Uniformly Dispersed Cu Nanoparticles over Mesoporous Silica as a Highly Selective and Recyclable Ethanol Dehydrogenation Catalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Uniformly Dispersed Cu Nanoparticles over Mesoporous Silica
2.2. Catalytic Performances of Mesoporous-Silica-Supported Cu Nanoparticles
2.3. The Thermal Treatment Atmosphere Effect on Catalytic Behaviors
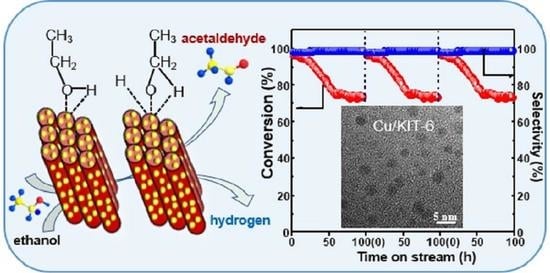
2.4. Stability and Regeneration
2.5. In Situ FTIR Studies
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.2.1. Synthesis of Mesoporous Silica
3.2.2. Cu Nanoparticles Supported on Various Silica Supports
3.2.3. Cu/KIT-6 Catalyst Treated under Various Atmospheres
3.3. Catalyst Characterization
3.4. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ob-eye, J.; Praserthdam, P.; Jongsomjit, B. Dehydrogenation of ethanol to acetaldehyde over different metals supported on carbon catalysts. Catalysts 2019, 9, 66. [Google Scholar] [CrossRef]
- Shan, J.; Liu, J.; Li, M.W.; Lustig, S.; Stephanopoulos, M.F. NiCu single atom alloys catalyze the C-H bond activation in the selective non-oxidative ethanol dehydrogenation reaction. Appl. Catal. B Environ. 2018, 226, 534–543. [Google Scholar] [CrossRef]
- Chen, A.; Yu, X.; Zhou, Y.; Sehested, J.; Wang, Y.; Shen, W. Structure of the catalytically active copper-ceria interfacial perimeter. Nat. Catal. 2019, 2, 334–341. [Google Scholar] [CrossRef]
- Rosset, M.; Perez-Lopez, O.W. Cu-Ca-Al catalysts derived from hydrocalumite and their application to ethanol dehydrogenation. React. Kinet. Mech. Catal. 2019, 126, 497–511. [Google Scholar] [CrossRef]
- Huang, Y.J.; Wang, B.; Yuan, H.K.; Sun, Y.B.; Yang, D.Y.; Cui, X.J.; Shi, F. The catalytic dehydrogenation of ethanol by heterogeneous catalysts. Catal. Sci. Technol. 2021, 11, 1652–1664. [Google Scholar] [CrossRef]
- Sato, A.G.; Volanti, D.P.; Meira, D.M.; Damyanova, S.; Longo, E.; Bueno, J.M.C. Effect of the ZrO2 phase on the structure and behavior of supported Cu catalysts for ethanol conversion. J. Catal. 2013, 307, 1–17. [Google Scholar] [CrossRef]
- Shiau, C.Y.; Lee, Y.R. Characterization and dehydrogenation activity of Cr-added electroless plated copper catalyst. Appl. Catal. A Gen. 2001, 220, 173–180. [Google Scholar] [CrossRef]
- Hanukovich, S.; Dang, A.; Christopher, P. Influence of metal oxide support acid sites on Cu-catalyzed nonoxidative dehydrogenation of ethanol to acetaldehyde. ACS Catal. 2019, 9, 3537–3550. [Google Scholar] [CrossRef]
- Fujita, S.; Iwasa, N.; Tani, H.; Nomura, W.; Arai, M.; Takezawa, N. Dehydrogenation of ethanol over Cu/ZnO catalysts prepared from various coprecipitated precursors. React. Kinet. Catal. Lett. 2001, 73, 367–372. [Google Scholar] [CrossRef]
- Gao, D.; Feng, Y.H.; Yin, H.B.; Wang, A.; Jiang, T.S. Coupling reaction between ethanol dehydrogenation and maleic anhydride hydrogenation catalyzed by Cu/Al2O3, Cu/ZrO2, and Cu/ZnO catalysts. Chem. Eng. J. 2013, 233, 349–359. [Google Scholar] [CrossRef]
- Phung, T.K. Copper-based catalysts for ethanol dehydrogenation and dehydrogenative coupling into hydrogen, acetaldehyde and ethyl acetate. Int. J. Hydrogen Energy 2021, 11, 253–268. [Google Scholar] [CrossRef]
- Wang, N.; Yu, X.; Shen, K.; Chu, W.; Qian, W. Synthesis, characterization and catalytic performance of MgO-coated Ni/SBA-15 catalysts for methane dry reforming to syngas and hydrogen. Int. J. Hydrogen Energy 2013, 38, 9718–9731. [Google Scholar] [CrossRef]
- Zhou, G.L.; Zhang, H.B.; Xie, H.M.; Wu, M.; Wei, M.Y. Ethanol catalytic oxidation on ordered mesoporous CuO/KIT-6 catalyst. Int. J. Chem. React. Eng. 2013, 11, 259–263. [Google Scholar] [CrossRef]
- Finger, P.H.; Osmari, T.A.; Cabral, N.M.; Bueno, J.C.; Gallo, J.M.R. Direct synthesis of Cu supported on mesoporous silica: Tailoring the Cu loading and the activity for ethanol dehydrogenation. Catal. Today 2021, 381, 26–33. [Google Scholar] [CrossRef]
- Gao, D.Z.; Wang, A.; Shen, L.Q.; Liu, S.X. Gas phase dehydrogenation of ethanol using maleic anhydride as hydrogen acceptor over Cu/hydroxylapatite, Cu/SBA-15, and Cu/MCM-41 catalysts. J. Ind. Eng. Chem. 2015, 26, 322–332. [Google Scholar] [CrossRef]
- Feng, Z.L.; Bai, X.F. 3D-mesoporous KIT-6 supported highly dispersed Pd nanocatalyst for dodecahydro-N-ethylcarbazole dehydrogenation. Micropor. Mesopor. Mat. 2022, 335, 111789–111800. [Google Scholar] [CrossRef]
- Zaccheria, F.; Scotti, N.; Marelli, M.; Psaro, R.; Ravasio, N. Unravelling the properties of supported copper oxide: Can the particle size induce acidic behaviour? Dalton Trans. 2013, 42, 1319–1328. [Google Scholar] [CrossRef]
- Wong, A.; Liu, Q.; Griffin, S.; Nicholls, A.; Regalbuto, J.R. Synthesis of ultrasmall, homogeneously alloyed, bimetallic nanoparticles on silica supports. Science 2017, 358, 1427–1430. [Google Scholar] [CrossRef]
- Zhang, H.W.; Tan, H.R.; Jaenicke, S.; Chuah, G.K. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 9–28. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Ikram, M.; Rehman, S. Enhanced desulfurization characteristics of Cu-KIT-6 for thiophene. Micropor. Mesopor. Mat. 2014, 199, 108–116. [Google Scholar] [CrossRef]
- Miao, K.K.; Luo, X.L.; Guo, J.L.; Cao, F.J.; Hu, Y.Q.; Feng, G.D. One-step synthesis of Cu-SBA-15 under neutral condition and its oxidation catalytic performance. Micropor. Mesopor. Mat. 2019, 289, 109640–109648. [Google Scholar] [CrossRef]
- Fu, Z.H.; Chen, J.H.; Yin, D.L.; Yin, D.H.; Zhang, L.X.; Zhang, Y.Y. Highly effective Cu-HMS catalyst for hydroxylation of phenol. Catal. Lett. 2000, 66, 105–108. [Google Scholar] [CrossRef]
- Pang, J.F.; Zheng, M.Y.; Yang, X.F.; Wang, Y.; Zhang, T. Hierarchical echinus-like Cu-MFI catalysts for ethanol dehydrogenation. ACS Catal. 2020, 10, 13624–13629. [Google Scholar] [CrossRef]
- Kim, D.; Becknell, N.; Yu, Y.; Yang, P. Room-temperature dynamics of vanishing copper nanoparticles supported on silica. Nano Lett. 2017, 17, 2732–2737. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Yu, T.; Zhu, S.C.; Shen, M.Q.; Li, W.; Wang, J.Q. The migration of Cu species over Cu–SAPO-34 and its effect on NH3 oxidation at high temperature. Catal. Sci. Technol. 2014, 4, 3004–3012. [Google Scholar] [CrossRef]
- Cassinelli, W.H.; Martins, L.; Passos, A.R.; Pulcinelli, S.H.; Rochet, A.; Briois, V.; Santilli, C.V. Correlation between structural and catalytic properties of copper supported on porous alumina for the ethanol dehydrogenation reaction. ChemCatChem 2015, 7, 1668–1677. [Google Scholar] [CrossRef]
- Reddy, K.P.; Choi, H.; Kim, D.; Ryoo, R.; Park, J.Y. Cu oxide deposited on shape-controlled ceria nanocrystals for CO oxidation: Influence of interface-driven oxidation states on catalytic activity. Catal. Sci. Technol. 2021, 11, 6134–6142. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.Q.; He, P.; Yuan, Y.Z. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Amokrane, S.; Boualouache, A.; Simon, P.; Capron, M.; Otmanine, G.; Allam, D.; Hocine, S. Effect of adding transition metals to copper on the dehydrogenation reaction of ethanol. Catal. Lett. 2021, 151, 2864–2883. [Google Scholar] [CrossRef]
- Li, M.Y.; Lu, W.D.; He, L.; Schüth, F.; Lu, A.H. Tailoring the surface structure of silicon carbide support for copper catalyzed ethanol dehydrogenation. ChemCatChem 2019, 11, 481–487. [Google Scholar] [CrossRef]
- Patel, A.; Shukla, P.; Rufford, T.; Wang, S.; Chen, J.; Rudolph, V.; Zhu, Z. Catalytic reduction of NO by CO over copper-oxide supported mesoporous silica. Appl. Catal. A Gen. 2011, 409–410, 55–65. [Google Scholar] [CrossRef]
- Li, L.D.; Guan, N.J. HC-SCR reaction pathways on ion exchanged ZSM-5 catalysts. Micropor. Mesopor. Mat. 2009, 117, 450–457. [Google Scholar] [CrossRef]
- Ming, S.; Wang, P.; Liu, P.; Duan, J.H.; Mei, F.M.; Pang, L.; Chen, Z.; Li, T. Promotional effect of metal cations doping on OMS-2 catalysts for NH3-SCR Reaction. Chem. Eng. J. 2020, 379, 122287–122297. [Google Scholar] [CrossRef]
- Gao, J.S.; Han, Y.L.; Mu, J.C.; Wu, S.C.; Tan, F.; Shi, Y.; Li, X.Y. 2D, 3D mesostructured silicas templated mesoporous manganese dioxide for selective catalytic reduction of NOx with NH3. J. Colloid Interf. Sci. 2018, 516, 254–262. [Google Scholar] [CrossRef]
- Sridevi, B.; Nagaiah, P.; Padmasri, A.H.; Raju, B.D.; Rao, K.S.R. Studies on dehydrogenation of cyclohexanol to cyclohexanone over mesoporous SiO2 supported copper catalysts. J. Chem. Sci. 2017, 129, 601–608. [Google Scholar] [CrossRef]
- Ding, T.; Tian, H.; Liu, J.; Wu, W.; Yu, J. Highly active Cu/SiO2 catalysts for hydrogenation of diethyl malonate to 1,3-propanediol. Chin. J. Catal. 2016, 37, 484–493. [Google Scholar] [CrossRef]
- Marino, F.J.; Cerrella, E.G.; Duhalde, S.; Jobbagy, M.; Laborde, M.A. Hydrogen from steam reforming of ethanol. characterization and performance of copper-nickel supported catalysts. Int. J. Hydrogen Energy 1998, 23, 1095–1101. [Google Scholar] [CrossRef]
- Li, F.J.; Wang, L.G.; Han, X.; Cao, Y.; He, P.H.; Li, Q. Selective hydrogenation of ethylene carbonate to methanol and ethylene glycol over Cu/SiO2 catalysts prepared by ammonia evaporation method. Int. J. Hydrogen Energy 2017, 42, 2144–2156. [Google Scholar] [CrossRef]
- Campisano, I.S.P.; Rodella, C.B.; Sousa, Z.S.B.; Henriques, C.A.; Silva, V.T. Influence of thermal treatment conditions on the characteristics of Cu-based metal oxides derived from hydrotalcite-like compounds and their performance in bio-ethanol dehydrogenation to acetaldehyde. Catal. Today 2018, 306, 111–120. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Zheng, H.; Li, Y. Promoting effect of boron oxide on Cu/SiO2 catalyst for glycerol hydrogenolysis to 1,2-propanediol. J. Catal. 2013, 303, 70–79. [Google Scholar] [CrossRef]
- Albert, K.B.; Fan, C.; Pang, L.; Chen, Z.; Ming, S.J.; Albert, T.; Li, T. The influence of chemical poisoning, hydrothermal aging and their coeffects on Cu-SAPO-34 catalyst for NOx reduction by NH3-SCR. Appl. Surf. Sci. 2019, 479, 1200–1211. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, J.; Liu, J.; Wang, D.; Zhao, Z.; Cheng, K.; Li, J. Enhanced hydrothermal stability of Cu-ZSM-5 catalyst via surface modification in the selective catalytic reduction of NO with NH3. Appl. Surf. Sci. 2016, 375, 186–195. [Google Scholar] [CrossRef]
- Manriquez, M.E.; Lopez, T.; Gomez, R.; Navarrete, J. Preparation of TiO2-ZrO2 mixed oxides with controlled acid-basic properties. J. Mol. Catal. A Gen. 2004, 220, 229–237. [Google Scholar] [CrossRef]
- van der Grift, C.J.G.; Wielers, A.F.H.; Mulder, A.; Geus, J.W. The reduction behaviour of silica-supported copper catalysts prepared by deposition-precipitation. Thermochim. Acta 1990, 171, 95–113. [Google Scholar] [CrossRef]
- Heemeier, M.; Stempel, S.; Shaikhutdinov, S.K.; Libuda, J.; Bäumer, M.; Oldman, R.J.; Jackson, S.D.; Freund, H.J. On the thermal stability of metal particles supported on a thin alumina film. Surf. Sci. 2003, 523, 103–110. [Google Scholar] [CrossRef]
- Raskó, J.; Kiss, J. Adsorption and surface reactions of acetaldehyde on TiO2, CeO2 and Al2O3. Appl. Catal. A Gen. 2005, 287, 252–260. [Google Scholar] [CrossRef]
- Hao, Y.; Hao, G.P.; Guo, D.C.; Lu, A.H. Bimetallic Au–Pd nanoparticles confined in tubular mesoporous carbon as highly selective and reusable benzyl alcohol oxidation catalysts. ChemCatChem 2012, 4, 1595–1602. [Google Scholar] [CrossRef]
- Wang, Q.N.; Shi, L.; Li, W.; Li, W.C.; Si, R.F.; Lu, A.H. Cu supported on thin carbon layer-coated porous SiO2 for efficient ethanol dehydrogenation. Catal. Sci. Technol. 2018, 8, 472–479. [Google Scholar] [CrossRef]
- Freitas, I.C.; Damyanova, S.; Oliveira, D.C.; Marques, C.M.P.; Bueno, J.M.C. Effect of Cu content on the surface and catalytic properties of Cu/ZrO2 catalyst for ethanol dehydrogenation. J. Mol. Catal. A Chem. 2014, 381, 26–37. [Google Scholar] [CrossRef]
- Chernov, A.N.; Astrakova, T.V.; Koltunov, K.Y.; Sobolev, V.I. Ethanol dehydrogenation to acetaldehyde over Co@N-Doped carbon. Catalysts 2021, 11, 1411. [Google Scholar] [CrossRef]
- Kustov, A.L.; Tarasov, A.L.; Tkachenko, O.P.; Mishin, I.V.; Kapustin, G.I.; Kustov, L.M. Ethanol to acetaldehyde conversion under thermal and microwave heating of ZnO-CuO-SiO2 modified with WC nanoparticles. Molecules 2021, 26, 1955. [Google Scholar] [CrossRef]
- Wang, Z.T.; Hoyt, R.A.; El-Soda, M.; Madix, R.J.; Kaxiras, E.; Sykes, E.C.H. Dry dehydrogenation of ethanol on Pt-Cu single atom alloys. Top. Catal. 2018, 61, 328–335. [Google Scholar] [CrossRef]
- Akti, F.; Balci, S.; Dogu, T. Role of synthesis media on properties of tin and copper incorporated SBA-15 catalysts and their activity in selective oxidation of ethanol. Mater. Chem. Phys. 2019, 223, 249–259. [Google Scholar] [CrossRef]
- He, F.; Luo, J.Q.; Liu, S.T. Novel metal loaded KIT-6 catalysts and their applications in the catalytic combustion of chlorobenzene. Chem. Eng. J. 2016, 294, 362–370. [Google Scholar] [CrossRef]
- Pauly, T.R.; Pinnavaia, T.J. Pore size modification of mesoporous HMS molecular sieve silicas with wormhole framework structures. Chem. Mater. 2001, 13, 987–993. [Google Scholar] [CrossRef]
- Ai, P.P.; Tan, M.H.; Yamane, N.; Liu, G.G.; Fan, R.G.; Yang, G.H.; Yoneyama, Y.; Yang, R.Q.; Tsubaki, N. Synergistic effect of a boron-doped carbon-nanotube-supported Cu catalyst for selective hydrogenation of dimethyl oxalate to ethanol. Chem. Eur. J. 2017, 23, 8252–8261. [Google Scholar] [CrossRef]











| Catalysts | Size (nm) | Cu Dispersion (%) a | SBET (m2/g) | Vpore (cm3/g) | Dpore (nm) | Acidity (mmol NH3 g−1) b | Xethanol (%) c | Sacetaldehyde (%) c | |
|---|---|---|---|---|---|---|---|---|---|
| Weak | Moderate | ||||||||
| Cu/KIT-6 | 2.01 | 62.3 | 920 | 1.25 | 4.74 | 0.122 | 0.051 | 96.8 | 99.8 |
| Cu/SBA-15 | 2.24 | 60.9 | 640 | 1.12 | 7.17 | 0.122 | 0.048 | 92.8 | 96.8 |
| Cu/HMS | 2.21 | 60.2 | 918 | 1.07 | 3.67 | 0.126 | 0.037 | 89.4 | 94.5 |
| Catalysts | Cu+/Cu0 a | D(H2/Cu) b | Cu Nanoparticle Size (nm) c | Acidity (mmol NH3 g−1) d | Xethanol (%) e | Sacetaldehyde (%) e | |
|---|---|---|---|---|---|---|---|
| Weak | Moderate | ||||||
| RO | 0.53 | 37.8% | 2.01 | 0.122 | 0.051 | 96.8 | 99.8 |
| R | 0.21 | 1.8% | 2.14 | 0.127 | - | 94.5 | 81.4 |
| OR | - | 0.7% | 2.62 | 0.121 | - | 40.4 | 81.0 |
| O | 0.72 | 30.7% | 4.00 | 0.126 | 0.102 | 4.10 | 89.9 |
| Catalysts | T (°C) | WHSV (h−1) | Xethanol (%) | Sacetaldehyde (%) | Yacetaldehyde (%) | Ref. |
|---|---|---|---|---|---|---|
| Cu/KIT-6 | 250 | 0.87 | 96.8 | 99.8 | 96.6 | This work |
| Cu/SiO2 | 250 | 0.87 | 3.26 | 100 | 3.26 | This work |
| Cu-MFI-AE a | 250 | 0.53 | 97.9 | 93.1 | 91.1 | [23] |
| Cu-MFI-IM b | 250 | 0.53 | 64.4 | 89.4 | 57.6 | [23] |
| Cu/MCM-41 | 300 | - | 90.0 | 90.0 | 81.0 | [14] |
| Cu/SBA-15 | 300 | 2.1 | 72.0 | 37.0 | 27.0 | [15] |
| Cu/SiO2 | 260 | 2.4 | 85.4 | 79.4 | 67.8 | [48] |
| Cu/C/SiO2 | 260 | 2.4 | 83.0 | 95.1 | 78.9 | [48] |
| Cu/ZnO | 300 | - | 16.4 | 67.6 | 11.1 | [10] |
| 10Cu/ZrO2 | 275 | 3.2 | 81.5 | 13.6 | 11.1 | [49] |
| Co@CN | 400 | 18,000 | 66.0 | 84.0 | 55.4 | [50] |
| ZnO-CuO- SiO2 | 400 | 3 | 92.8 | 72.7 | 67.5 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Zhao, D.; Liu, W.; Zhang, M.; Lou, Y.; Wang, Z.; Tang, Q.; Yang, J. Uniformly Dispersed Cu Nanoparticles over Mesoporous Silica as a Highly Selective and Recyclable Ethanol Dehydrogenation Catalyst. Catalysts 2022, 12, 1049. https://doi.org/10.3390/catal12091049
Hao Y, Zhao D, Liu W, Zhang M, Lou Y, Wang Z, Tang Q, Yang J. Uniformly Dispersed Cu Nanoparticles over Mesoporous Silica as a Highly Selective and Recyclable Ethanol Dehydrogenation Catalyst. Catalysts. 2022; 12(9):1049. https://doi.org/10.3390/catal12091049
Chicago/Turabian StyleHao, Yan, Dajie Zhao, Wen Liu, Min Zhang, Yixiao Lou, Zhenzhen Wang, Qinghu Tang, and Jinghe Yang. 2022. "Uniformly Dispersed Cu Nanoparticles over Mesoporous Silica as a Highly Selective and Recyclable Ethanol Dehydrogenation Catalyst" Catalysts 12, no. 9: 1049. https://doi.org/10.3390/catal12091049