X. MOF-derived Zn–Co–Ni sulfides with hollow nanosword arrays for high-efficiency overall water and urea electrolysis. Green Energy Environ. 2023, 8, 798–811. [Google Scholar] [CrossRef]
- Xu, S.; Li, M.; Wang, H.; Sun, Y.; Liu, W.; Duan, J.; Chen, S. High-Entropy Metal–Organic Framework Arrays Boost Oxygen Evolution Electrocatalysis. J. Phys. Chem. C 2022, 126, 14094–14102. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Ruiz-Montoya, J.G. Emerging high-entropy coordination compounds and their derivatives for energy application. J. Mater. Chem. A 2023, 11, 20872–20885. [Google Scholar] [CrossRef]
- Ede, S.R.; Luo, Z. Tuning the intrinsic catalytic activities of oxygen-evolution catalysts by doping: A comprehensive review. J. Mater. Chem. A 2021, 9, 20131–20163. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Santos, É.A.; Martins, P.R.; Silva, C.G.; Zanin, H. Emerging medium- and high-entropy materials as catalysts for lithium-sulfur batteries. Energy Stor. Mater. 2023, 63, 102999. [Google Scholar] [CrossRef]
- Ritter, T.G.; Phakatkar, A.H.; Rasul, M.G.; Saray, M.T.; Sorokina, L.V.; Shokuhfar, T.; Gonçalves, J.M.; Shahbazian-Yassar, R. Electrochemical synthesis of high entropy hydroxides and oxides boosted by hydrogen evolution reaction. Cell Rep. Phys. Sci. 2022, 3, 100847. [Google Scholar] [CrossRef]
- He, B.; Zu, Y.; Mei, Y. Design of advanced electrocatalysts for the high-entropy alloys: Principle, progress, and perspective. J. Alloys Compd. 2023, 958, 170479. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Hennemann, A.L.; Ruiz-Montoya, J.G.; Martins, P.R.; Araki, K.; Angnes, L.; Shahbazian-Yassar, R. Metal-glycerolates and their derivatives as electrode materials: A review on recent developments, challenges, and future perspectives. Coord. Chem. Rev. 2023, 477, 214954. [Google Scholar] [CrossRef]
- Horn, M.; Horns, U. Alkoxides, metal. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Phakatkar, A.H.; Gonçalves, J.M.; Zhou, J.; Ritter, T.G.; Tamadoni Saray, M.; Sorokina, L.V.; Amiri, A.; Angnes, L.; Shokuhfar, T.; Shahbazian-Yassar, R. Enhanced Bacterial Growth by Polyelemental Glycerolate Particles. ACS Appl. Bio Mater. 2023, 6, 1515–1524. [Google Scholar] [CrossRef]
- Ooka, H.; Huang, J.; Exner, K.S. The Sabatier Principle in Electrocatalysis: Basics, Limitations, and Extensions. Front. Energy Res. 2021, 9, 654460. [Google Scholar] [CrossRef]
- Wang, C.; Yan, B.; Chen, Z.; You, B.; Liao, T.; Zhang, Q.; Lu, Y.; Jiang, S.; He, S. Recent advances in carbon substrate supported nonprecious nanoarrays for electrocatalytic oxygen evolution. J. Mater. Chem. A 2021, 9, 25773–25795. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Abu-Jdayil, B. Crude Glycerol as a Potential Feedstock for Future Energy via Thermochemical Conversion Processes: A Review. Sustainability 2021, 13, 12813. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Su, Y.H.; Lin, C.C.; Ruan, J.; Ting, J.M. A New High Entropy Glycerate for High Performance Oxygen Evolution Reaction. Adv. Sci. 2021, 8, 2002446. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, J.; Ai, L. Layered Bimetallic Iron–Nickel Alkoxide Microspheres as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. ACS Sustain. Chem. Eng. 2018, 6, 6117–6125. [Google Scholar] [CrossRef]
- Reinoso, D.M.; Damiani, D.E.; Tonetto, G.M. Zinc glycerolate as a novel heterogeneous catalyst for the synthesis of fatty acid methyl esters. Appl. Catal. B 2014, 144, 308–316. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, W.; Xiao, Y.; Wang, Y.; Luan, C.; Qin, C.; Dong, Y.; Li, M.; Dai, X.; Zhang, X. One-Pot-Synthesized CoFe-Glycerate Hollow Spheres with Rich Oxyhydroxides for Efficient Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2020, 8, 5464–5477. [Google Scholar] [CrossRef]
- Larcher, D.; Sudant, G.; Patrice, R.; Tarascon, J.M. Some Insights on the Use of Polyols-Based Metal Alkoxides Powders as Precursors for Tailored Metal-Oxides Particles. Chem. Mater. 2003, 15, 3543–3551. [Google Scholar] [CrossRef]
- Gomes, J.F.; Garcia, A.C.; Gasparotto, L.H.S.; de Souza, N.E.; Ferreira, E.B.; Pires, C.; Tremiliosi-Filho, G. Influence of silver on the glycerol electro-oxidation over AuAg/C catalysts in alkaline medium: A cyclic voltammetry and in situ FTIR spectroscopy study. Electrochim. Acta 2014, 144, 361–368. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Lima, I.S.; Phakatkar, A.H.; Pereira, R.S.; Martins, P.R.; Araki, K.; Angnes, L.; Shahbazian-Yassar, R. What are the Tower’s method products: Metal-hydroxides or metal-glycerolates? Mater. Charact. 2023, 196, 112636. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Ghorbani, A.; Ritter, T.G.; Lima, I.S.; Saray, M.T.; Phakatkar, A.H.; Silva, V.D.; Pereira, R.S.; Yarin, A.L.; Angnes, L.; et al. Multimetallic glycerolate as a precursor template of spherical porous high-entropy oxide microparticles. J. Colloid Interface Sci. 2023, 641, 643–652. [Google Scholar] [CrossRef]
- Ding, S.; An, J.; Ding, D.; Zou, Y.; Zhao, L. Micron-sized NiMn-glycerate solid spheres as cathode materials for all-solid-state asymmetric supercapacitor with superior energy density and cycling life. Chem. Eng. J. 2022, 431, 134100. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Fathoni, K.B.; Kani, K.; Allah, A.E.; Yuliarto, B.; Nugraha; Dipojono, H.K.; Alothman, Z.A.; Golberg, D.; et al. Self-Assembly of Two-Dimensional Bimetallic Nickel–Cobalt Phosphate Nanoplates into One-Dimensional Porous Chainlike Architecture for Efficient Oxygen Evolution Reaction. Chem. Mater. 2020, 32, 7005–7018. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Zhang, Y.; Rao, G.; Wu, C.; Hu, Y.; Wang, X.; Lu, R.; Li, Y.; Xiong, J. Identification of Key Reversible Intermediates in Self-Reconstructed Nickel-Based Hybrid Electrocatalysts for Oxygen Evolution. Angew. Chem. 2019, 131, 17619–17625. [Google Scholar] [CrossRef]
- Zhao, Z.; Lamoureux, P.S.; Kulkarni, A.; Bajdich, M. Trends in Oxygen Electrocatalysis of 3 d-Layered (Oxy)(Hydro)Oxides. ChemCatChem 2019, 11, 3423–3431. [Google Scholar] [CrossRef]
- Foruzin, L.J.; Rezvani, Z.; Shishavan, Y.H.; Habibi, B. Ni2Zn0.5Fe-LDH modified carbon paste electrode as an efficient electrocatalyst for water oxidation in neutral media. Int. J. Hydrogen Energy 2018, 43, 150–160. [Google Scholar] [CrossRef]
- Wang, H.; Feng, E.-M.; Liu, Y.-M.; Zhang, C.-Y. High-performance hierarchical ultrathin sheet-based CoOOH hollow nanospheres with rich oxygen vacancies for the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 7777–7783. [Google Scholar] [CrossRef]
- Anantharaj, S.; Noda, S. Appropriate Use of Electrochemical Impedance Spectroscopy in Water Splitting Electrocatalysis. ChemElectroChem 2020, 7, 2297–2308. [Google Scholar] [CrossRef]
- Samanta, R.; Panda, P.; Mishra, R.; Barman, S. IrO2-Modified RuO2 Nanowires/Nitrogen-Doped Carbon Composite for Effective Overall Water Splitting in All pH. Energy Fuels 2022, 36, 1015–1026. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, H.; Han, J.; Yan, F.; Sun, C. Manganese-Doped Hollow Layered Double (Ni, Co) Hydroxide Microcuboids as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. ChemElectroChem 2020, 7, 3852–3858. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Guo, Y.; Septiani, N.L.W.; Iqbal, M.; Jiang, X.; Takei, T.; Yuliarto, B.; Alothman, Z.A.; Golberg, D.; Yamauchi, Y. Self-templated fabrication of hierarchical hollow manganese-cobalt phosphide yolk-shell spheres for enhanced oxygen evolution reaction. Chem. Eng. J. 2021, 405, 126580. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Guo, Y.; Yuliarto, B.; Jiang, X.; Ide, Y.; Nugraha, N.; Dipojono, H.K.; Yu, A.; Sugahara, Y.; et al. Holey Assembly of Two-Dimensional Iron-Doped Nickel-Cobalt Layered Double Hydroxide Nanosheets for Energy Conversion Application. ChemSusChem 2020, 13, 1645–1655. [Google Scholar] [CrossRef]
- Moradi, M.; Hasanvandian, F.; Afshar, M.G.; Larimi, A.; Khorasheh, F.; Niknam, E.; Setayesh, S.R. Incorporation of Fe in mixed CoCu-alkoxide hollow sphere for enhancing the electrochemical water oxidation performance. Mater. Today Chem. 2021, 22, 100586. [Google Scholar] [CrossRef]
- Enman, L.J.; Burke, M.S.; Batchellor, A.S.; Boettcher, S.W. Effects of Intentionally Incorporated Metal Cations on the Oxygen Evolution Electrocatalytic Activity of Nickel (Oxy)hydroxide in Alkaline Media. ACS Catal. 2016, 6, 2416–2423. [Google Scholar] [CrossRef]







| Material | Ni (At%) | Mn (At%) | Zn (At%) | Mg (At%) | Co (At%) |
|---|---|---|---|---|---|
| NiG | 100 | - | - | - | - |
| NiMnG | 51.20 | 48.79 | - | - | - |
| NiMnZnG | 32.78 | 33.17 | 34.05 | - | - |
| NiMnZnMgCoG | 19.36 | 21.65 | 20.01 | 18.21 | 20.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, I.S.; Pereira, R.S.; Ritter, T.G.; Shahbazian-Yassar, R.; Gonçalves, J.M.; Angnes, L. Nickel Glycerolate Overcoming a High-Entropy Configuration for High-Performance Oxygen Evolution Reaction. Catalysts 2023, 13, 1371. https://doi.org/10.3390/catal13101371
Lima IS, Pereira RS, Ritter TG, Shahbazian-Yassar R, Gonçalves JM, Angnes L. Nickel Glycerolate Overcoming a High-Entropy Configuration for High-Performance Oxygen Evolution Reaction. Catalysts. 2023; 13(10):1371. https://doi.org/10.3390/catal13101371
Chicago/Turabian StyleLima, Irlan S., Rafael S. Pereira, Timothy G. Ritter, Reza Shahbazian-Yassar, Josué M. Gonçalves, and Lúcio Angnes. 2023. "Nickel Glycerolate Overcoming a High-Entropy Configuration for High-Performance Oxygen Evolution Reaction" Catalysts 13, no. 10: 1371. https://doi.org/10.3390/catal13101371