Conversion of Sugarcane Trash to Nanocrystalline Cellulose and its Life Cycle Assessment
Abstract
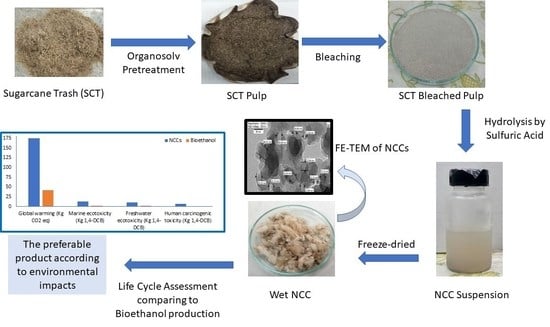
:1. Introduction
2. Results and Discussion
2.1. Effects of Reaction Temperature and Catalyst for Organosolv Pretreatment
2.2. Bleaching
2.3. Morphological Analysis
2.4. X-ray Diffraction Analysis (XRD)
2.5. Fourier-Transform Infrared Spectroscopy (FTIR) Characterization
2.6. Life Cycle Impact Assessment
2.6.1. Global Warming Impact on NCC Production
2.6.2. Marine Ecotoxicity Impact of NCC Production
2.6.3. Freshwater Ecotoxicity Impact of NCC Production
2.6.4. Human Carcinogenic Toxicity Impact of NCC Production
3. Materials and Methods
3.1. Materials
3.2. Pretreatment Using Acid Catalyst
3.3. NCC Production
3.3.1. Bleaching
3.3.2. Extraction
3.3.3. Characterization Procedures
3.4. Life Cycle Assessment
3.4.1. Goal and Scope Definition
3.4.2. Life Cycle Inventory
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walter, D.; Virender, K.S.; Mengshan, L.; Govind, N.; Rajender, S.V. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Chapter one: Insights into lignin degradation and its potential industrial applications. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Elsevier Press: Amsterdam, The Netherlands, 2013; pp. 1–28. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Characterization of non-edible lignocellulosic biomass in terms of their candidacy towards alternative renewable fuels. Biomass Convers. Biorefinery 2018, 8, 799–812. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pretreatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [Green Version]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Maw Maw, T.; Dagmar, J.; Myo Min, W.; Aung Myat, T.; Tomáš, P. Biomass Energy: An Overview of Biomass Sources, Energy Potential, and Management in Southeast Asian Countries. Resources 2019, 8, 81. [Google Scholar]
- Jimén, D.J.; Dini-Andreote, F.; Van Elsas, J.D. Metataxonomic profiling and prediction of functional behaviour of wheat straw degrading microbial consortia. Biotechnol. Biofuels 2014, 7, 2–30. [Google Scholar]
- Photphisutthiphong, Y.; Vatanyoopaisarn, S. Dyadobacter and Sphingobacterium isolated from herbivore manure in Thailand and their cellulolytic activity in various organic waste substrates. Agric. Nat. Resour. 2019, 53, 89–98. [Google Scholar]
- Powar, R.V.; Mehetre, S.A.; Powar, T.R.; Patil, S.B. End-Use Applications of Sugarcane Trash: A Comprehensive Review. Sugar. Tech. 2022, 24, 699–714. [Google Scholar] [CrossRef]
- Cardoen, D.; Joshi, P.; Diels, L.; Sarma, P.M.; Pant, D. Agriculture biomass in India: Part 1. Estimation and characterization. Resour. Conserv. Recycl. 2015, 102, 39–48. [Google Scholar] [CrossRef]
- Salimi, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of Nanocellulose and Its Applications in Drug Delivery: A Critical Review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Thomas, B.; Raj, M.C.; Athira, K.B.; Rubiah, M.H.; Joy, J.; Moores, A.; Drisko, G.L.; Sanchez, C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575–11625. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; De Lannoy, C.F.; Wiesner, M.R. Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef]
- Manhas, N.; Balasubramanian, K.; Prajith, P.; Rule, P.; Nimje, S. PCL/PVA Nanoencapsulated Reinforcing Fillers of Steam Exploded/Autoclaved Cellulose Nanofibrils for Tissue Engineering Applications. RSC Adv. 2015, 5, 23999–24008. [Google Scholar] [CrossRef]
- Vilarinho, F.; Sanches Silva, A.; Vaz, M.F.; Farinha, J.P. Nanocellulose in green food packaging. Crit. Rev. Food Sci. Nutr. 2018, 58, 1526–1537. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from Lignocellulosic Biomass: Current Findings Determine Research Priorities. Sci. World J. 2014, 2014, 298153. [Google Scholar] [CrossRef] [PubMed]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Hanaki, K.; Portugal-Pereira, J. The Effect of Biofuel Production on Greenhouse Gas Emission Reductions. In Biofuels and Sustainability. Science for Sustainable Societies; Takeuchi, K., Shiroyama, H., Saito, O., Matsuura, M., Eds.; Springer: Tokyo, Japan, 2018; pp. 53–71. [Google Scholar]
- Clauser, N.M.; González, G.; Mendieta, C.M.; Kruyeniski, J.; Area, M.C.; Vallejos, M.E. Biomass Waste as Sustainable Raw Material for Energy and Fuels. Sustainability 2021, 13, 794. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Zheng, D.; Li, M.; Yue, J. Isolation and characterization of nanocellulose crystals via acid hydrolysis from agricultural waste-tea stalk. Int. J. Biol. Macromol. 2020, 163, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Inkrod, C.; Raita, M.; Laosiripojana, N.; Champreda, V. Characteristics of Lignin Extracted from Different Lignocellulosic Materials via Organosolv Fractionation. Bioenergy Res. 2018, 11, 277–290. [Google Scholar] [CrossRef]
- Chin, D.W.K.; Lim, S.; Pang, Y.L.; Lim, C.H.; Shuit, S.H.; Lee, K.M.; Chong, C.T. Effects of Organic Solvents on the Organosolv Pretreatment of Degraded Empty Fruit Bunch for Fractionation and Lignin Removal. Sustainability 2021, 13, 6757. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Yadama, V.; Lowell, E.; Espinoza-Herrera, R. A review of wood thermal pretreatments to improve wood composite properties. Wood Sci. Technol. 2013, 47, 1285–1319. [Google Scholar] [CrossRef]
- Klamrassamee, T.; Champreda, V.; Reunglek, V.; Laosiripojana, N. Comparison of homogeneous and heterogeneous acid promoters in single-step aqueous-organosolv fractionation of eucalyptus wood chips. Bioresour. Technol. 2013, 147, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Mariano, M.; Rabelo, S.C.; Gouveia, R.F.; Lona, L.M.F. Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: From a micro- to a nano-scale view. Appl. Surf. Sci. 2018, 436, 1113–1122. [Google Scholar] [CrossRef]
- Mân Vu, T.H.; Pakkanen, H.; Alén, R. Delignification of bamboo (Bambusa procera acher): Part 1. Kraft pulping and the subsequent oxygen delignification to pulp with a low kappa number. Ind. Crops Prod. 2004, 19, 49–57. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Rocha, G.J.M.; Roberto, I.C. Hydrogen peroxide bleaching of cellulose pulps obtained from brewer’s spent grain. Cellulose 2008, 15, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Muangmeesri, S.; Li, N.; Georgouvelas, D.; Ouagne, P.; Placet, V.; Mathew, A.P.; Samec, J.S.M. Holistic Valorization of Hemp through Reductive Catalytic Fractionation. ACS Sustain. Chem. Eng. 2021, 9, 17207–17213. [Google Scholar] [CrossRef]
- Dong, X.M.; Revol, J.-F.; Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G. Crystalline Nanocellulose—Preparation, Modification, and Properties. In Cellulose—Fundamental Aspects and Current Trends; IntechOpen: London, UK, 2015. [Google Scholar]
- Saïd Azizi Samir, M.A.; Alloin, F.; Paillet, M.; Dufresne, A. Tangling Effect in Fibrillated Cellulose Reinforced Nanocomposites. Macromolecules 2004, 37, 4313–4316. [Google Scholar] [CrossRef]
- Kusmono; Listyanda, R.F.; Wildan, M.W.; Ilman, M.N. Preparation and characterization of cellulose nanocrystal extracted from ramie fibers by sulfuric acid hydrolysis. Heliyon 2020, 6, e05486. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, W.T.; Rochliadi, A.; Arcana, I.M. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012045. [Google Scholar] [CrossRef]
- Zianor Azrina, Z.A.; Beg, M.D.H.; Rosli, M.Y.; Ramli, R.; Junadi, N.; Alam, A.K.M.M. Spherical nanocrystalline cellulose (NCC) from oil palm empty fruit bunch pulp via ultrasound assisted hydrolysis. Carbohydr. Polym. 2017, 162, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Mehanny, S.; Magd, E.E.A.-E.; Ibrahim, M.; Farag, M.; Gil-San-Millan, R.; Navarro, J.; Habbak, A.E.H.E.; El-Kashif, E. Extraction and characterization of nanocellulose from three types of palm residues. J. Mater. Res. Technol. 2021, 10, 526–537. [Google Scholar] [CrossRef]
- Holilah, H.; Bahruji, H.; Ediati, R.; Asranudin, A.; Jalil, A.A.; Piluharto, B.; Nugraha, R.E.; Prasetyoko, D. Uniform rod and spherical nanocrystalline celluloses from hydrolysis of industrial pepper waste (Piper nigrum L.) using organic acid and inorganic acid. Int. J. Biol. Macromol. 2022, 204, 593–605. [Google Scholar] [CrossRef]
- Gong, J.; Li, J.; Xu, J.; Xiang, Z.; Mo, L. Research on cellulose nanocrystals produced from cellulose sources with various polymorphs. RSC Adv. 2017, 7, 33486–33493. [Google Scholar] [CrossRef] [Green Version]
- Csiszar, E.; Kalic, P.; Kobol, A.; Ferreira Ede, P. The effect of low frequency ultrasound on the production and properties of nanocrystalline cellulose suspensions and films. Ultrason. Sonochem 2016, 31, 473–480. [Google Scholar] [CrossRef]
- El Achaby, M.; El Miri, N.; Aboulkas, A.; Zahouily, M.; Bilal, E.; Barakat, A.; Solhy, A. Processing and properties of eco-friendly bio-nanocomposite films filled with cellulose nanocrystals from sugarcane bagasse. Int. J. Biol. Macromol. 2017, 96, 340–352. [Google Scholar] [CrossRef]
- Khawas, P.; Deka, S.C. Isolation and characterization of cellulose nanofibers from culinary banana peel using high-intensity ultrasonication combined with chemical treatment. Carbohydr. Polym. 2016, 137, 608–616. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Ahmad, I.; Abdullah, I.; Dufresne, A.; Zainudin, S.Y.; Sheltami, R.M. Effects of hydrolysis conditions on the morphology, crystallinity, and thermal stability of cellulose nanocrystals extracted from kenaf bast fibers. Cellulose 2012, 19, 855–866. [Google Scholar] [CrossRef]
- Listyanda, R.F.; Kusmono Wildan, M.W.; Ilman, M.N. Extraction and characterization of nanocystalline cellulose (NCC) from ramie fiber by suphuric acid hydrolysis. AIP Conf. Proc. 2020, 2217, 030069. [Google Scholar]
- Asrofi, M.; Abral, H.; Kasim, A.; Pratoto, A. XRD and FTIR Studies of Nanocrystalline Cellulose from Water Hyacinth (Eichornia crassipes) Fiber. J. Metastable Nanocrystalline Mater. 2017, 29, 9–16. [Google Scholar] [CrossRef]
- Sun, J.X.; Xu, F.; Sun, X.F.; Xiao, B.; Sun, R.C. Physico-chemical and thermal characterization of cellulose from barley straw. Polym. Degrad. Stab. 2005, 88, 521–531. [Google Scholar] [CrossRef]
- Sungsinchai, S. Production and Characterization of Nanocellulose and Its Applications as Food Thickener and Emulsifier. In Chemical Engineering; Kasersart Uiniversity: Bangkok, Thailand, 2021. [Google Scholar]
- Burhani, D.; Septevani, A.A.; Setiawan, R.; Djannah, L.M.; Putra, M.A.; Kusumah, S.S.; Sondari, D. Self-Assembled Behavior of Ultralightweight Aerogel from a Mixture of CNC/CNF from Oil Palm Empty Fruit Bunches. Polymers 2021, 13, 2649. [Google Scholar] [CrossRef] [PubMed]
- Casas, A.; Alonso, M.V.; Oliet, M.; Santos, T.M.; Rodriguez, F. Characterization of Cellulose regenerated from solutions of pine and eucalyptus woods in 1-allyl-3-methilimidazolium chloride. Carbohydr. Polym. 2013, 92, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Ingole, V.H.; Vuherer, T.; Maver, U.; Vinchurkar, A.; Ghule, A.V.; Kokol, V. Mechanical Properties and Cytotoxicity of Differently Structured Nanocellulose-hydroxyapatite Based Composites for Bone Regeneration Application. Nanomaterials 2019, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Sungsinchai, S.; Niamnuy, C.; Seubsai, A.; Prapainainar, P.; Wattanapan, P.; Thakhiew, W.; Raghavan, V.; Devahastin, S. Comparative evaluation of the effect of microfluidisation on physicochemical properties and usability as food thickener and Pickering emulsifier of autoclaved and Tempo-oxidized nanofibrillated cellulose. Int. J. Food Sci. Technol. 2021, 56, 4298–4315. [Google Scholar] [CrossRef]
- Murakami, T. Creating Better Social Acceptance for Electric Power Infrastructure. ERIA Res. Proj. Rep. 2017, 2017, 3–18. [Google Scholar]
- Andres, R.J.; Boden, T.A.; Bréon, F.M.; Ciais, P.; Davis, S.; Erickson, D.; Gregg, J.S.; Jacobson, A.; Marland, G.; Miller, J.; et al. A synthesis of carbon dioxide emissions from fossil-fuel combustion. Biogeosciences 2012, 9, 1845–1871. [Google Scholar] [CrossRef] [Green Version]
- Raghuvanshi, S.; Babu, B.V. Biofiltration for r0emoval of methyl isobutyl ketone (MIBK): Experimental studies and kinetic modelling. Environ. Technol. 2010, 31, 29–40. [Google Scholar] [CrossRef]
- Tsai, W.T. Fate of Chloromethanes in the Atmospheric Environment: Implications for Human Health, Ozone Formation and Depletion, and Global Warming Impacts. Toxics 2017, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Heijungs, R.; Koning, A.D. Improvement of LCA Characterization Factors and LCA Practice for Metals; Institute of Environmental Sciences—Leiden University: Apeldoorn, The Netherlands, 2004. [Google Scholar]
- Stringer, D.A. Joint Assessment Commodity of Chemicals No. 8 Methyl Isobutyl Ketone CAS: 108-10-1; ECETOC: Brussels, Belgium, 1987. [Google Scholar]
- Chipman, K. Enviromental Health Crileria 117 MethyI Isobutyl Ketone; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Atilgan, B.; Azapagic, A. Assessing the Environmental Sustainability of Electricity Generation in Turkey on a Life Cycle Basis. Energies 2016, 9, 31. [Google Scholar] [CrossRef] [Green Version]
- Albanese, S.; Cicchella, D. Legacy Problems in Urban Geochemistry. Elements 2012, 8, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Hameed, S.; Dignon, J. Global Emissions of Nitrogen and Sulfur Oxides in Fossil Fuel Combustion 1970–1986. J. Air Waste Manag. Assoc. 2012, 42, 159–163. [Google Scholar] [CrossRef]
- Amoatey, P.; Omidvarborna, H.; Baawain, M.S.; Al-Mamun, A. Emissions and exposure assessments of SOX, NOX, PM10/2.5 and trace metals from oil industries: A review study (2000–2018). Process. Saf. Environ. Prot. 2019, 123, 215–228. [Google Scholar] [CrossRef]
- Johnson, W., Jr. Safety assessment of MIBK (methyl isobutyl ketone). Int. J. Toxicol. 2004, 23, 29–57. [Google Scholar] [PubMed]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food. Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Michel, K.; Sluiter, J.; Payne, C.; Ness, R.; Thornton, B.; Reed, M.; Schwartz, A.; Wolfrum, E. Determination of Cellulosic Glucan Content in Starch Containing Feedstocks; National Renewable Energy Laboratory: Golden, CO, USA, 2021. [Google Scholar]
- Peng, Y.; Gardner, D.J.; Han, Y.; Cai, Z.; Tshabalala, M.A. Influence of drying method on the surface energy of cellulose nanofibrils determined by inverse gas chromatography. J. Colloid Interface Sci. 2013, 405, 85–95. [Google Scholar] [CrossRef]
- Kaushik, M.; Chen, W.C.; Ven, T.G.M.V.D.; Moores, A. An Improved Methodology for Imaging Cellulose Nanocrystals by Transmission Electron Microscopy. Nord. Pulp Pap. Res. J. 2014, 25, 77–84. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose using the X-ray Diffractomete. Text. Res. J. 1957, 25, 786–794. [Google Scholar]








| Temperature (°C) | Solid Yield (%) | Pulp Composition (%) | Cellulose Recovery (%) | Removal Efficiency (%) | Cellulose Purity (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cellulose | Lignin | Hemicellulose | Ash | Hemicellulose | Lignin | ||||
| Untreated SCT | - | 33.35 ± 0.2 | 22.70 ± 0.8 | 20.26 ± 0.4 | 7.13 ± 0.1 | - | - | - | - |
| 140 | 46.4 ± 0.26 | 63.20 ± 2.6 | 14.47 ± 1.3 | 7.90 ± 0.5 | 9.33 ± 0.4 | 97.56 ± 4.0 | 83.83 ± 1.8 | 66.99 ± 1.5 | 66.60 ± 0.5 |
| 160 | 41.6 ± 0.18 | 64.45 ± 2.8 | 11.66 ± 0.6 | 4.63 ± 0.3 | 11.54 ± 0.8 | 89.09 ± 1.2 | 91.54 ± 0.5 | 76.08 ± 2.1 | 69.82 ± 1.7 |
| 180 | 34.7 ± 0.08 | 69.04 ± 0.1 | 11.01± 0.6 | 1.14 ± 0.4 | 12.73 ± 0.2 | 79.92 ± 1.3 | 98.24 ± 1.0 | 81.15 ± 1.3 | 73.51 ± 1.1 |
| Pretreated and Bleached SCT (PB-SCT) | 18.2 ± 0.05 | 76.22 ± 1.5 | 3.05 ± 0.9 | 0.15 ± 0.02 | 0.47 ± 0.1 | 27.94 ± 0.7 | 99.92 ± 0.01 | 98.48 ± 0.4 | 95.42 ± 1.1 |
| No | Process | Input | Output | ||
|---|---|---|---|---|---|
| Feeding | Amount | Product | Amount (Kg) | ||
| 1 | Pretreatment | MIBK (Kg) | 6.82 | Pulp | 0.35 |
| Ethanol (Kg) | 3.31 | Lignin | 0.16 | ||
| H2SO4 (Kg) | 0.04 | Water | 1.1 | ||
| SCT (Kg) | 1 | Waste vapor | 6.74 | ||
| Water (Kg) | 49.89 | Wastewater | 52.69 | ||
| Energy (J) | 1.0 × 107 | ||||
| 2 | Bleaching | Pretreated SCT (Kg) | 0.35 | Pulp bleached | 0.1818 |
| NaOH (Kg) | 0.844 | H2O | 0.517 | ||
| H2O2 (Kg) | 3.997 | Wastewater | 66.292 | ||
| H2O (Kg) | 61.79 | ||||
| Energy (J) | 3.6 × 106 | ||||
| 3 | Extraction and Sonication | PB-SCT (Kg) | 0.1818 | NCC suspension | 1.14 |
| H2SO4 (Kg) | 4.158675 | Wastewater | 825.833 | ||
| H2O (Kg) | 822.632 | ||||
| Energy (J) | 6.1 × 105 | ||||
| 4 | Freeze-drying | NCC suspension (Kg) | 1.14 | NCC | 0.011 |
| Energy (j) | 6.5 × 108 | 1.13 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wibowo, A.; Chiarasumran, N.; Thanapimmetha, A.; Saisriyoot, M.; Srinophakun, P.; Suriyachai, N.; Champreda, V. Conversion of Sugarcane Trash to Nanocrystalline Cellulose and its Life Cycle Assessment. Catalysts 2022, 12, 1215. https://doi.org/10.3390/catal12101215
Wibowo A, Chiarasumran N, Thanapimmetha A, Saisriyoot M, Srinophakun P, Suriyachai N, Champreda V. Conversion of Sugarcane Trash to Nanocrystalline Cellulose and its Life Cycle Assessment. Catalysts. 2022; 12(10):1215. https://doi.org/10.3390/catal12101215
Chicago/Turabian StyleWibowo, Agung, Nutchapon Chiarasumran, Anusith Thanapimmetha, Maythee Saisriyoot, Penjit Srinophakun, Nopparat Suriyachai, and Verawat Champreda. 2022. "Conversion of Sugarcane Trash to Nanocrystalline Cellulose and its Life Cycle Assessment" Catalysts 12, no. 10: 1215. https://doi.org/10.3390/catal12101215