1. Introduction
With the concern of using or generating toxic and hazardous materials though chemical processes, people are trying to find sustainable, mild, and environmental-friendly methods for industrial manufacturing [
1]. Enzyme catalysis is a promising solution to overcome the above limitations since enzymes are produced by living organisms and usually working in a mild environment [
2,
3]. However, using soluble enzymes in their original form may face the problem of no recyclability and low stability; the separation or recovery of active enzymes from the reaction mixture is also difficult and uneconomic [
3,
4,
5].
Fortunately, these limitations can be overcome by enzyme immobilization [
6]. Different from free enzymes, the immobilized enzymes are insoluble in the reaction mixture; therefore, they can be removed and recycled easily from the reaction solution [
7]. Meanwhile, the immobilized enzymes usually benefit from the improved stability against harsh environments [
8]. Enzyme immobilization methods have been developed rapidly in recent years [
9]. The main principles of these methods include adsorption, covalent binding, encapsulation or entrapment, and cross-linking [
10]. In addition to the immobilization method, choosing an appropriate support material is another key aspect to realize optimal enzyme immobilization [
11]. With the advantages of non-toxicity, biocompatibility, biodegradability, and flexibility, organic biopolymers, including alginate, chitosan, cellulose, gelatin, etc., have been widely selected as the support material for enzyme immobilization [
12].
In our case, we are interested in the immobilization of the enzyme diacetylchitobiose deacetylase (Dac), which is an important enzyme for the biosynthesis of glucosamine (GlcN). GlcN is an important monosaccharide in the human body, especially in cartilage tissue. It has been widely utilized as a medicine or health product for osteoarthritis treatment and cartilage tissue repairing [
13,
14]. Compared with traditional hydrolysis of chitin or chitosan, the enzymatic catalysis method with Dac shows the advantage of mild condition, low pollution, and no allergies caused by raw material (crab or shrimp shells) [
15,
16]. However, although many attempts have been made to improve the yield of Dac or catalytic activity of Dac, further improvements for greater economic advantage against other methods are still necessary [
17,
18]. Therefore, increasing the reusability of Dac by enzyme immobilization could be a potential method to reduce its cost for the industrial manufacturing of GlcN.
As an enzyme derived from hyperthermophilic bacteria, Dac shows its highest catalytic activity at ~80 °C, which is much higher than most common enzymes [
19,
20]. Actually, many biopolymers, such as agarose, cellulose, agar-agar, and gelatin, exhibit excellent ability against high temperature [
21,
22,
23,
24,
25,
26]. However, the immobilized enzyme activity declined during the repeated catalytic cycles at high temperature. This activity loss might be caused by the imperfect immobilizing method or thermal-unstable enzyme itself.
In our preliminary experiment, we chose gelatin for the immobilization of Dac due to its excellent biodegradability and low cytotoxicity [
27]. Gelatin can be formed by reversible hydrogel with low temperature or form irreversible hydrogel with a cross-linker (e.g., genipin). Firstly, we generated the hydrogel by adding genipin into the gelatin solution (containing Dac) directly and fabricated hydrogel particles via emulsion. However, we found the activity of immobilized Dac decreased dramatically during the repeated catalytic cycles. Then, we tried a different cross-linking strategy. The hydrogel particles with Dac solution were firstly generated by low temperature and cross-linked by genipin in the buffer solution. Although the enzyme activity could be maintained at high temperature, the initial enzyme activity of the immobilized Dac was only ~53%.
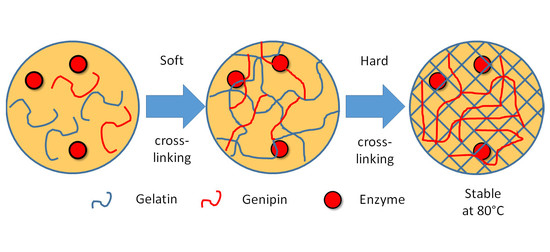
To overcome these limitations, we combined two methods and proposed a two-step cross-linked gelatin hydrogel for the immobilization of highly thermostable biocatalysis (
Figure 1). The enzyme was firstly cross-linked in the emulsion state and then cross-linked in the aqueous buffer. As a proof of concept, we chose Dac as an example to demonstrate the enzyme immobilization. Firstly, the cross-linking durations, concentrations of gelatin, and concentrations of cross-linker genipin were optimized. Then, we characterized our hydrogel with different conditions to demonstrate the advantage of the proposed method.
2. Results
2.1. Optimization of the Cross-Linking Duration
Firstly, to validate the necessity of both cross-linking steps, we fabricated hydrogels with only 1 day of “soft cross-linking”, 1 day of “hard cross-linking”, and 1 day for the remaining cross-linking. For the “soft cross-linked-only” hydrogel, the hydrogel was washed with a buffer and demulsifier directly after 1-day “soft cross-linking”. For “hard cross-linked-only” hydrogel, hydrogel was firstly formed by low temperature and “hard cross-linked” for 1 day after washing. Activities of the immobilized Dac with different hydrogels were measured for 10 rounds; activities for leaking Dac were also measured (round 0 of Dac leaking activity was measuring the washing solution). Activity of crude Dac solution (1556 ± 54 U/mL, 40 °C) was considered as the reference enzyme activity for the calculation of relative enzyme activity.
Here, we used “a-b” to represent the hydrogel with “a” day of “soft cross-linking” and “b” day of “hard cross-linking”. For example, 0-1 hydrogel was cross-linked with 0 days of “soft cross-linking” and 1 day of “hard cross-linking”. As shown in
Figure 2A, the Dac activity of “soft cross-linked-only” (1-0) hydrogel for the first round of catalysis was relatively high (~68%). However, during the catalyzing process, most Dac molecules escaped from hydrogel; the leaking Dac activity reached up to ~60% after the first round of catalysis. Therefore, at the second round of catalysis, the remaining Dac activity was less than 10% and the measurement was stopped accordingly. This result indicated that although “soft cross-linking” could immobilize most Dac molecules with limited leaking, the immobilization was not thermally stable while most Dac molecules were released from hydrogel, even at 40 °C.
On the contrary, the Dac activity was basically maintained during 10 rounds of catalysis for the “hard cross-linked-only” (0-1) hydrogel; the leaking Dac activities were almost 0% after the first round of catalysis. However, the initial immobilizing efficiency was not satisfactory, as around half the Dac escaped the immobilization and was washed away by buffer. This result indicated “hard cross-linking” could improve the thermal stability of Dac immobilization, maintaining Dac activity during repeated catalysis. However, for this cross-linking method, only around half the Dac molecules were immobilized. With both “soft cross-linking” and “hard cross-linking” (1-1), the hydrogel showed both high initial Dac immobilizing efficiency (~81%) and thermal stability: the average Dac activity for 10 rounds of catalysis was ~77%.
Actually, the cross-linking duration of hydrogel has great influence on the mechanical properties of gelatin hydrogels [
28]. Therefore, we tried to improve the Dac immobilization by optimizing the incubating duration. Accordingly, we fabricated hydrogels with different “soft cross-linking” and “hard cross-linking” durations, ranging from 1 day, 3 days, to 5 days, respectively. The average Dac activities for 10 rounds of catalysis with different hydrogels are shown in
Figure 2B; the specific Dac activities and leaking Dac activities for each round are shown in
Figure S2. The trends with different cross-linking durations were clear: with longer “soft cross-linking”, the activities of hydrogels were lower and with longer “hard cross-linking”, the activities of hydrogels were higher.
To explain this phenomenon, we hypothesized that hydrogel with longer “hard cross-linking” acquired stronger structural stability, which decreased Dac leaking during the washing step. As for the hydrogel with long “soft cross-linking”, we found that the hydrogel particles were aggregated, which might limit the diffusion in the substrate and thereby, decrease the catalyzing activity of the hydrogel. We further took images of “1-5” and “5-5” hydrogels by microscope (
Figure 2C) to support our finding. With this experiment, we determined the optimal cross-linking duration as 1-day “soft cross-linking” and 5-day “hard cross-linking” for downstream experiments.
2.2. Concentration Optimizations of Gelatin and Genipin
We learned of the initial concentrations of gelatin and genipin from our previous work [
29]. To optimize the concentrations of gelation and genipin for this work, we started the testing from 10% (
w/
v) of gelatin and 500 µg/mL of genipin. Firstly, we fixed the genipin concentration to 500 µg/mL and changed the gelatin concentration to 2%, 5%, 15%, and 20% (
w/
v); then, we fixed the gelatin concentration to 10% (
w/
v) and changed the genipin concentration to 100, 250, 750, and 1000 µg/mL. All hydrogels were “soft cross-linked” for 1 day and “hard cross-linked” for 5 days, according to the previous optimization. Similarly, all hydrogels were tested 10 times and the relative enzyme activities were measured. The results for hydrogels with different gelatin concentrations and different genipin concentrations are presented in
Figure 3A,B, respectively.
With the fixed concentration of cross-linker genipin (
Figure 3A), an appropriate concentration of gelatin was necessary. As shown in
Figure 3C, with only 2% gelatin, the hydrogel was basically not formed (the enzyme activity measurement was stopped after the first round of catalysis); for hydrogel with 5% gelatin, around half of the Dac molecules escaped from hydrogel during the washing step. From these two results, we could infer that a certain concentration of gelatin was necessary to complete the hydrogel structure. However, if the gelatin concentration was too high, the immobilized Dac activity also dropped. We inferred that high concentration of gelatin resulted in the imbalance in the gelatin and genipin ratio, since the concentration of genipin was fixed.
For the hydrogels with fixed concentration of gelatin, we found that a minimal concentration of genipin was necessary for the immobilization of Dac (
Figure 3B). We noticed that when genipin concentration was 250 µg/mL or lower, Dac molecules were released into the washing buffer (
Figure 3C). In addition, we noticed that two hydrogels with the same gelatin–genipin ratio (20% gelatin, 500 µg/mL genipin hydrogel and 10% gelatin, 250 µg/mL hydrogel) showed similar performance, which supported the previous finding that an appropriate gelatin–genipin ratio could improve the immobilizing efficiency. However, when the concentration of genipin reached 500 µg/mL, more genipin showed limited effects on the immobilization. To avoid an unnecessary waste of genipin, we set the optimal concentration for gelatin to 10% and concentration for genipin to 500 µg/mL.
2.3. Tests for Harsh Conditions
After the optimization of cross-linking time and concentrations of gelatin and genipin, we tested four harsh conditions, which were heating at 80 °C for 10 min, dehydrating at 40 °C, recycling 50 times, and storing at room temperature for 50 days. For the heating test, the tube with prepared hydrogel was put into the water at 80 °C for 10 min and cooled down to room temperature by water. The hydrogel for the dehydrating test was collected in a tube and heated to 40 °C with an opening cap for 24 h. No free water was observed after the treatment. Then, the hydrogel was rehydrated by adding PB (pH = 8.0) solution and gently shaking. The enzyme activity tests for both treated hydrogels were performed for 10 rounds; the reference for both hydrogels was the crude enzyme solution without heating or dehydrating. As shown in
Figure 4A, their activities were basically maintained, as in comparison with the untreated hydrogel. Since Dac is a highly thermostable enzyme, heating at 80 °C would not reduce its activity. This result also proved the structure of hydrogel and the immobilization were thermally stable at 80 °C. For the dehydrating experiment, since hydrogel was capable of locking water, Dac was protected to maintain the activity.
Interestingly, we found the volumes of both hydrogels were shrunk to around 50% after the treatment, compared with the untreated hydrogel. To verify the shrinking of hydrogels, we fabricated the hydrogel via droplet microfluidics and performed the same treatment. As shown in
Figure 4B, before the treatment, the diameter for hydrogel was 199.2 ± 13.0 µm (i); after the heating treatment, the diameter was decreased to 163.1 ± 8.0 µm (ii); after the dehydrating treatment, the diameter was decreased to 155.8 ± 9.3 µm (iii). From the measurement, we could calculate that the diameters of heating and dehydrating hydrogels were ~81.9% and ~78.2% of the untreated hydrogel. Therefore, the volumes of heating and dehydrating hydrogels were ~54.9% and ~47.8% of the untreated hydrogel, which were consistent with our observation. These results indicated that the hydrogel particles were shrunk and “concentrated” during the heating or dehydrating processes, while most Dac molecules were still encapsulated in the hydrogel.
In the previous tests, hydrogels were recycled for 10 rounds and a limited decline in enzyme activity was observed. To test the enzyme-activity-maintaining ability of the optimized hydrogel, we performed measurements for 50 rounds. The result is shown in
Figure 4C. After 50 rounds of catalysis, the relative enzyme activity was decreased from ~87.2% to ~63%, with an average declining speed of ~0.48% per round. One possible reason for this decline could be the instability of the hydrogel itself; Dac molecules were released continuously during the catalysis. Meanwhile, we found that only a small amount of hydrogel particles were washed away during the washing steps. Since the sizes of hydrogel particles were heterogeneous, some small particles were often floating in the solution rather than sinking down. Therefore, these hydrogel particles with encapsulated Dac were easy to discard, which might be another reason for the loss of enzyme activity. We also measured the remaining activity in the washing solution during 50 rounds and an average of ~0.3% Dac activity was detected, which supported the hypothesis that some Dac molecules were released in the washing solution.
Next, we tested the long-time storing ability of the optimized hydrogel. Both hydrogel and crude Dac solution were stored at room temperature for 50 days. Since the fabrication of hydrogel took 6 days, we performed the measurement at both Day 6 and Day 50. The relative Dac activities in the optimized hydrogel after 50-day storing are shown in
Figure 4D and the average results are shown in
Figure 4E. Different from normal enzymes, Dac has extraordinary thermal stability. Therefore, even after a 50-day incubation at room temperature, its catalyzing activity was only reduced to 90.4% ± 1.3% of the original activity. With the protection of hydrogel, the catalyzing activity of immobilized Dac was slightly decreased to ~99.2% for the first time of catalyzing and to ~99.3% for the average of 10 rounds of catalyzing, comparing with the fresh prepared hydrogels (6-day storing). This result suggested that our hydrogel could provide extra protection for the encapsulated enzymes. Therefore, the relative enzyme activity was even improved after long-time storing.
2.4. Catalyzing at High Temperature
The above experiments for catalyzing GlcNAc by Dac were performed at 40 °C. Actually, Dac showed the highest catalyzing activity at 80 °C, which was too high for the GlcN to remain stable [
20]. Therefore, the current temperature for catalysis was a compromise of Dac activity and GlcN stability. At 40 °C, Dac could catalyze GlcNAc to GlcN at a relatively high activity with an acceptable degrading speed of GlcN.
Nevertheless, we were interested in the performance of hydrogel working at high temperatures, 60 °C and 80 °C, to maximize the catalyzing efficiency of immobilized Dac. To minimize the degradation of GlcN, only substrate solution and hydrogel solution were preheated to 60 °C or 80 °C for 3 min. After a 10 min reaction, the supernatant was added into the PB buffer immediately, which was kept at room temperature. Since the dilution ratio was set to 20, the GlcN also cooled down immediately. Similarly, the measurements were performed for 10 rounds.
As shown in
Figure 5A, the activities of crude Dac solution were increased from 1556 ± 54 U/mL (40 °C) to 2627 ± 35 U/mL (60 °C) and 3405 ± 67 U/mL (80 °C). Similarly, the immobilized Dac activities increased from 1357 ± 31 U/mL (40 °C) to 2267 ± 62 U/mL (60 °C) and 2895 ± 85 U/mL (80 °C) at the first time of catalysis. After catalyzing for 10 rounds (at least 100 min in total), the relative enzyme activities of hydrogels decreased to 79.6% ± 1.1% (60 °C) and 65.8% ± 2.2% (80 °C). Compared with the catalysis and recycling at 40 °C, we found that when increasing the temperature to 60 °C, the immobilization efficiency and activity losing speed were similar to the hydrogel at 40 °C. At 80 °C, the enzyme activity declined faster; the detected leaking activity for 80 °C was also higher than 60 °C. Nevertheless, after 10 rounds of catalysis, the immobilized activity was still ~77.3%, compared with the first round of catalysis and the average activity for 10 rounds of catalysis was 74.8%. These results indicated that our hydrogel had good thermal stability and reusability, with high enzyme immobilizing efficiency.
2.5. Characterization of Enzyme Immobilization
We further characterized the enzymatic properties of the immobilized enzyme. Firstly, we measured the kinetic parameters of free Dac and immobilized Dac by non-linear fitting (
Figure 6A). With different concentrations of substrate, the pH was maintained at 8.0 and the catalyzing temperature was 80 °C. The kinetic parameters were calculated by non-linear fitting. The results showed that K
m for immobilized Dac slightly declined from 14.8 ± 1.1 mM to 10.6 ± 0.6 mM, while V
max value declined from 839.7 ± 41.1 µM/s to 543.0 ± 20.1 µM/s. The decline in K
m indicated the immobilization of Dac increased its affinity to the substrate and the decline in V
max was consistent with the decreased enzyme activity. Similar results were also reported in [
26,
30].
Then, we tested free and immobilized Dac at different pHs, ranging from 5.0 to 9.0. Since GlcN was unstable at high temperature and high pH, to avoid the interference of GlcN degradation, we performed catalysis at 40 °C. As shown in
Figure 6B, the optimal pH for both free and immobilized Dac was still around 8.0. However, when pH was 5.0, the immobilized Dac showed slightly higher relative enzyme activity (
p < 0.1), which might benefit from the hydrogel to maintain structural conformational stability [
31]. We also tested their catalytic activities with different temperatures, ranging from 60 °C to 90 °C. As shown in
Figure 6C, the optimal temperature for immobilized Dac increased from 80 °C to 90 °C. The principle of this phenomenon was similar to the improved acid resistance. However, even activity for immobilized Dac was improved at 90 °C, though it might not be suitable for GlcN production, since GlcN degradation was also accelerated.
3. Materials and Methods
3.1. Preparation of Crude Dac Solution
As the substrate for enzyme immobilization, Dac was synthesized by the recombinant
Bacillus subtilis. The plasmid for Dac expression was constructed by following our previous work [
18]. The original strain was
B. subtilis WB600 and the transformation was performed according to the standard procedures (MoBiTec). The antibiotic used for the culture of the recombinant strain was kanamycin with a concentration of 10 μg/mL. Then, the recombination
B. subtilis was cultured in Luria-Bertani medium (LB) at 37 °C with 220 rpm shaking for overnight culture. Next, 2% of the culture medium was inoculated into the Terrific broth (TB) medium at 37 °C with 220 rpm shaking. After 60 h culture, the medium was centrifuged at 12,000×
g for 3 min. The supernatant was collected as the crude Dac solution for enzyme immobilization.
3.2. Two-Step Cross-Linking for Dac Immobilization
The Dac immobilization was performed by the two-step crosslinking of gelatin hydrogel. Here, we take 1 mL of Dac solution as example. Firstly, 100 mg (10% (w/v)) gelatin powder (Type A gelatin, Maokangbio, Shanghai, China) was added in the crude Dac solution and dissolved at 40 °C. Then, 10 µL (1% (v/v)) of cross-linker genipin (Aladdin, Shanghai, China), which was dissolved in dimethyl sulfoxide at 50 mg/mL, was added in the solution with a final concentration of 500 µg/mL. Next, 1mL fluorocarbon oil HFE 7500 (3M, Saint Paul, MN, USA) with 2% (v/v) Pico-Surf surfactant (5% in HFE 7500, Sphere Fluidics, Cambridge, UK), i.e., the final concentration of the surfactant was 0.1%, was added in the solution immediately. Then, the mixing solution was treated by high-speed vortex shaking for 5 min. Since the hydrogel solution (crude Dac solution, gelation, and genipin) was insoluble in fluorocarbon oil, it formed hydrogel emulsion with the protection of surfactant. Then, the hydrogel was incubated at room temperature with 220 rpm shaking for one day. Since this cross-linking could immobilize most enzyme but suffered from weak thermal stability, we named this step as “soft cross-linking”.
After the first cross-linking, 8 mL of phosphate buffer (PB, 50 mM, pH = 8.0) and 100 µL of demulsifier 1H,1H,2H,2H-perfluoro-1-octanal (Aladdin, Shanghai, China) were added into the hydrogel solution to break the hydrogel emulsion and wash the hydrogel. With gentle shaking and short incubation, oil phase and aqueous phase were separated and hydrogel particles formed sediment in the aqueous phase. Then, 5 mL aqueous phase with hydrogel sediment was collected. Genipin (50 µL) was added in the solution again with the same final concentration (500 µg/mL). The solution was then incubated at room temperature with 220 rpm shaking for 5 days. After that, hydrogel particles gained strong thermal stability; therefore, we named this step as “hard cross-linking”.
Finally, the two-step cross-linked hydrogel was washed by PB (pH = 8.0) 3 times to remove the free Dac, genipin, and other solutes in the solution. It was noteworthy that the volume of hydrogel solution was almost doubled comparing with original crude Dac solution, i.e., 1 mL crude Dac solution would obtain ~2 mL hydrogel solution. To have a fair comparation between immobilized and free Dac, we diluted both solutions (hydrogel and crude Dac solution) to 2 mL by PB (pH = 8.0).
3.3. Enzyme Activity Measurement of Immobilized Dac and Crude Dac Solution
The enzyme activity of immobilized and free Dac was measured through the deacetylation reaction of GlcNAc [
32]. Firstly, 500 μL hydrogel solution or crude Dac solution was mixed with 1 mL 66.7 g/L GlcNAc solution (dissolved in PB (50 mM, pH = 8.0)) and incubated at 40 °C with 1000 rpm shaking for 9 min. Both solutions were preheated before the mixing. Then, the shaking was stopped and the mixture was incubated for 1 min to let the hydrogel sink. Next, 10 μL supernatant was collected and added into 190 μL of PB (pH = 8.0) solution. After the mixing, 5 μL solution was added into 100 μL o-Phthalaldehyde (OPA) GlcN detecting reagent, which contained 50 µg OPA (dissolved in 100 mM sodium carbonate buffer, pH = 10.5), 1 μL ethanol, and 50 nL of 2.0 M dithiothreitol (DTT). After 2 min shaking, the absorbance of the mixture was measured at 330 nm by spectrophotometer (Thermo Scientific, Vantaa, Finland). Standard GlcN solutions (dissolved in PB solution, pH = 8.0) had concentrations ranging from 0.1 to 5.0 g/L and were used to get standard curve. One Dac activity unit was defined as producing 1 μmol GlcN in 1 h.
To test the reusability of immobilized Dac, the reaction mixture was centrifuged at 1000× g for 2 min. The supernatant was discarded and PB (pH = 8.0) solution was used to wash the remaining hydrogel particles. This washing step was repeated 3 times to fully remove the GlcNAc and GlcN in the solution. Then, the volume of the hydrogel solution was adjusted to 500 μL by PB (pH = 8.0) for next-round testing.
To measure the leaking of enzyme, same procedure for enzyme activity was performed except the addition of GlcNAc was deleted. Firstly, 500 μL hydrogel solution was mixed with 1 mL PB buffer. After 10 min incubation, 500 μL supernatant was collected and mixed with the same 1 mL 66.7 g/L GlcNAc solution for enzyme activity measurement. Another 500 μL supernatant was used as the reference. It should be noted that the activity of the leaking Dac was calculated according to the diluting ratio. The rest of the hydrogel (500 μL) was collected by centrifugation for the next round of testing.
3.4. Generation of Homogenous Hydrogel Particles via Droplet Microfluidics
To generate homogenous hydrogel particles, we replaced vortex shaking by generating hydrogel droplets via droplet microfluidics. The design of the droplet-generating chip was cross-junction with 2 inlets (one for Dac solution, one for spacing oil); the height and width of channel were both 150 µm (
Figure S1). The fabrication and preparation steps for microfluidic chips followed our previous work [
33]. In this work, crude Dac solution with 10% (
w/
v) gelatin and spacing oil (described in
Section 3.2) were loaded into 1 mL syringes, respectively. Both syringes were mounted onto syringe pumps (Harvard, PHD2000) and the outlet of the syringes were connected to the droplet-generating chip by polyethylene tubing with an inner diameter of 0.38 mm. To maintain the liquid state of the hydrogel solution, an electric heating carpet was used to heat its syringe and PE tubing. Flow rates for infusing gelatin solution and spacing oil were set at 5 µL/min and 10 µL/min, respectively. The generated droplets were collected in a 1.5 mL tube, which was placed in an ice box to maintain the solidification of hydrogel particles. Then, genipin was added in the solution with the same final concentration of 500 µg/mL. After 1-day incubation, hydrogel particles were washed out with PB (pH = 8.0) solution. The remaining steps for “hard cross-linking” and washing of the hydrogel were the same as described in
Section 3.2.
3.5. Statistical Analysis
All the experiments were independently conducted at least three times and the results were expressed as means ± standard deviations (n = 3).
4. Discussion
In this work, we proposed an enzyme-immobilizing strategy, for highly thermostable biocatalysis, which was based on encapsulating an enzyme in gelatin hydrogel. For the hydrogel material, we chose gelatin and genipin, since they show the advantages of biocompatibility and non/lower toxicity [
34,
35]. Actually, many researchers utilized biopolymers to immobilize enzymes, with the common features of recycling ability, improved thermal stability, acid resistance, long-time storing ability, etc. [
12,
22,
23,
24]. Differently, for a highly thermostable enzyme (e.g., Dac), the key feature is the recycling ability at high temperature. Although highly thermostable enzymes will not lose their activity quickly at high temperature, the immobilization may not be stable.
With the two-step cross-linking strategy and further optimization, our proposed method demonstrated good enzyme-immobilizing and recycling ability, which is important for industrial manufacturing of GlcN with Dac. Dac showed the highest catalyzing activity at 80 °C; however, GlcN degrades gradually at high temperatures. The current attempt for producing GlcN by Dac was using free Dac to catalyze GlcNAc at 40 °C, which could maintain the existence of GlcN. However, this condition faced the problem of limited enzyme activity. If catalyzing at 80 °C, the enzyme cost would be another problem. To maintain the existence of GlcN, reaction duration should be controlled, which requires a larger amount of free Dac and leads to extensive waste. With our proposed immobilization method for Dac, a large amount of Dac could be used for catalysis and recycled with limited activity lost. Therefore, the reaction time could be controlled to keep GlcN stable and the reusability of Dac could reduce its cost dramatically when using a large amount of Dac.
In addition, our strategy showed excellent storability compared to existing studies: ~99.1% immobilized enzyme activity maintained after a 44-day incubation (Day 50 and Day 6) at room temperature. Although our demonstration benefited from a highly thermostable enzyme itself, whose activity remained at 90.4% after the same incubation, our hydrogel still showed excellent ability in providing extra protection for the immobilized enzyme. Meanwhile, the thermal stability and acid resistance were also improved. We hypothesized that two-step cross-linking played an important role for these features. During the “soft cross-linking”, genipin cross-linked both gelatin and the enzyme. Since the cross-linking was performed in the emulsion format, a phase barrier blocked the release of the enzyme. Although the “soft cross-linking” was not stable enough to resist high temperature, it immobilized most enzyme molecules to avoid leaking during the “hard cross-linking”. The “hard cross-linking” strengthened the hydrogel structure to allow high temperature catalysis to occur.
However, the current method to generate hydrogel particles was based on high-speed vortex shaking. Although it was a convenient approach, the size of hydrogel particles was not controlled. Actually, particle size was important for enzyme catalytic efficiency; larger size may induce higher mass transfer resistance [
36]. A standard procedure to fabricate homogeneous hydrogel particles is necessary in the future.
In conclusion, we developed a two-step cross-linked hydrogel immobilization strategy for Dac. Our hydrogel showed both high Dac immobilizing efficiency and strong thermal stability. When catalyzing at 40 °C, our hydrogel showed 87.2% activity for first-round catalysis and 83.1% average activity for 10 rounds of catalysis. Our hydrogel also showed good stability against heating, dehydrating, long-time storing, and massive recycling. Notably, at 80 °C, our hydrogel showed 85.0% relative enzyme activity at 80 °C and remained at 65.8% after 10 rounds of catalysis (74.8% for average). The above experiments demonstrated the advantages of our enzyme immobilization strategy and could serve as a sample for similar highly thermostable enzymes.