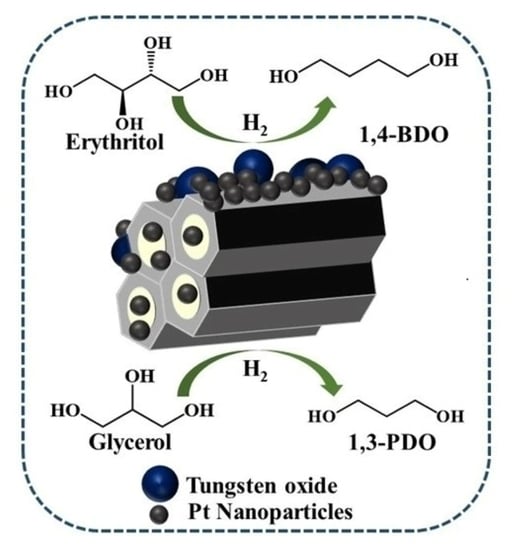
C–O Hydrogenolysis of C3–C4 Polyols Selectively to Terminal Diols over Pt/W/SBA-15 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Spectroscopic Characterization
2.2. Catalytic Activity
2.2.1. Control Experiments and Mechanistic Pathway
2.2.2. Effect of Reaction Conditions on Erythritol Hydrogenolysis
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization Techniques
3.3. Reaction Procedure and Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic Conversion of Biomass to Biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Ruppert, A.M.; Weinberg, K.; Palkovits, R. Hydrogenolysis Goes Bio: From Carbohydrates and Sugar Alcohols to PlatformChemicals. Angew. Chem. Int. Ed. 2012, 51, 2564–2601. [Google Scholar] [CrossRef] [PubMed]
- Besson, M.; Gallezot, P.; Pinel, C. Conversion of Biomass into Chemicals over Metal Catalysts. Chem. Rev. 2014, 114, 1827–1870. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.S.; Berdugo-Díaz, C.E.; Flaherty, D.W. Advances in Understanding the Selective Hydrogenolysis of Biomass Derivatives. ACS Catal. 2021, 11, 11193–11232. [Google Scholar] [CrossRef]
- Tomishige, K.; Yabushita, M.; Cao, J.; Nakagawa, Y. Hydrodeoxygenation of Potential Platform Chemicals Derived from Biomass to Fuels and Chemicals. Green Chem. 2022, 24, 5652–5690. [Google Scholar] [CrossRef]
- Top Value Added Chemicals from Biomass, Vol. 1—Results of Screening for Possible Candidates from Sugars and Synthesis Gas, U.S. Department of Energy: Energy Efficiency and Renewable Energy. Available online: https://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on 1 August 2022).
- Bhowmik, S.; Darbha, S. Advances in Solid Catalysts for Selective Hydrogenolysis of Glycerol to 1,3-Propanediol. Catal. Rev. Sci. Eng. 2021, 63, 639–703. [Google Scholar] [CrossRef]
- Dias da Silva Ruy, A.; Maria de Brito Alves, R.; Lewis Reis Hewer, T.; De Aguiar Pontes, D.; Sena Gomes Teixeira, L.; Antônio Magalhães Pontes, L. Catalysts for Glycerol Hydrogenolysis to 1,3-Propanediol: A Review of Chemical Routes and Market. Catal. Today 2021, 381, 243–253. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Kasumi, T.; Ogihara, J.; Tamura, M.; Arai, T.; Tomishige, K. Erythritol: Another C4 Platform Chemical in Biomass Refinery. ACS Omega 2020, 5, 2520–2530. [Google Scholar] [CrossRef]
- Weitz, H.M.; Schnabel, R.; Platz, R. Preparation of Butane-1,4-diol. U.S. Patent No. 4361710, 30 November 1982. [Google Scholar]
- Ji, X.J.; Huang, H.; Ouyang, P.K. Microbiol 2,3-Butanediol Production: A State-of-the-Art Review. Biotechnol. Adv. 2011, 29, 351–364. [Google Scholar] [CrossRef]
- Nimlos, M.R.; Blanksby, S.J.; Qian, X.; Himmel, M.E.; Johnson, D.K. Mechanisms of Glycerol Dehydration. J. Phys. Chem. A 2006, 110, 6145–6156. [Google Scholar] [CrossRef]
- Robinson, A.M.; Hensley, J.E.; Medlin, J.W. Bifunctional Catalysts for Upgrading of Biomass-Derived Oxygenates: A Review. ACS Catal. 2016, 6, 5026–5043. [Google Scholar] [CrossRef]
- Virgilio, E.M.; Padró, C.L.; Sad, M.E. Butanediols Production from Erythritol on Rh Promoted Catalyst. Lat. Am. Appl. Res. 2020, 50, 89–94. [Google Scholar]
- Sadier, A.; Perret, N.; Da Silva Perez, D.; Besson, M.; Pinel, C. Efffect of Carbon Chain Length on Catalytic C–O Bond Clevage of Polyols over Rh-ReOx/ZrO2 in Aqueous Phase. Appl. Catal. A Gen. 2019, 586, 117213. [Google Scholar] [CrossRef]
- Said, A.; Da Silva Perez, D.; Perret, N.; Pinel, C.; Besson, M. Selective C–O Hydrogenolysis of Erythritol over Supported Rh-ReOx Catalysts in the Aqueous Phase. ChemCatChem 2017, 9, 2768–2783. [Google Scholar] [CrossRef]
- Liu, L.; Cao, J.; Nakagawa, Y.; Betchaku, M.; Tamura, M.; Yabushita, M.; Tomishige, K. Hydrodeoxygenation of C4-C6 Sugar Alcohols to Diols or Mono-Alcohols with Retension of the Carbon Chain Over A Silica-Supported Tungsten Oxide-Modified Platinum Catalyst. Green Chem. 2021, 23, 5665–5679. [Google Scholar] [CrossRef]
- Gu, M.; Lice, L.; Nagakawa, Y.; Li, C.; Tamura, M.; Shen, Z.; Zhou, X.; Zhang, Y.; Tomishiga, K. Selective Hydrogenolysis of Erythritol Over Ir-ReOx/Rutile-TiO2 Catalyst. ChemSusChem 2021, 14, 642–654. [Google Scholar] [CrossRef]
- Ota, N.; Tamura, M.; Nakagawa, Y.; Okumura, K.; Tomishige, K. Hydrodeoxygenation of Vicinal OH Groups over Heterogeneous Rhenium Catalyst Promoted by Palladium and Ceria Support. Angew. Chem. Int. Ed. 2015, 54, 1897–1900. [Google Scholar] [CrossRef]
- Liu, L.; Asano, T.; Nakagawa, Y.; Gu, M.; Li, C.; Tamura, M.; Tomishige, K. Structure and Performance Relationship of Silica-Supported Platinum-Tungsten Catalysts in Selective C–O Hydrogenolysis of Glycerol and 1,4-Anhydroerythritol. Appl. Catal. B Environ. 2021, 292, 120164. [Google Scholar] [CrossRef]
- Liu, L.; Asano, T.; Nakagawa, Y.; Tamura, M.; Tomishige, K. One-Pot Synthesis of 1,3-Butanediol by 1,4-Anhydroerythritol Hydrogenolysis Over A Tungsten-Modified Platinum on Silica Catalysts. Green Chem. 2020, 22, 2375–2380. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Tamura, M.; Nakagawa, Y.; Hiyoshi, N.; Tomishige, K. One-Pot Catalytic Selective Synthesis of 1,4-Butanediol from 1,4-Anhydroerythritol and Hydrogen. Green Chem. 2018, 20, 2547–2557. [Google Scholar] [CrossRef]
- ShanthiPriya, S.; PavanKumar, V.; Lakshmi Kantam, M.; Bhargava, S.K.; Srikanth, A.; Chary, K.V.R. High Efficiency Conversion of Glycerol to 1,3-Propanediol Using a Novel Platinum–Tungsten Catalyst Supported on SBA-15. Ind. Eng. Chem. Res. 2015, 54, 9104–9115. [Google Scholar]
- Fan, Y.; Cheng, S.; Wang, H.; Ye, D.; Xie, S.; Pei, Y.; Hu, H.; Hua, W.; Li, Z.H.; Qiao, M.; et al. Nanoparticulate Pt on Mesoporous SBA-15 Doped with Extremely Low Amount of W as a Highly Selective Catalyst for Glycerol Hydrogenolysis to 1,3-Propanediol. Green Chem. 2017, 19, 2174–2183. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, B.; Liang, Y.; Liu, L.; Dong, J. Improving Selectivity to 1,3-Propanediol for Glycerol Hydrogenolysis Using W- and Al-Incorporated SBA-15 as Support for Pt Nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 2661–2671. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Ross-Medgaarden, E.I.; Wachs, I.E. Structural Determination of Bulk and Surface Tungsten Oxides with UV–Vis Diffuse Reflectance Spectroscopy and Raman Spectroscopy. J. Phys. Chem. C 2007, 111, 15089–15099. [Google Scholar] [CrossRef]
- Horsley, J.A.; Wachs, I.E.; Brown, J.M.; Via, G.H.; Hardcastle, F.D. Structure of Surface Tungsten Oxide Species in the Tungsten Trioxide/Alumina Supported Oxide System from X-Ray Absorption Near-Edge Spectroscopy and Raman Spectroscopy. J. Phys. Chem. 1987, 91, 4014–4020. [Google Scholar] [CrossRef]
- Barton, D.G.; Shtein, M.; Wilson, R.D.; Soled, S.L.; Iglesia, E. Structure and Electronic Properties of Solid Acids Based on Tungsten Oxide Nanostructures. J. Phys. Chem. B 1999, 103, 630–640. [Google Scholar] [CrossRef]
- Guntida, A.; Suriye, K.; Panpranot, J.; Praserthdam, P. Lewis Acid Transformation to Brönsted Acid Sites over Supported Tungsten Oxide Catalysts Containing Different Surface WOx Structures. Catal. Today 2020, 358, 354–369. [Google Scholar] [CrossRef]
- Cheng, S.; Fan, Y.; Zhang, X.; Zeng, Y.; Xie, S.; Pei, Y.; Zeng, G.; Qiao, M.; Zong, B. Tungsten-Doped Siliceous Mesocellular Foams-Supported Platinum Catalyst for Glycerol Hydrogenolysis to 1,3-Propanediol. Appl. Catal. B Environ. 2021, 297, 120428. [Google Scholar] [CrossRef]
- Amada, Y.; Watanabe, H.; Hirai, Y.; Kajikawa, Y.; Nakagawa, Y.; Tomishige, K. Production of Biobutanediols by the Hydrogenolysis of Erythritol. ChemSusChem 2012, 5, 1991–1999. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Wang, X.; Yao, D.; Wang, Y.; Huang, S.; Li, W.; Zhao, Y.; Wang, S.; Ma, X. Insight into the Nature of Brönsted Acidity of Pt-(WOx)n-H Model Catalysts in Glycerol Hydrogenolysis. J. Catal. 2020, 388, 154–163. [Google Scholar] [CrossRef]
- Wang, T.; Tamura, M.; Nakagawa, Y.; Tomishige, K. Preparation of Highly Active Monometallic Rhenium Catalysts for Selective Synthesis of 1,4-Butanediol from 1,4-Anhydroerythritol. ChemSusChem 2019, 12, 3615–3626. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Nakagawa, Y.; Tamura, M.; Okumura, K.; Tomishige, K. Tungsten–Zirconia-Supported Rhenium Catalyst Combined with a Deoxy Dehydration Catalyst for the One-Pot Synthesis of 1,4-Butanediol from 1,4-Anhydroerythritol. React. Chem. Eng. 2020, 5, 1237–1250. [Google Scholar] [CrossRef]
- Bhowmik, S.; Enjamuri, N.; Darbha, S. Hydrogenolysis of Glycerol in an Aqueous Medium over Pt/WO3/Zirconium Phosphate Catalysts Studied by 1H NMR Spectroscopy. New J. Chem. 2021, 45, 5013–5022. [Google Scholar] [CrossRef]









| Catalyst | B.E. (eV) | |||
|---|---|---|---|---|
| Pt 4f7/2 | Pt 4f5/2 | W 4f7/2 | W 4f5/2 | |
| 0.5Pt/1W/SBA-15 | 71.2 | 74.6 | - | - |
| 1Pt/1W/SBA-15 | 71.5 | 74.5 | - | - |
| 2Pt/1W/SBA-15 | 71.6 | 74.6 | 36.5 | 38.2 |
| 3Pt/1W/SBA-15 | 71.4 | 74.6 | 35.8 | 37.8 |
| 4Pt/1W/SBA-15 | 71.5 | 74.7 | 35.7 | 37.8 |
| Entry | Catalyst | Erythritol Conversion (%) | Product Selectivity (%) | Yield of 1,4-BDO (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BTOs | BDOs | BOs | Others | |||||||||
| 1,2,3- | 1,2,4- | 1,4- | 2,3- | 1-BO | 2-BO | |||||||
| 1 | 1W/SBA-15 | 6.2 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | ||
| 2 | 4Pt/SBA-15 | 4.8 | 11.6 | 18.4 | 37.1 | 0 | 0 | 0.4 | 32.5 | 1.8 | ||
| 3 | 4Pt/1W/SBA-15 | 58.1 | 7.4 | 32.2 | 34.8 | 10.1 | 7.4 | 2.8 | 5.3 | 20.2 | ||
| 4 | 4Pt/1W/SBA-15 | 82.2 | 5.8 | 18.3 | 37.6 | 12.9 | 9.2 | 3.3 | 12.9 | 30.9 | ||
| Entry | Substrate | Catalyst | Solvent | Reaction Conditions | Conversion (%) | Yield of 1,4-BDO (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Erythritol | Pt-W/SBA-15 (4 wt% Pt, 1 wt% W) | Water | Substrate = 0.375 g, H2O = 12.125 g, catalyst = 0.3125 g, p(H2) = 50 bar, T = 190 °C, t = 24 h | 82.2 | 30.9 | Present work |
| 2 | Erythritol | Pt-WOx/SiO2 (4 wt% Pt, W/Pt = 0.25) | Water | Substrate = 0.5 g, H2O = 4 g, catalyst = 0.2 g, p(H2) = 80 bar, T = 140 °C, t = 24 h (50 h) | 82 (99.3) | 32 (51.4) | [17] |
| 3 | Erythritol | ReOx-Pd/CeO2 (2 wt% Re, 0.3 wt% Pd) | 1,4-Dioxane | Substrate = 0.5 g, 1,4-dioxane = 4g, catalyst = 0.15 g, p(H2) = 80 bar, T = 160 °C, t = 24 h | 98 | 12 | [19] |
| 4 | Erythritol | Ir-ReOx/SiO2 (4 wt% Ir, 3.9 wt% Re) | H2SO4 + Water | Substrate = 1 g, water = 4 g, H2SO4 (H+/Ir = 1), catalyst = 0.3 g, p(H2) = 80 bar, T = 100 °C, t = 24 h | 74 | 24 | [32] |
| 5 | Erythritol | Ir-ReOx/TiO2 (4 wt% Ir, 1.0 wt% Re) | Water | Substrate = 1 g, water = 4 g, catalyst = 0.3 g, p(H2) = 80 bar, T = 100 °C, t = 12 h | 62 | 23 | [18] |
| 6 | 1,4-Anhydroerythritol | Pt-WOx/SiO2 (4 wt% Pt, 0.9 wt% W) | Water | Substrate = 0.5 g, water = 4 g, catalyst = 0.2 g, p(H2) = 80 bar, T = 140 °C, t = 80 h | 100 | 54 | [21] |
| 7 | 1,4-Anhydroerythritol | ReOx-Au/CeO2 + ReOx/C (1 wt% Re, 0.3 wt% Au; 3 wt% Re) | 1,4-Dioxane | Substrate = 0.5 g, 1,4-dioxane = 4 g, catalyst = 0.15 g, p(H2) = 80 bar, T = 140 °C, t = 24 h | 100 | 86 | [22] |
| 8 | 1,4-Anhydroerythritol | ReOx/CeO2 + ReOx/C (1 wt% Re + 3 wt% Re) | 1,4-Dioxane | Substrate = 0.5 g, 1,4-dioxane = 4 g, catalyst = 0.15 g + 0.15 g, p(H2) = 80 bar, T = 140 °C, t = 24 h | 97 | 83 | [34] |
| 9 | 1,4-Anhydroerythritol | ReOx-Au/CeO2 + ReOx/WO3-ZrO2 (Re = 1 wt%, Au = 0.3 wt%; Re = 1 wt%, WO3 = 10 wt%) | 1,4-Dioxane | Substrate = 0.3 g, 1,4-dioxane = 4 g, catalyst = 0.15 g + 0.15 g, p(H2) = 80 bar, T = 140 °C, t = 48 h | 100 | 53 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhowmik, S.; Enjamuri, N.; Marimuthu, B.; Darbha, S. C–O Hydrogenolysis of C3–C4 Polyols Selectively to Terminal Diols over Pt/W/SBA-15 Catalysts. Catalysts 2022, 12, 1070. https://doi.org/10.3390/catal12091070
Bhowmik S, Enjamuri N, Marimuthu B, Darbha S. C–O Hydrogenolysis of C3–C4 Polyols Selectively to Terminal Diols over Pt/W/SBA-15 Catalysts. Catalysts. 2022; 12(9):1070. https://doi.org/10.3390/catal12091070
Chicago/Turabian StyleBhowmik, Susmita, Nagasuresh Enjamuri, Banu Marimuthu, and Srinivas Darbha. 2022. "C–O Hydrogenolysis of C3–C4 Polyols Selectively to Terminal Diols over Pt/W/SBA-15 Catalysts" Catalysts 12, no. 9: 1070. https://doi.org/10.3390/catal12091070