Formation and Intramolecular Capture of α-Imino Gold Carbenoids in the Au(I)-Catalyzed [3 + 2] Reaction of Anthranils, 1,2,4-Oxadiazoles, and 4,5-Dihydro-1,2,4-Oxadiazoles with Ynamides
Abstract
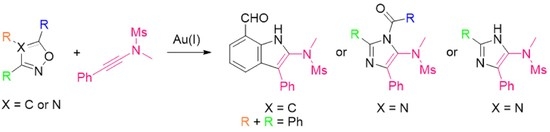
:1. Introduction
2. Results
2.1. Reaction of Anthranil with Ynamide
2.2. Reaction of 1,2,4-Oxadiazole with Ynamide
2.3. Reaction of 4,5-Dihydro-1,2,4-Oxadiazole with Ynamide
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorin, D.J.; Davis, N.R.; Toste, F.D. Gold(I)-Catalyzed Intramolecular Acetylenic Schmidt Reaction. J. Am. Chem. Soc. 2005, 127, 11260–11261. [Google Scholar]
- Benitez, D.; Shapiro, N.D.; Tkatchouk, E.; Wang, Y.; Goddard, W.A.; Toste, F.D. A Bonding Model for Gold(I) Carbene Complexes. Nat. Chem. 2009, 1, 482–486. [Google Scholar]
- Gorin, D.J.; Toste, F.D. Relativistic Effects in Homogeneous Gold Catalysis. Nature 2007, 446, 395–403. [Google Scholar]
- Brouwer, H.; Stothers, J.B. 13 C Nuclear Magnetic Resonance Studies. XX. 13 C Shieldings of Several Allyl Alcohols. Geometric Dependence of 13 C Shieldings. Can. J. Chem. 1972, 50, 1361–1370. [Google Scholar]
- Seidel, G.; Fürstner, A. Structure of a Reactive Gold Carbenoid. Angew. Chemie Int. Ed. 2014, 53, 4807–4811. [Google Scholar]
- Seidel, G.; Mynott, R.; Fürstner, A. Elementary Steps of Gold Catalysis: NMR Spectroscopy Reveals the Highly Cationic Character of a “Gold Carbenoid”. Angew. Chemie Int. Ed. 2009, 48, 2510–2513. [Google Scholar]
- Marion, N.; Nolan, S.P. N-Heterocyclic Carbenes in Gold Catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. [Google Scholar]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar]
- Hong, F.-L.; Ye, L.-W. Transition Metal-Catalyzed Tandem Reactions of Ynamides for Divergent N-Heterocycle Synthesis. Acc. Chem. Res. 2020, 53, 2003–2019. [Google Scholar]
- Chen, Y.-B.; Qian, P.-C.; Ye, L.-W. Brønsted Acid-Mediated Reactions of Ynamides. Chem. Soc. Rev. 2020, 49, 8897–8909. [Google Scholar]
- Luo, J.; Chen, G.-S.; Chen, S.-J.; Yu, J.-S.; Li, Z.-D.; Liu, Y.-L. Exploiting Remarkable Reactivities of Ynamides: Opportunities in Designing Catalytic Enantioselective Reactions. ACS Catal. 2020, 10, 13978–13992. [Google Scholar]
- Hu, Y.-C.; Zhao, Y.; Wan, B.; Chen, Q.-A. Reactivity of Ynamides in Catalytic Intermolecular Annulations. Chem. Soc. Rev. 2021, 50, 2582–2625. [Google Scholar]
- Shandilya, S.; Protim Gogoi, M.; Dutta, S.; Sahoo, A.K. Gold-Catalyzed Transformation of Ynamides. Chem. Rec. 2021, 21, 4123–4149. [Google Scholar]
- Madhavan, S.; Keshri, S.K.; Kapur, M. Transition Metal-Mediated Functionalization of Isoxazoles: A Review. Asian J. Org. Chem. 2021, 10, 3127–3165. [Google Scholar]
- Gao, Y.; Nie, J.; Huo, Y.; Hu, X.-Q. Anthranils: Versatile Building Blocks in the Construction of C–N Bonds and N-Heterocycles. Org. Chem. Front. 2020, 7, 1177–1196. [Google Scholar]
- Aguilar, E.; Santamaría, J. Gold-Catalyzed Heterocyclic Syntheses through α-Imino Gold Carbene Complexes as Intermediates. Org. Chem. Front. 2019, 6, 1513–1540. [Google Scholar]
- Zhao, X.; Rudolph, M.; Asiri, A.M.; Hashmi, A.S.K. Easy Access to Pharmaceutically Relevant Heterocycles by Catalytic Reactions Involving α-Imino Gold Carbene Intermediates. Front. Chem. Sci. Eng. 2020, 14, 317–349. [Google Scholar]
- Zeng, Z.; Jin, H.; Sekine, K.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-Catalyzed Regiospecific C–H Annulation of o-Ethynylbiaryls with Anthranils: π-Extension by Ring-Expansion En Route to N-Doped PAHs. Angew. Chemie Int. Ed. 2018, 57, 6935–6939. [Google Scholar]
- Zeng, Z.; Jin, H.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold(III)-Catalyzed Site-Selective and Divergent Synthesis of 2-Aminopyrroles and Quinoline-Based Polyazaheterocycles. Angew. Chemie Int. Ed. 2018, 57, 16549–16553. [Google Scholar]
- González, J.; Santamaría, J.; Suárez-Sobrino, Á.L.; Ballesteros, A. One-Pot and Regioselective Gold-Catalyzed Synthesis of 2-Imidazolyl-1-Pyrazolylbenzenes from 1-Propargyl-1 H -Benzotriazoles, Alkynes and Nitriles through α-Imino Gold(I) Carbene Complexes. Adv. Synth. Catal. 2016, 358, 1398–1403. [Google Scholar]
- Zeng, Z.; Jin, H.; Xie, J.; Tian, B.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. α-Imino Gold Carbenes from 1,2,4-Oxadiazoles: Atom-Economical Access to Fully Substituted 4-Aminoimidazoles. Org. Lett. 2017, 19, 1020–1023. [Google Scholar]
- Xu, W.; Wang, G.; Sun, N.; Liu, Y. Gold-Catalyzed Formal [3 + 2] Cycloaddition of Ynamides with 4,5-Dihydro-1,2,4-Oxadiazoles: Synthesis of Functionalized 4-Aminoimidazoles. Org. Lett. 2017, 19, 3307–3310. [Google Scholar]
- Rani, N.; Sharma, A.; Singh, R. Trisubstituted Imidazole Synthesis: A Review. Mini Rev. Org. Chem. 2014, 12, 34–65. [Google Scholar]
- Strelnikova, J.O.; Rostovskii, N.V.; Starova, G.L.; Khlebnikov, A.F.; Novikov, M.S. Rh(II)-Catalyzed Transannulation of 1,2,4-Oxadiazole Derivatives with 1-Sulfonyl-1,2,3-Triazoles: Regioselective Synthesis of 5-Sulfonamidoimidazoles. J. Org. Chem. 2018, 83, 11232–11244. [Google Scholar]
- Ermolat’ev, D.S.; Van der Eycken, E.V. A Divergent Synthesis of Substituted 2-Aminoimidazoles from 2-Aminopyrimidines. J. Org. Chem. 2008, 73, 6691–6697. [Google Scholar]
- Guo, X.; Chen, W.; Chen, B.; Huang, W.; Qi, W.; Zhang, G.; Yu, Y. One-Pot Three-Component Strategy for Functionalized 2-Aminoimidazoles via Ring Opening of α-Nitro Epoxides. Org. Lett. 2015, 17, 1157–1159. [Google Scholar]
- Guchhait, S.K.; Hura, N.; Shah, A.P. Synthesis of Polysubstituted 2-Aminoimidazoles via Alkene-Diamination of Guanidine with Conjugated α-Bromoalkenones. J. Org. Chem. 2017, 82, 2745–2752. [Google Scholar]
- Francini, C.M.; Fallacara, A.L.; Artusi, R.; Mennuni, L.; Calgani, A.; Angelucci, A.; Schenone, S.; Botta, M. Identification of Aminoimidazole and Aminothiazole Derivatives as Src Family Kinase Inhibitors. ChemMedChem 2015, 10, 2027–2041. [Google Scholar]
- Francini, C.M.; Musumeci, F.; Fallacara, A.L.; Botta, L.; Molinari, A.; Artusi, R.; Mennuni, L.; Angelucci, A.; Schenone, S. Optimization of Aminoimidazole Derivatives as Src Family Kinase Inhibitors. Molecules 2018, 23, 2369. [Google Scholar]
- Bennett, L.L., Jr.; Baker, H.T. Synthesis of Potential Anticancer Agents. IV. 4-Nitro- and 4-Amino-5-Imidazole Sulfones. J. Am. Chem. Soc. 1957, 79, 2188–2191. [Google Scholar]
- Zhang, L.; Brodney, M.A.; Candler, J.; Doran, A.C.; Duplantier, A.J.; Efremov, I.V.; Evrard, E.; Kraus, K.; Ganong, A.H.; Haas, J.A.; et al. 1-[(1-Methyl-1H-Imidazol-2-Yl)Methyl]-4-Phenylpiperidines as MGluR2 Positive Allosteric Modulators for the Treatment of Psychosis. J. Med. Chem. 2011, 54, 1724–1739. [Google Scholar]
- Mehrabi, H.; Alizadeh-Bami, F.; Meydani, A.; Besharat, S. An Eco-Friendly Approach for the Synthesis of 1,2,5-Trisubstituted and 4-Amino-1,2,5-Tetrasubstituted Imidazoles via a Multi-Component Condensation. Arkivoc 2021, 2021, 86–95. [Google Scholar]
- Hashmi, A.S.K. Introduction: Gold Chemistry. Chem. Rev. 2021, 121, 8309–8310. [Google Scholar]
- Mato, M.; Franchino, A.; García-Morales, C.; Echavarren, A.M. Gold-Catalyzed Synthesis of Small Rings. Chem. Rev. 2021, 121, 8613–8684. [Google Scholar]
- Reyes, R.L.; Iwai, T.; Sawamura, M. Construction of Medium-Sized Rings by Gold Catalysis. Chem. Rev. 2021, 121, 8926–8947. [Google Scholar]
- Wang, T.; Hashmi, A.S.K. 1,2-Migrations onto Gold Carbene Centers. Chem. Rev. 2021, 121, 8948–8978. [Google Scholar]
- Zheng, Z.; Ma, X.; Cheng, X.; Zhao, K.; Gutman, K.; Li, T.; Zhang, L. Homogeneous Gold-Catalyzed Oxidation Reactions. Chem. Rev. 2021, 121, 8979–9038. [Google Scholar]
- Hendrich, C.M.; Sekine, K.; Koshikawa, T.; Tanaka, K.; Hashmi, A.S.K. Homogeneous and Heterogeneous Gold Catalysis for Materials Science. Chem. Rev. 2021, 121, 9113–9163. [Google Scholar]
- Wang, W.; Ji, C.-L.; Liu, K.; Zhao, C.-G.; Li, W.; Xie, J. Dinuclear Gold Catalysis. Chem. Soc. Rev. 2021, 50, 1874–1912. [Google Scholar]
- Zhou, A.-H.; He, Q.; Shu, C.; Yu, Y.-F.; Liu, S.; Zhao, T.; Zhang, W.; Lu, X.; Ye, L.-W. Atom-Economic Generation of Gold Carbenes: Gold-Catalyzed Formal [3 + 2] Cycloaddition between Ynamides and Isoxazoles. Chem. Sci. 2015, 6, 1265–1271. [Google Scholar]
- Zhang, Y.; Luo, T.; Yang, Z. Strategic Innovation in the Total Synthesis of Complex Natural Products Using Gold Catalysis. Nat. Prod. Rep. 2014, 31, 489–503. [Google Scholar]
- Campeau, D.; León Rayo, D.F.; Mansour, A.; Muratov, K.; Gagosz, F. Gold-Catalyzed Reactions of Specially Activated Alkynes, Allenes, and Alkenes. Chem. Rev. 2021, 121, 8756–8867. [Google Scholar]
- Rocchigiani, L.; Bochmann, M. Recent Advances in Gold(III) Chemistry: Structure, Bonding, Reactivity, and Role in Homogeneous Catalysis. Chem. Rev. 2021, 121, 8364–8451. [Google Scholar]
- Lu, Z.; Li, T.; Mudshinge, S.R.; Xu, B.; Hammond, G.B. Optimization of Catalysts and Conditions in Gold(I) Catalysis-Counterion and Additive Effects. Chem. Rev. 2021, 121, 8452–8477. [Google Scholar]
- Chintawar, C.C.; Yadav, A.K.; Kumar, A.; Sancheti, S.P.; Patil, N.T. Divergent Gold Catalysis: Unlocking Molecular Diversity through Catalyst Control. Chem. Rev. 2021, 121, 8478–8558. [Google Scholar]
- Ye, L.-W.; Zhu, X.-Q.; Sahani, R.L.; Xu, Y.; Qian, P.-C.; Liu, R.-S. Nitrene Transfer and Carbene Transfer in Gold Catalysis. Chem. Rev. 2021, 121, 9039–9112. [Google Scholar]
- Melekhova, A.A.; Smirnov, A.S.; Novikov, A.S.; Panikorovskii, T.L.; Bokach, N.A.; Kukushkin, V.Y. Copper(I)-Catalyzed 1,3-Dipolar Cycloaddition of Ketonitrones to Dialkylcyanamides: A Step toward Sustainable Generation of 2,3-Dihydro-1,2,4-Oxadiazoles. ACS Omega 2017, 2, 1380–1391. [Google Scholar]
- Breugst, M.; Reissig, H.U. The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar]
- Ess, D.H.; Houk, K.N. Distortion/Interaction Energy Control of 1,3-Dipolar Cycloaddition Reactivity. J. Am. Chem. Soc. 2007, 129, 10646–10647. [Google Scholar]
- Stylianakis, I.; Litinas, I.; Nieto Faza, O.; Kolocouris, A.; Silva López, C. On the Mechanism of the Au(I)-Mediated Addition of Alkynes to Anthranils to Furnish 7-Acylindoles. J. Phys. Org. Chem. 2022, e4333. [Google Scholar] [CrossRef]
- Hansen, T.; Vermeeren, P.; Bickelhaupt, F.M.; Hamlin, T.A. Origin of the α-Effect in SN2 Reactions. Angew. Chemie Int. Ed. 2021, 60, 20840–20848. [Google Scholar]
- Schleyer, P.V.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J.R. Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar]
- Moll, J.F.; Pemberton, R.P.; Gutierrez, M.G.; Castro, C.; Karney, W.L.J. Configuration Change in [14]Annulene Requires Möbius Antiaromatic Bond Shifting. Am. Chem. Soc. 2007, 129, 274–275. [Google Scholar]
- Fallah-Bagher-Shaidaei, H.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.V.R. Which NICS Aromaticity Index for Planar π Rings Is Best? Org. Lett. 2006, 8, 863–866. [Google Scholar]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P. von R. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar]
- Morao, I.; Cossio, F.P. A Simple Ring Current Model for Describing In-Plane Aromaticity in Pericyclic Reactions. J. Org. Chem. 1999, 64, 1868–1874. [Google Scholar]
- López, C.S.; Nieto Faza, O.; Freindorf, M.; Kraka, E.; Cremer, D. Solving the Pericyclic–Pseudopericyclic Puzzle in the Ring-Closure Reactions of 1,2,4,6-Heptatetraene Derivatives. J. Org. Chem. 2016, 81, 404–414. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, 1133–1138. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Bauernschmitt, R.; Ahlrichs, R.J. Stability Analysis for Solutions of the Closed Shell Kohn-Sham Equation. Chem. Phys. 1996, 104, 9047–9052. [Google Scholar]
- Faza, O.N.; Rodríguez, R.Á.; López, C.S. Performance of Density Functional Theory on Homogeneous Gold Catalysis. Theor. Chem. Acc. 2011, 128, 647–661. [Google Scholar]
- Faza, O.; López, C. Computational approaches to homogeneous gold catalysis. Top. Curr. Chem. 2014, 357, 213–283. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar]
- York, D.M.; Karplus, M.J. A Smooth Solvation Potential Based on the Conductor-like Screening Model. Phys. Chem. A 1999, 103, 11060–11079. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]






| 1st Step | ΔG‡ | 2nd Step | ΔG‡ | 3rd Step | ΔG‡ | 4th Step | ΔG‡ | 5th Step | ΔG‡ |
|---|---|---|---|---|---|---|---|---|---|
| TS3a1 | 21.4 | TS4a1 | 2.1 | TS5a1_anti | 19.2 | TS6a1_anti | 10.6 | TS6a1_anti’ | 13.3 |
| TS5a1_syn | 30.3 | TS6a1_syn | 13.2 | TS6a1_syn’ | 9.7 | ||||
| TS3a2 | 9.1 | TS4a2 | 8.8 | TS5a2_anti | 10.4 | TS6a2_anti | 16.6 | TS6a2_anti’ | 16.6 |
| TS5a2_syn | 11.0 | TS6a2_syn | 18.5 | TS6a2_syn’ | 15.8 |
| 1st Step | ΔG‡ | 2nd Step | ΔG‡ | 3rd Step | ΔG‡ | 4th Step | ΔG‡ | 5th Step | ΔG‡ |
|---|---|---|---|---|---|---|---|---|---|
| TS3b1 | 14.5 | TS4b1 | 5.9 | TS5b1 | 28.9 | TS6b1A | 11.0 | - | |
| TS6b1B | 7.0 | TS6b1B’ | 7.3 | ||||||
| TS3b2 | 16.6 | TS4b2 | 5.6 | TS5b2 | 31.7 | TS6b2A | 15.2 | - | |
| TS6b2B | 6.1 | TS6b2B’ | 10.8 | ||||||
| TS3b3 | 16.2 |
| 1st Step | ΔG‡ | 2nd Step | ΔG‡ | 3rd Step | ΔG‡ | 4th Step | ΔG‡ | 5th Step | ΔG‡ | 6th Step | ΔG‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TS3c1 | 10.2 | TS4c1A | 13.3 | TS4c1B | 1.6 | TS5c1A | 29.3 | TS6c1A | 6.9 | TS7c1A | 1.2 |
| TS6c1B | 1.1 | TS7c1B | 7.1 | ||||||||
| TS3c2 | 18.2 | TS4c2A | 9.9 | TS4c2B | -- | TS5c2A | 32.4 | TS6c2A | 14.9 | TS7c2A | 1.5 |
| TS6c2B | 10.2 | TS7c2B | 11.4 | ||||||||
| TS3c3 | 18.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stylianakis, I.; Litinas, I.; Kolocouris, A.; Silva López, C. Formation and Intramolecular Capture of α-Imino Gold Carbenoids in the Au(I)-Catalyzed [3 + 2] Reaction of Anthranils, 1,2,4-Oxadiazoles, and 4,5-Dihydro-1,2,4-Oxadiazoles with Ynamides. Catalysts 2022, 12, 915. https://doi.org/10.3390/catal12080915
Stylianakis I, Litinas I, Kolocouris A, Silva López C. Formation and Intramolecular Capture of α-Imino Gold Carbenoids in the Au(I)-Catalyzed [3 + 2] Reaction of Anthranils, 1,2,4-Oxadiazoles, and 4,5-Dihydro-1,2,4-Oxadiazoles with Ynamides. Catalysts. 2022; 12(8):915. https://doi.org/10.3390/catal12080915
Chicago/Turabian StyleStylianakis, Ioannis, Iraklis Litinas, Antonios Kolocouris, and Carlos Silva López. 2022. "Formation and Intramolecular Capture of α-Imino Gold Carbenoids in the Au(I)-Catalyzed [3 + 2] Reaction of Anthranils, 1,2,4-Oxadiazoles, and 4,5-Dihydro-1,2,4-Oxadiazoles with Ynamides" Catalysts 12, no. 8: 915. https://doi.org/10.3390/catal12080915