Photocatalytic Filtration of Zinc Oxide-Based Membrane with Enhanced Visible Light Responsiveness for Ibuprofen Removal
Abstract
:1. Introduction
2. Result and Discussion
2.1. Characterization of ZnO/Ag2CO3/Ag2O Photocatalyst
2.2. Membrane Morphology and Structure
2.3. Membrane Wettability and Porosity
2.4. Membrane Surface Charge
2.5. The IBF Flux Properties of MMMs for Photocatalytic Filtration Performance
2.6. Ibuprofen Removal Efficiency
2.7. Membrane Recyclability Studies
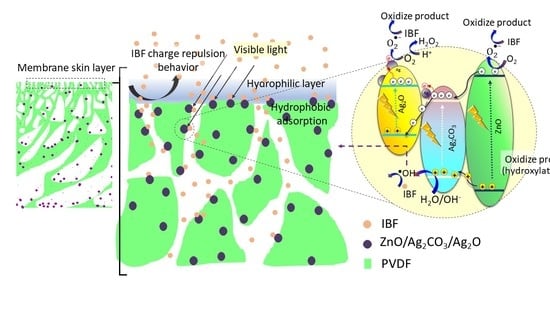
2.8. Mechanism for Enhanced Antifouling Performance
3. Methodology and Characteristics
3.1. Materials
3.2. Preparation of the Photocatalyst
3.3. Preparation of Mixed Matrix Membrane (MMMs)
3.4. Photocatalyst Characteristics
3.5. Membrane Characteristics
3.6. Membrane Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Richardson, B.J.; Lam, P.K.; Martin, M. Emerging chemicals of concern: Pharmaceuticals and personal care products (PPCPs) in Asia, with particular reference to Southern China. Mar. Pollut. Bull. 2005, 50, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Roy, S.; Nigam, V.K. Advances in protein/enzyme-based biosensors for the detection of pharmaceutical contaminants in the environment. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 207–229. [Google Scholar]
- Celiz, M.D.; Tso, J.; Aga, D.S. Pharmaceutical metabolites in the environment: Analytical challenges and ecological risks. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, E.; Perrodin, Y.; Keck, G.; Blanchard, J.-M.; Vermande, P. Ecotoxicological risk assessment of hospital wastewater: A proposed framework for raw effluents discharging into urban sewer network. J. Hazard. Mater. 2005, 117, 1–11. [Google Scholar] [CrossRef]
- Tauxe-Wuersch, A.; De Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Res. 2005, 39, 1761–1772. [Google Scholar] [CrossRef]
- Brozinski, J.-M.; Lahti, M.; Meierjohann, A.; Oikari, A.; Kronberg, L. The anti-inflammatory drugs diclofenac, naproxen and ibuprofen are found in the bile of wild fish caught downstream of a wastewater treatment plant. Environ. Sci. Technol. 2013, 47, 342–348. [Google Scholar] [CrossRef]
- Bragança, I.; Plácido, A.; Paíga, P.; Domingues, V.F.; Delerue-Matos, C. QuEChERS: A new sample preparation approach for the determination of ibuprofen and its metabolites in soils. Sci. Total Environ. 2012, 433, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Weigel, S.; Berger, U.; Jensen, E.; Kallenborn, R.; Thoresen, H.; Hühnerfuss, H. Determination of selected pharmaceuticals and caffeine in sewage and seawater from Tromsø/Norway with emphasis on ibuprofen and its metabolites. Chemosphere 2004, 56, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Fontela, M.; Galceran, M.T.; Ventura, F. Occurrence and removal of pharmaceuticals and hormones through drinking water treatment. Water Res. 2011, 45, 1432–1442. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Advanced oxidation processes for textile wastewater treatment. Int. J. Photochem. Photobiol. 2017, 2, 85–93. [Google Scholar]
- Tran, D.-T.; Mendret, J.; Méricq, J.-P.; Faur, C.; Brosillon, S. Study of permeate flux behavior during photo-filtration using photocatalytic composite membranes. Chem. Eng. 2020, 148, 107781. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, C.; He, A.; Yang, S.J.; Wu, G.P.; Darling, S.B.; Xu, Z.K. Photocatalytic nanofiltration membranes with self-cleaning property for wastewater treatment. Adv. Funct. Mater. 2017, 27, 1700251. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, Z.-P.; Shi, L.; Cheng, R.; Yuan, D.-H. Photocatalytic membrane reactors (PMRs) in water treatment: Configurations and influencing factors. Catalysts 2017, 7, 224. [Google Scholar] [CrossRef]
- Lee, A.; Libera, J.A.; Waldman, R.Z.; Ahmed, A.; Avila, J.R.; Elam, J.W.; Darling, S.B. Conformal nitrogen-doped TiO2 photocatalytic coatings for sunlight-activated membranes. Adv. Sustain. Syst. 2017, 1, 1600041. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Li, X.; Yu, C.; Zeng, D.; Chen, F.; Zhang, K.; Huang, W.; Ji, H. Review on heterophase/homophase junctions for efficient photocatalysis: The case of phase transition construction. Chin. J. Catal. 2019, 40, 796–818. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Razali, N.A.M. Heterojunction-based photocatalyst. In Photocatalytic Systems by Design; Elsevier: Amsterdam, The Netherlands, 2021; pp. 85–130. [Google Scholar]
- Yang, F.; Yan, L.; Zhang, B.; He, X.; Li, Y.; Tang, Y.; Ma, C.; Li, Y. Fabrication of ternary NaTaO3/g-C3N4/G heterojunction photocatalyst with enhanced activity for Rhodamine B degradation. J. Alloys Compd. 2019, 805, 802–810. [Google Scholar] [CrossRef]
- Liang, H.; Guo, J.; Yu, M.; Zhou, Y.; Zhan, R.; Liu, C.; Niu, J. Porous loofah-sponge-like ternary heterojunction g-C3N4/Bi2WO6/MoS2 for highly efficient photocatalytic degradation of sulfamethoxazole under visible-light irradiation. Chemosphere 2021, 279, 130552. [Google Scholar] [CrossRef] [PubMed]
- Nasir, R.; Mukhtar, H.; Man, Z.; Mohshim, D.F. Material advancements in fabrication of mixed-matrix membranes. Chem. Eng. Technol. 2013, 36, 717–727. [Google Scholar] [CrossRef]
- Bhol, P.; Yadav, S.; Altaee, A.; Saxena, M.; Misra, P.K.; Samal, A.K. Graphene-Based Membranes for Water and Wastewater Treatment: A Review. ACS Appl. Nano Mater. 2021, 4, 3274–3293. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; Di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Jamalludin, M.R.; Harun, Z.; Hubadillah, S.K.; Basri, H.; Ismail, A.F.; Othman, M.H.D.; Shohur, M.F.; Yunos, M.Z. Antifouling polysulfone membranes blended with green SiO2 from rice husk ash (RHA) for humic acid separation. Chem. Eng. Res. Des. 2016, 114, 268–279. [Google Scholar] [CrossRef]
- Subramaniam, M.; Goh, P.; Lau, W.; Tan, Y.; Ng, B.; Ismail, A. Hydrophilic hollow fiber PVDF ultrafiltration membrane incorporated with titanate nanotubes for decolourization of aerobically-treated palm oil mill effluent. Chem. Eng. J. 2017, 316, 101–110. [Google Scholar] [CrossRef]
- Rosman, N.; Salleh, W.N.W.; Mohamed, M.A.; Harun, Z.; Ismail, A.F.; Aziz, F. Constructing a compact heterojunction structure of Ag2CO3/Ag2O in-situ intermediate phase transformation decorated on ZnO with superior photocatalytic degradation of ibuprofen. Sep. Purif. Technol. 2020, 117391. [Google Scholar] [CrossRef]
- Méricq, J.-P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Zhang, R.; Luis, P.; Arsuaga, J.M.; Kim, J.; Van der Bruggen, B. Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES–TiO2 membranes. J. Colloid Interface Sci. 2011, 363, 540–550. [Google Scholar] [CrossRef]
- Kahrs, C.; Gühlstorf, T.; Schwellenbach, J. Influences of different preparation variables on polymeric membrane formation via nonsolvent induced phase separation. J. Appl. Polym. Sci. 2019, 48852. [Google Scholar] [CrossRef] [Green Version]
- Hołda, A.K.; Vankelecom, I.F. Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, L.; Shen, F.; Zhang, B.; Jiang, H.; Li, J.; Luo, J.; Wu, H.; Khan, R.; Wan, Y. Fabrication of PES-based membranes with a high and stable desalination performance for membrane distillation. RSC Adv. 2016, 6, 107840–107850. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Mollahosseini, A.; Rajaeian, B. Structural and performance properties of UV-assisted TiO2 deposited nano-composite PVDF/SPES membranes. Desalination 2012, 285, 31–38. [Google Scholar] [CrossRef]
- Rizza, M.A.; Wijayanti, W.; Hamidi, N.; Wardana, I. Role of Intermolecular Forces on the Contact Angle of Vegetable Oil Droplets during the Cooling Process. Sci. World J. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Zhang, D.; Dai, F.; Zhang, P.; Ana, Z.; Zhao, Y.; Chen, L. The photodegradation of methylene blue in water with PVDF/GO/ZnO composite membrane. Mater. Sci. Eng. C 2019, 96, 684–692. [Google Scholar] [CrossRef]
- Salim, N.E.; Nor, N.; Jaafar, J.; Ismail, A.; Qtaishat, M.; Matsuura, T.; Othman, M.; Rahman, M.A.; Aziz, F.; Yusof, N. Effects of hydrophilic surface macromolecule modifier loading on PES/Og-C3N4 hybrid photocatalytic membrane for phenol removal. Appl. Surf. Sci. 2019, 465, 180–191. [Google Scholar] [CrossRef]
- Ayyaru, S.; Dinh, T.T.L.; Ahn, Y.-H. Enhanced antifouling performance of PVDF ultrafiltration membrane by blending zinc oxide with support of graphene oxide nanoparticle. Chemosphere 2020, 241, 125068. [Google Scholar] [CrossRef]
- Zinadini, S.; Rostami, S.; Vatanpour, V.; Jalilian, E. Preparation of antibiofouling polyethersulfone mixed matrix NF membrane using photocatalytic activity of ZnO/MWCNTs nanocomposite. J. Membr. Sci. 2017, 529, 133–141. [Google Scholar] [CrossRef]
- Yu, L.Y.; Shen, H.M.; Xu, Z.L. PVDF–TiO2 composite hollow fiber ultrafiltration membranes prepared by TiO2 sol–gel method and blending method. J. Appl. Polym. Sci. 2009, 113, 1763–1772. [Google Scholar] [CrossRef]
- Shukla, A.K.; Alam, J.; Alhoshan, M.; Dass, L.A.; Muthumareeswaran, M. Development of a nanocomposite ultrafiltration membrane based on polyphenylsulfone blended with graphene oxide. Sci. Rep. 2017, 7, 41976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Lu, X.; Wu, C.; Gao, Q.; Zhao, L.; Zhang, H.; Liu, Z. Functional surface modification of PVDF membrane for chemical pulse cleaning. J. Membr. Sci. 2017, 524, 389–399. [Google Scholar] [CrossRef]
- Mahlangu, O.; Nackaerts, R.; Mamba, B.; Verliefde, A. Development of hydrophilic GO-ZnO/PES membranes for treatment of pharmaceutical wastewater. Water Sci. Technol. 2017, 76, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Bocchini, S.; Morlat-Thérias, S.; Gardette, J.-L.; Camino, G. Influence of nanodispersed boehmite on polypropylene photooxidation. Polym. Degrad. Stab. 2007, 92, 1847–1856. [Google Scholar] [CrossRef]
- Qiu, S.; Wu, L.; Pan, X.; Zhang, L.; Chen, H.; Gao, C. Preparation and properties of functionalized carbon nanotube/PSF blend ultrafiltration membranes. J. Membr. Sci. 2009, 342, 165–172. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.M.; Jang, A.; Jang, M.; Hernández-Maldonado, A.J.; Yu, M.; Heo, J.; Yoon, Y. Removal of selected pharmaceuticals in an ultrafiltration-activated biochar hybrid system. J. Membr. Sci. 2019, 570, 77–84. [Google Scholar] [CrossRef]
- Subramaniam, M.; Goh, P.; Lau, W.; Ng, B.; Ismail, A. AT-POME colour removal through photocatalytic submerged filtration using antifouling PVDF-TNT nanocomposite membrane. Sep. Purif. Technol. 2018, 191, 266–275. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, L.; Lin, H.; Yu, W.; Xu, Y.; Li, R.; Sun, T.; He, Y. A novel strategy based on magnetic field assisted preparation of magnetic and photocatalytic membranes with improved performance. J. Membr. Sci. 2020, 612, 118378. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Park, G.-Y.; Lee, J.H.; Kim, I.S.; Cho, J. Pharmaceutical rejection by membranes for wastewater reclamation and reuse. Water Sci. Technol. 2004, 50, 239–244. [Google Scholar] [CrossRef]
- Cho, H.-H.; Huang, H.; Schwab, K. Effects of solution chemistry on the adsorption of ibuprofen and triclosan onto carbon nanotubes. Langmuir 2011, 27, 12960–12967. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Yadav, V.; Purkait, M.K. Cu2O photocatalyst modified antifouling polysulfone mixed matrix membrane for ultrafiltration of protein and visible light driven photocatalytic pharmaceutical removal. Sep. Purif. Technol. 2019, 212, 191–204. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Martincigh, B.S.; Skelton, A.A. Interplay between electrostatic and hydrophobic interactions in the pH-dependent adsorption of ibuprofen onto acid-functionalized multiwalled carbon nanotubes. J. Phys. Chem. C 2018, 122, 22556–22568. [Google Scholar] [CrossRef]
- Mahmoudi, E.; Ng, L.Y.; Ang, W.L.; Chung, Y.T.; Rohani, R.; Mohammad, A.W. Enhancing morphology and separation performance of polyamide 6, 6 membranes by minimal incorporation of silver decorated graphene oxide nanoparticles. Sci. Rep. 2019, 9, 1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustakas, N.; Katsaros, F.; Kontos, A.; Romanos, G.E.; Dionysiou, D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Albiter, E.; Valenzuela, M.; Alfaro, S.; Valverde-Aguilar, G.; Martínez-Pallares, F. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef] [Green Version]
- Mino, L.; Pellegrino, F.; Rades, S.; Radnik, J.R.; Hodoroaba, V.-D.; Spoto, G.; Maurino, V.; Martra, G. Beyond shape engineering of TiO2 nanoparticles: Post-synthesis treatment dependence of surface hydration, hydroxylation, Lewis acidity and photocatalytic activity of TiO2 anatase nanoparticles with dominant {001} or {101} facets. ACS Appl. Nano Mater. 2018, 1, 5355–5365. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Zou, J.-J.; Zhang, X.; Wang, L. Water-mediated promotion of dye sensitization of TiO2 under visible light. J. Am. Chem. Soc. 2011, 133, 10000–10002. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, S.; Okada, K.; Morisugi, T.; Azuma, M.; Ohue, H.; Kim, T.-H.; Matsuoka, M.; Anpo, M. Effect of surface treatment on the selective photocatalytic oxidation of benzyl alcohol into benzaldehyde by O2 on TiO2 under visible light. Top. Catal. 2010, 53, 578–583. [Google Scholar] [CrossRef]
- Wu, H.; Fu, Q.; Li, Y.; Cui, Y.; Wang, R.; Su, N.; Lin, L.; Dong, A.; Ning, Y.; Yang, F. Controlled growth of uniform two-dimensional ZnO overlayers on Au (111) and surface hydroxylation. Nano Res. 2019, 12, 2348–2354. [Google Scholar] [CrossRef]
- Newberg, J.T.; Goodwin, C.; Arble, C.; Khalifa, Y.; Boscoboinik, J.A.; Rani, S. ZnO (1010) surface Hydroxylation under ambient water vapor. J. Phys. Chem. B 2018, 122, 472–478. [Google Scholar] [CrossRef]
- Wang, M.; Yang, G.; Jin, P.; Tang, H.; Wang, H.; Chen, Y. Highly hydrophilic poly (vinylidene fluoride)/meso-titania hybrid mesoporous membrane for photocatalytic membrane reactor in water. Sci. Rep. 2016, 6, 19148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosman, N.; Salleh, W.N.W.; Razali, N.A.M.; Ahmad, S.Z.N.; Ismail, N.H.; Aziz, F.; Harun, Z.; Ismail, A.F.; Yusof, N. Ibuprofen removal through photocatalytic filtration using antifouling PVDF-ZnO/Ag2CO3/Ag2O nanocomposite membrane. Mater. Today Proc. 2021, 42, 69–74. [Google Scholar] [CrossRef]











| Peak Assignment | Map Sum Spectrum (wt%) | |||
|---|---|---|---|---|
| PVDF–ZAA0.5 | PVDF–ZAA1 | PVDF–ZAA2 | PVDF–ZAA3 | |
| Fluoride (F) | 55.3 | 54.6 | 53.3 | 51.4 |
| Carbon (C) | 42.6 | 41.3 | 40.4 | 59.2 |
| Oxygen (O) | 1.3 | 1.5 | 2.5 | 3.9 |
| Zinc (Zn) | 0.5 | 1.4 | 2.3 | 3.7 |
| Silver (Ag) | 0.3 | 1.3 | 1.6 | 1.8 |
| Membranes | Ra (Mean Surface Roughness) nm | Rq (Root Mean Square Roughness) nm | Rz (Difference between High Peak and Low Valley) nm |
|---|---|---|---|
| PVDF | 36.930 ± 2.551 | 49.957 ± 1.858 | 463.866 ± 161.025 |
| PVDF-ZAA0.5 | 35.726 ± 3.323 | 45.057 ± 4.538 | 382.682 ± 35.404 |
| PVDF-ZAA1 | 47.763 ± 4.767 | 61.203 ± 8.012 | 520.012 ± 89.788 |
| PVDF-ZAA2 | 48.013 ± 6.858 | 59.815 ± 7.543 | 440.444 ± 18.170 |
| PVDF-ZAA3 | 60.012 ± 9. 99 | 77.525 ± 13.314 | 611.874 ± 138.050 |
| Membrane | Water Uptake (g) | Porosity (%) | Contact Angle (° s−1) |
|---|---|---|---|
| PVDF | 0.420 ± 0.0063 | 43.83 | 0.036 |
| PVDF-ZAA0.5 | 0.463 ± 0.0037 | 54.99 | 0.092 |
| PVDF-ZAA1 | 0.450 ± 0.0987 | 58.55 | 0.085 |
| PVDF-ZAA2 | 0.325 ± 0.0737 | 48.24 | 0.051 |
| PVDF-ZAA3 | 0.221 ± 0.0049 | 45.52 | 0.068 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosman, N.; Wan Salleh, W.N.; Jaafar, J.; Harun, Z.; Aziz, F.; Ismail, A.F. Photocatalytic Filtration of Zinc Oxide-Based Membrane with Enhanced Visible Light Responsiveness for Ibuprofen Removal. Catalysts 2022, 12, 209. https://doi.org/10.3390/catal12020209
Rosman N, Wan Salleh WN, Jaafar J, Harun Z, Aziz F, Ismail AF. Photocatalytic Filtration of Zinc Oxide-Based Membrane with Enhanced Visible Light Responsiveness for Ibuprofen Removal. Catalysts. 2022; 12(2):209. https://doi.org/10.3390/catal12020209
Chicago/Turabian StyleRosman, Nurafiqah, Wan Norharyati Wan Salleh, Juhana Jaafar, Zawati Harun, Farhana Aziz, and Ahmad Fauzi Ismail. 2022. "Photocatalytic Filtration of Zinc Oxide-Based Membrane with Enhanced Visible Light Responsiveness for Ibuprofen Removal" Catalysts 12, no. 2: 209. https://doi.org/10.3390/catal12020209