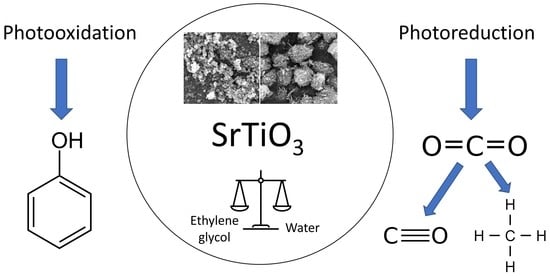
Dependence of Photocatalytic Activity on the Morphology of Strontium Titanates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. XRD, N2 Adsorption, and DRS Measurements
2.1.2. SEM Measurements
2.2. Photocatalytic Activity Measurements
2.2.1. Photocatalytic Oxidation of Phenol
2.2.2. Photocatalytic Reduction of CO2
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Characterization Methods and Instrumentation
3.4. Photocatalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Phoon, B.L.; Lai, C.W.; Juan, J.C.; Show, P.L.; Chen, W.H. A review of synthesis and morphology of SrTiO3 for energy and other applications. Int. J. Energy Res. 2019, 43, 5151–5174. [Google Scholar] [CrossRef]
- Yousefi, R.; Jamali-Sheini, F.; Cheraghizade, M.; Khosravi-Gandomani, S.; Sáaedi, A.; Huang, N.M.; Basirun, W.J.; Azarang, M. Enhanced visible-light photocatalytic activity of strontium-doped zinc oxide nanoparticles. Mater. Sci. Semicond. Process. 2015, 32, 152–159. [Google Scholar] [CrossRef]
- Xu, J.; Wei, Y.; Huang, Y.; Wang, J.; Zheng, X.; Sun, Z.; Fan, L.; Wu, J. Solvothermal synthesis nitrogen doped SrTiO3 with high visible light photocatalytic activity. Ceram. Int. 2014, 40, 10583–10591. [Google Scholar] [CrossRef]
- Gao, X.; Li, M.; Zhou, F.; Wang, X.; Chen, S.; Yu, J. Flexible zirconium doped strontium titanate nanofibrous membranes with enhanced visible-light photocatalytic performance and antibacterial activities. J. Colloid Interface Sci. 2021, 600, 127–137. [Google Scholar] [CrossRef]
- Sukpanish, P.; Lertpanyapornchai, B.; Yokoi, T.; Ngamcharussrivichai, C. Lanthanum-doped mesostructured strontium titanates synthesized via sol–gel combustion route using citric acid as complexing agent. Mater. Chem. Phys. 2016, 181, 422–431. [Google Scholar] [CrossRef]
- Kiss, B.; Manning, T.D.; Hesp, D.; Didier, C.; Taylor, A.; Pickup, D.M.; Chadwick, A.V.; Allison, H.E.; Dhanak, V.R.; Claridge, J.B.; et al. Nano-structured rhodium doped SrTiO3–Visible light activated photocatalyst for water decontamination. Appl. Catal. B Environ. 2017, 206, 547–555. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Z.; Wang, R.; Zhou, C.; Sun, C. Synthesis and post-annealing of Ag nanoparticles decorated urchin-like SrTiO3 particles for enhanced electron/hole separation and photocatalytic activity. Ceram. Int. 2020, 46, 19460–19468. [Google Scholar] [CrossRef]
- Taşyürek, L.B.; Aydoğan, Ş.; Sevim, M.; Çaldıran, Z. Synthesis of nickel nanoparticles-deposited strontium titanate nanocubes (Ni-STO) and heterojunction electrical applications over a wide temperature range. Mater. Sci. Eng. B 2021, 274, 115479. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, J.; Zhang, Y.; Jiang, M.; Xu, S.; Liang, Q.; Li, Z. Shape-controlled synthesis of golf-like, star-like, urchin-like and flower-like SrTiO3 for highly efficient photocatalytic degradation and H2 production. J. Alloy. Compd. 2020, 817, 152796. [Google Scholar] [CrossRef]
- Kuang, Q.; Yang, S. Template synthesis of single-crystal-like porous SrTiO3 nanocube assemblies and their enhanced photocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2013, 5, 3683–3690. [Google Scholar] [CrossRef]
- Dong, W.; Li, X.; Yu, J.; Guo, W.; Li, B.; Tan, L.; Li, C.; Shi, J.; Wang, G. Porous SrTiO3 spheres with enhanced photocatalytic performance. Mater. Lett. 2012, 67, 131–134. [Google Scholar] [CrossRef]
- Liu, Y.; Qian, Q.; Li, J.; Zhu, X.; Zhang, M.; Zhang, T. Photocatalytic Properties of SrTiO3 Nanocubes Synthesized Through Molten Salt Modified Pechini Route. J. Nanosci. Nanotechnol. 2016, 16, 12321–12325. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; Fresno, F.; Suárez, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Meng, D.; Xing, Y.; Wang, C.; Li, F.; Wang, Y.; Wu, X. Enhanced photocatalytic activity of hierarchically structured BiVO4 oriented along {040} facets with different morphologies. Mater. Lett. 2015, 147, 1–3. [Google Scholar] [CrossRef]
- Kedves, E.-Z.; Bárdos, E.; Gyulavári, T.; Pap, Z.; Hernadi, K.; Baia, L. Dependence of cationic dyes’ adsorption upon α-MoO3 structural properties. Appl. Surf. Sci. 2022, 573, 151584. [Google Scholar] [CrossRef]
- Bardos, E.; Marta, V.A.; Fodor, S.; Kedves, E.Z.; Hernadi, K.; Pap, Z. Hydrothermal Crystallization of Bismuth Oxychlorides (BiOCl) Using Different Shape Control Reagents. Materials 2021, 14, 2261. [Google Scholar] [CrossRef]
- Balázs, N.; Mogyorósi, K.; Srankó, D.F.; Pallagi, A.; Alapi, T.; Oszkó, A.; Dombi, A.; Sipos, P. The effect of particle shape on the activity of nanocrystalline TiO2 photocatalysts in phenol decomposition. Appl. Catal. B Environ. 2008, 84, 356–362. [Google Scholar] [CrossRef]
- Wahi, R.K.; Yu, W.W.; Liu, Y.; Mejia, M.L.; Falkner, J.C.; Nolte, W.; Colvin, V.L. Photodegradation of Congo Red catalyzed by nanosized TiO2. J. Mol. Catal. A Chem. 2005, 242, 48–56. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Li, B.; Li, P. Morphology dependent photocatalytic efficacy of zinc ferrite probed for methyl orange degradation. J. Serb. Chem. Soc. 2018, 83, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Xiang, L.; Zhao, X. Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties. Nanomaterials 2017, 7, 310. [Google Scholar] [CrossRef] [Green Version]
- Gyulavári, T.; Kovács, K.; Kovács, Z.; Bárdos, E.; Kovács, G.; Baán, K.; Magyari, K.; Veréb, G.; Pap, Z.; Hernadi, K. Preparation and characterization of noble metal modified titanium dioxide hollow spheres—New insights concerning the light trapping efficiency. Appl. Surf. Sci. 2020, 534, 147327. [Google Scholar] [CrossRef]
- Gao, H.; Yang, H.; Wang, S. Hydrothermal synthesis, growth mechanism, optical properties and photocatalytic activity of cubic SrTiO3 particles for the degradation of cationic and anionic dyes. Optik 2018, 175, 237–249. [Google Scholar] [CrossRef]
- Ramos-Sanchez, J.E.; Camposeco, R.; Lee, S.-W.; Rodríguez-González, V. Sustainable synthesis of AgNPs/strontium-titanate-perovskite-like catalysts for the photocatalytic production of hydrogen. Catal. Today 2020, 341, 112–119. [Google Scholar] [CrossRef]
- Huang, S.-T.; Lee, W.W.; Chang, J.-L.; Huang, W.-S.; Chou, S.-Y.; Chen, C.-C. Hydrothermal synthesis of SrTiO3 nanocubes: Characterization, photocatalytic activities, and degradation pathway. J. Taiwan Inst. Chem. Eng. 2014, 45, 1927–1936. [Google Scholar] [CrossRef]
- Le, M.-V.; Vo, N.-Q.-D.; Le, Q.-C.; Tran, V.A.; Phan, T.-Q.-P.; Huang, C.-W.; Nguyen, V.-H. Manipulating the Structure and Characterization of Sr1−xLaxTiO3 Nanocubes toward the Photodegradation of 2-Naphthol under Artificial Solar Light. Catalysts 2021, 11, 564. [Google Scholar] [CrossRef]
- Asgari-Fard, Z.; Sabet, M.; Salavati-Niasari, M. Synthesis and Characterization of Strontium Carbonate Nanostructures via Simple Hydrothermal Method. High. Temp. Mater. Processes 2016, 35, 215–220. [Google Scholar] [CrossRef]
- Xu, G.; Huang, X.; Zhang, Y.; Deng, S.; Wei, X.; Shen, G.; Han, G. Self-assembly and formation mechanism of single-crystal SrTiO3 nanosheets via solvothermal route with ethylene glycol as reaction medium. CrystEngComm 2013, 15, 7206. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, Z.; Fu, Q.; Wang, H.; Hu, Z.; Tao, H.; Zhang, X.; Ma, Z.; Jia, T. Enhanced photocatalytic performance of SrTiO3 crystals with (100), (110) and (111) orientations treated by N2 (H2) plasma. J. Mater. Sci. 2018, 53, 15340–15347. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Wang, J.; Chen, C.; Peng, T. SrCO3-modified brookite/anatase TiO2 heterophase junctions with enhanced activity and selectivity of CO2 photoreduction to CH4. Appl. Surf. Sci. 2019, 476, 937–947. [Google Scholar] [CrossRef]
- Li-Mei, G.U.O.; Yuan-Jiang, K.; Xiao-Dan, Y.; Yan-Long, Y.U.; Jiang-Hong, Y.A.O.; Ya-An, C.A.O. Investigation on Photocatalytic Reduction of CO2 into CH4 Using SrB2O4/SrCO3 Composite Catalyst. Acta Phys.-Chim. Sin. 2013, 29, 1558–1565. [Google Scholar] [CrossRef]
- Jin, S.; Dong, G.; Luo, J.; Ma, F.; Wang, C. Improved photocatalytic NO removal activity of SrTiO3 by using SrCO3 as a new co-catalyst. Appl. Catal. B Environ. 2018, 227, 24–34. [Google Scholar] [CrossRef]
- Weiss, C.V.; Zhang, J.; Spies, M.; Abdallah, L.S.; Zollner, S.; Cole, M.W.; Alpay, S.P. Bulk-like dielectric properties from metallo-organic solution–deposited SrTiO3 films on Pt-coated Si substrates. J. Appl. Phys. 2012, 111, 054108. [Google Scholar] [CrossRef] [Green Version]
- Trepakov, V.; Dejneka, A.; Markovin, P.; Lynnyk, A.; Jastrabik, L. A ‘soft electronic band’ and the negative thermooptic effect in strontium titanate. New J. Phys. 2009, 11, 083024. [Google Scholar] [CrossRef]
- Gyulavári, T.; Veréb, G.; Pap, Z.; Dombi, A.; Hernádi, K. Associating low crystallinity with peroxo groups for enhanced visible light active photocatalysts. Catal. Today 2018, 313, 231–238. [Google Scholar] [CrossRef] [Green Version]
- Bastida, M.A.C.; Maldonado, Y.G.; Vidal, Y.R.; Lopez, M.S.; Gonzalez, E.C.; Ayala, F.E. Sodium Titanate Synthesis for the Photocatalytic Degradation of NO. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Hai, C.; Li, S.; Zhou, Y.; Zeng, J.; Ren, X.; Li, X. Roles of ethylene glycol solvent and polymers in preparing uniformly distributed MgO nanoparticles. J. Asian Ceram. Soc. 2018, 5, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Fodor, S.; Kovács, G.; Hernádi, K.; Danciu, V.; Baia, L.; Pap, Z. Shape tailored Pd nanoparticles’ effect on the photocatalytic activity of commercial TiO2. Catal. Today 2017, 284, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Lowekamp, J.B.; Rohrer, G.S.; Morris Hotsenpiller, P.A.; Bolt, J.D.; Farneth, W.E. Anisotropic Photochemical Reactivity of Bulk TiO2 Crystals. J. Phys. Chem. B 1998, 102, 7323–7327. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, L.; Luo, L.; Huang, R.; Tang, Z.; Xiao, P.; Zhang, Y. Combining Bulk Charge Transport and Surface Charge Transfer to Design Titanium-Doped Hematite Homojunction Photoanodes. J. Phys. Chem. C 2022, 126, 4296–4305. [Google Scholar] [CrossRef]
- Parambil, J.A.; Mujeeb, A.V.M.; Parayil, S.K. P123 assisted sol-gel combustion synthesis of mesoporous strontium titanate nanomaterials for photocatalytic degradation of methylene blue. Indian J. Chem. A-Inorgh. Bio-Inorg. Phys. Theor. Anal. Chem. 2021, 60, 1443–1451. [Google Scholar]
- Nakagawa, K.; Yamaguchi, K.; Yamada, K.; Sotowa, K.I.; Sugiyama, S.; Adachi, M. Synthesis and Characterization of Surface-Functionalized Layered Titanate Nanosheets Using Lamellar Self-Assembly as a Template. Eur. J. Inorg. Chem. 2012, 2012, 2741–2748. [Google Scholar] [CrossRef]
- Yao, W.; Chen, Y.; Li, J.; Yang, J.; Ren, S.; Liu, W.; Liu, Q. Photocatalytic degradation of methyl orange by Ca doped β-In2S3 with varying Ca concentration. Res. Chem. Intermed. 2022, 48, 1813–1829. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 photo-reduction: Insights into CO2 activation and reaction on surfaces of photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Wachs, I.E.; Phivilay, S.P.; Roberts, C.A. Reporting of Reactivity for Heterogeneous Photocatalysis. ACS Catal. 2013, 3, 2606–2611. [Google Scholar] [CrossRef]
- Ahuja, S.; Kutty, T.R.N. Nanoparticles of SrTiO3 prepared by gel to crystallite conversion and their photocatalytic activity in the mineralization of phenol. J. Photochem. Photobiol. A 1996, 97, 99–107. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynska, A. TiO2/SrTiO3 and SrTiO3 microspheres decorated with Rh, Ru or Pt nanoparticles: Highly UV–vis responsible photoactivity and mechanism. J. Catal. 2017, 350, 159–173. [Google Scholar] [CrossRef]
- Bi, Y.; Ehsan, M.F.; Huang, Y.; Jin, J.; He, T. Synthesis of Cr-doped SrTiO3 photocatalyst and its application in visible-light-driven transformation of CO2 into CH4. J. CO2 Util. 2015, 12, 43–48. [Google Scholar] [CrossRef]
- Luo, C.; Zhao, J.; Li, Y.; Zhao, W.; Zeng, Y.; Wang, C. Photocatalytic CO2 reduction over SrTiO3: Correlation between surface structure and activity. Appl. Surf. Sci. 2018, 447, 627–635. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Zhou, H.; Haminhngoc; Zhu, F.; Guo, Q.; Zhao, Z. Molten Salt Synthesis of SrTiO3 Using TiO2 with Different Morphology as a Precursor. Integr. Ferroelectr. 2015, 162, 113–121. [Google Scholar] [CrossRef]





| Sample Name | Size (nm) | Specific Surface Area (m2∙g−1) | SrCO3 Content (%) | I(111)/(110) | Band Gap (eV) | r0,phenol (10−9 M∙s−1) | TORs,phenol (µmol/m2/h) | r0,CO₂ (10−11 mol∙s−1) | TORs,CO₂ (µmol/m2/h) | CH4 Selectivity (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| SrTiO3_0.25 | 30.6 | 26 | 1.23 | 0.22 | 3.29 | 1.46 | 2.0 × 10–2 | 9.35 | 1.3 × 10−2 | 1.99 |
| SrTiO3_0.5 | 32.9 | 14 | 0.29 | 0.21 | 3.28 | 1.34 | 3.5 × 10–2 | 7.66 | 2.0 × 10−2 | 3.99 |
| SrTiO3_1 | 17.2 | 55 | 0.18 | 0.24 | 3.34 | 0.99 | 6.5 × 10–3 | 10.17 | 6.7 × 10−3 | 3.52 |
| SrTiO3_2 | 8.4 | 146 | 3.53 | 0.27 | 3.33 | 1.01 | 2.5 × 10–3 | 8.49 | 2.1 × 10−3 | 4.76 |
| SrTiO3_4 | 9.8 | 123 | 4.62 | 0.25 | 3.34 | 1.20 | 3.5 × 10–3 | 8.86 | 2.6 × 10−3 | 4.64 |
| SrTiO3_8 | 10.5 | 117 | 10.94 | 0.26 | 3.36 | 1.20 | 3.7 × 10–3 | 9.97 | 3.1 × 10−3 | 3.72 |
| Sample Name | EG (mL) | Ti(IV) Butoxide (mL) | NaOH (mL) | Sr(NO3)2 (mL) | H2O (mL) | EG:H2O Ratio |
|---|---|---|---|---|---|---|
| SrTiO3_0.25 | 18.3 | 8.3 | 24.5 | 48.9 | 73.4 | 0.25 |
| SrTiO3_0.5 | 30.0 | 6.8 | 20.0 | 40.0 | 60.0 | 0.5 |
| SrTiO3_1 | 47.1 | 5.3 | 15.7 | 31.4 | 47.1 | 1.0 |
| SrTiO3_2 | 63.0 | 3.6 | 10.5 | 21.0 | 31.5 | 2.0 |
| SrTiO3_4 | 78.0 | 2.2 | 6.5 | 13.0 | 19.5 | 4.0 |
| SrTiO3_8 | 87.6 | 1.2 | 3.7 | 7.3 | 11.0 | 8.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyulavári, T.; Dusnoki, D.; Márta, V.; Yadav, M.; Abedi, M.; Sápi, A.; Kukovecz, Á.; Kónya, Z.; Pap, Z. Dependence of Photocatalytic Activity on the Morphology of Strontium Titanates. Catalysts 2022, 12, 523. https://doi.org/10.3390/catal12050523
Gyulavári T, Dusnoki D, Márta V, Yadav M, Abedi M, Sápi A, Kukovecz Á, Kónya Z, Pap Z. Dependence of Photocatalytic Activity on the Morphology of Strontium Titanates. Catalysts. 2022; 12(5):523. https://doi.org/10.3390/catal12050523
Chicago/Turabian StyleGyulavári, Tamás, Daniella Dusnoki, Viktória Márta, Mohit Yadav, Mahsa Abedi, András Sápi, Ákos Kukovecz, Zoltán Kónya, and Zsolt Pap. 2022. "Dependence of Photocatalytic Activity on the Morphology of Strontium Titanates" Catalysts 12, no. 5: 523. https://doi.org/10.3390/catal12050523