Enzymatic Desymmetrisation of Prochiral Phosphines and Phosphine P-Sulfides as a Route to P-Chiral Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Bis (2-hydroxymethylphenyl)phosphines 12 and 13
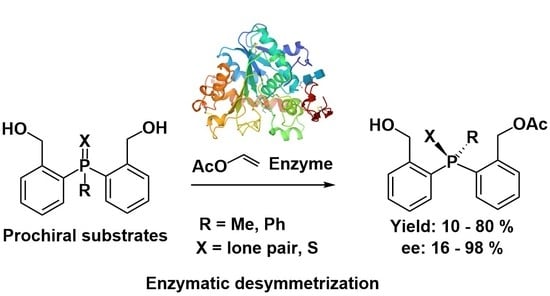
2.2. Desymmetrisation of Bis (2-hydroxymethylphenyl)phosphines 12 and 13
2.3. Desymmetrisation of Bis (2-hydroxymethylphenyl)phosphine Sulfides
2.4. Determination of the Absolute Configuration of Monoacetates 14 and 24
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Bis (2-hydroxymethylphenyl)phosphines 12 and 13
3.2.1. Synthesis of Bis [2-(2′-tetrahydropyranyloxy)methylphenyl]phosphine 10 and 11
- Bis [2-(2′-tetrahydropyranyloxy)methylphenyl]methylphosphine 10
- Crude yield: 1.970 g, 90%
- 31P NMR (CDCl3): δ = −48.3
- Bis [2-(2′-tetrahydropyranyloxy)methylphenyl]phenylphosphine 11
- Crude yield: 2.535 g, 94%
- 31P NMR (CDCl3): δ = −25.7
3.2.2. Synthesis of Bis (2-hydroxymethylphenyl)phosphines 12 and 13
- Bis (2-hydroxymethylphenyl)methylphosphine 12
- Oil, isolated yield: 0.439 g, 25%
- 31P NMR (CDCl3): δ = −50.8;
- 1H NMR (CDCl3): δ = 1.59 (d, J = 3.8 Hz, 3H), 4.67–4.90 (m, 4H), 7.27–7.39 (m, 8H);
- MS (+ESI): m/z = 261 (M + H);
- HRMS (+ESI): m/z = 261.1049, calcd for C15H18PO2 (M + H), 261.1044.
- Bis (2-hydroxymethylphenyl)phenylphosphine 13
- Oil, isolated yield: 0.098 g, 56%
- 31P NMR (CDCl3): δ = −27.5;
- 1H NMR (CDCl3): δ = 4.75–5.04 (m, 4H), 6.94–7.52 (m, 13H);
- MS (+ESI): m/z = 323 (M + H);
- HRMS (+ESI): m/z = 323.1208, calcd for C20H20PO2 (M + H), 323.1201.
3.3. Synthesis of Bis (2-hydroxymethylphenyl)phosphine Sulfides 22 and 23
- Bis (2-hydroxymethylphenyl)methylphosphine sulfide 22
- Yellowish solid, m. p. 92–94 °C, isolated yield: 0.124 g, 61%
- 31P NMR (CDCl3): δ = 34.2;
- 1H NMR (CDCl3): δ = 2.34 (d, J = 13.2 Hz, 3H), 3.71 (br. s, 1H), 4.39–4.75 (m, 4H), 7.11–7.72 (m, 8H);
- 13C NMR (CDCl3): δ = 23.68 (d, JP-Me = 60.5 Hz), 62.93 (d, JP-CH2OH = 6.0 Hz), 128.15, 131.03, 131.12, 131.73, 132.22, 132.30, 132.36, 132.74, 143.97, 144.04 (Aryl);
- MS (+ESI): m/z = 293 (M + H);
- HRMS (+ESI): m/z = 293.0771, calcd for C15H18PO2S (M + H), 293.0765.
- Bis (2-hydroxymethylphenyl)phenylphosphine sulfide 23
- Yellowish solid, m. p. 132–134 °C, isolated yield: 0.111 g, 76%
- 31P NMR (CDCl3): δ = 40.7;
- 1H NMR (CDCl3): δ = 3.85 (br. s, 1H), 4.67–4.82 (m, 4H), 5.29–5.49 (m, 2H), 6.78–7.72 (m, 13H);
- MS (CI): m/z = 355 (M + H);
- HRMS (CI): m/z = 354.0845, calcd for C20H19PO2S (M + H), 354.0843.
3.4. General Procedure for the Enzymatic Desymmetrization of Bis (2-hydroxymethylphenyl)phosphines 12 and 13 and Bis (2-hydroxymethylphenyl)phosphine Sulfides 22 and 23
- (2-acetoxymethylphenyl)(2′-hydroxymethylphenyl)methylphosphine 14
- 31P NMR (CDCl3): δ = −48.4;
- 1H NMR (CDCl3): δ = 1.59 (d, J = 3.6 Hz, 3H), 1.86 (s, 3H), 4.69–4.89 (m, 4H), 7.19–7.48 (m, 8H);
- MS (CI): m/z = 303 (M + H);
- HRMS (+ESI): m/z = 303.1158, calcd for C17H20PO3 (M + H), 303.1150.
- (2-acetoxymethylphenyl)(2′-hydroxymethylphenyl)phenylphosphine 15
- 31P NMR (CDCl3): δ = −26.3;
- 1H NMR (CDCl3): δ = 1.62 (s, 3H), 4.71–4.82 (m, 2H), 5.29–5.49 (m, 2H), 6.81–7.44 (m, 13H);
- MS (FAB): m/z = 365 (M + H);
- HRMS (FAB): m/z = 365.1317, calcd for C22H22PO3 (M + H), 365.1306.
- (2-acetoxymethylphenyl)(2′-hydroxymethylphenyl)methylphosphine sulfide 24
- 31P NMR (CDCl3): δ = 33.7;
- 1H NMR (CDCl3): δ = 1.89 (s, 3H), 2.38 (d, J = 13.2 Hz, 3H), 4.31–4.75 (m, 2H), 4.94–5.09 (m, 2H), 7.36–8.15 (m, 8H);
- MS (+ESI): m/z = 335 (M + H);
- HRMS (+ESI): m/z = 335.0876, calcd for C17H20PO3S (M + H), 335.0871.
- (2-acetoxymethylphenyl)(2′-hydroxymethylphenyl)phenylphosphine sulfide 25
- 31P NMR (CDCl3): δ = 41.2;
- 1H NMR (CDCl3): δ = 1.84 (s, 3H), 4.01 (br. s, 1H), 4.60–4.82 (m, 2H), 5.38–5.55 (m, 2H), 6.89–7.73 (m, 13H);
- MS (CI): m/z = 397 (M + H);
- HRMS (+ESI): m/z = 397.1023, calcd for C22H22PO3S (M + H), 397.1027.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Enders, D.; Hoffmann, R.W. Asymmetrische Synthese. Chem. Unserer Zeit 1985, 19, 177–199. [Google Scholar] [CrossRef]
- Börner, A. (Ed.) Phosphorus Ligands in Asymmetric Catalysis; Wiley-VCH: Karlsruhe, Germany, 2008. [Google Scholar]
- Gammon, J.J.; Viktoria, H.; Gessner, V.H.; Barker, G.R.; Granander, J.; Whitwood, A.C.; Strohmann, C.; O’Brien, P.; Brian, K.B. Synthesis of P-Stereogenic Compounds via Kinetic Deprotonation and Dynamic Thermodynamic Resolution of Phosphine Sulfides: Opposite Sense of Induction Using (-)-Sparteine. J. Am. Chem. Soc. 2010, 132, 13922–13927. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-H.; Zhou, Q.-Y.; Yang, C.; Chen, L.; Cheng, J.-P.; Li, X. Access to P-stereogenic compounds via desymmetrizing enantioselective bromination. Chem. Sci. 2021, 12, 4582. [Google Scholar] [CrossRef] [PubMed]
- Kiełbasiński, P.; Żurawiński, R.; Albrycht, M.; Mikołajczyk, M. The first enzymatic desymmetrizations of prochiral phosphine oxides. Tetrahedron Asymmetry 2003, 14, 3379–3384. [Google Scholar] [CrossRef]
- Wiktelius, D.; Johansson, M.J.; Luthmann, K.; Kann, N. A Biocatalytic Route to P-Chirogenic Compounds by Lipase-Catalyzed Desymmetrization of a Prochiral Phosphine−Borane. Org. Lett. 2005, 7, 4991–4994. [Google Scholar] [CrossRef] [PubMed]
- Kiełbasiński, P.; Rachwalski, M.; Kwiatkowska, M.; Mikołajczyk, M.; Wieczorek, W.M.; Szyrej, M.; Sieroń, L.; Rutjes, F.P.J.T. Enzyme-promoted desymmetrisation of prochiral bis(cyanomethyl)phenylphosphine oxide. Tetrahedron Asymmetry 2007, 18, 2108–2112. [Google Scholar] [CrossRef]
- Kaczmarczyk, S.; Kwiatkowska, M.; Madalińska, L.; Barbachowska, A.; Rachwalski, M.; Błaszczyk, J.; Sieroń, L.; Kiełbasiński, P. Enzymatic Synthesis of Enantiopure Precursors of Chiral Bidentate and Tridentate Phosphorus Catalysts. Adv. Synth. Catal. 2011, 353, 2446–2454. [Google Scholar] [CrossRef]
- Otocka, S.; Kwiatkowska, M.; Madalińska, L.; Kiełbasiński, P. Chiral Organosulfur Ligands/Catalysts with a Stereogenic Sulfur Atom: Applications in Asymmetric Synthesis. Chem. Rev. 2017, 117, 4147–4181. [Google Scholar] [CrossRef] [PubMed]
- Rachwalski, M.; Kwiatkowska, M.; Drabowicz, J.; Kłos, M.; Wieczorek, W.M.; Szyrej, M.; Sieroń, L.; Kiełbasiński, P. Enzyme-promoted Desymmetrization of Bis(2-hydroxymethylphenyl)sulfoxide as a Route to Tridentate Chiral Catalysts. Tetrahedron Asymmetry 2008, 19, 2096–2101. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Sznajder, E.; Kiełbasiński, P. New Highly Efficient Aziridine-functionalized Tridentate Sulfinyl Catalysts for Enantioselective Diethylzinc Addition to Carbonyl Compounds. Tetrahedron Asymmetry 2009, 20, 2311–2314. [Google Scholar] [CrossRef]
- Kiełbasiński, P.; Kwiatkowska, M.; Cierpiał, T.; Rachwalski, M.; Leśniak, S. The Sulfinyl Group: Its Importance for Asymmetric Synthesis and Biological Activity. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 649–653. [Google Scholar] [CrossRef]
- Kaczmarczyk, S.; Madalińska, L.; Kiełbasiński, P. Unexpected Racemization of 2-Hydroxymethylphenylphosphine Oxides. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 249–253. [Google Scholar] [CrossRef]
- Madalińska, L.; Kwiatkowska, M.; Kaczmarczyk, S.; Kiełbasiński, P.; Division of Organic Chemistry, Centre of Molecular and Macromolecular Studies, Polish Academy of Sciences, Sienkiewicza, Poland. 2022; (unpublished results).
- Kolodiazhna, A.O.; Kolodiazhnyi, O.I. Asymmetric Electrophilic Reactions in Phosphorus Chemistry. Symmetry 2020, 12, 108. [Google Scholar] [CrossRef] [Green Version]







| Entry | Diol | R | Enzyme (a) | Solvent | Time | Monoacetate | |||
|---|---|---|---|---|---|---|---|---|---|
| [Days] | Symbol | Yield [%] | [α]D (b) | Ee | |||||
| [%] (c) | |||||||||
| 1 | 12 | Me | CAL-B | CH2Cl2 | 8 | 14 | 53 | −5.40 | 40 |
| 2 | 12 | Me | CAL-B | i-Pr2O | 1 | 14 | 52 | −7.10 | 53 |
| 3 | 12 | Me | CAL-B | TBME | 1 | 14 | 49 | −8.70 | 64 |
| 4 | 12 | Me | CAL-B | toluene | 11 | 14 | 25 | −9.85 | 73 |
| 5 | 13 | Ph | CAL-B | TBME + pyridine | 21 | 15 | 10 | 4.49 | 98 |
| 6 | 13 | Ph | CAL-B | i-Pr2O + toluene + pyridine | 23 | 15 | 10 | 4.42 | 95 |
| 7 | 13 | Ph | CAL-B | acetone | 14 | 15 | 52 | 2.74 | 60 |
| 8 | 13 | Ph | CAL-B | toluene + pyridine | 15 | 15 | 38 | 2.74 | 60 |
| 9 | 13 | Ph | CAL-B | toluene | 6 | 15 | 27 | 2.28 | 50 |
| 10 | 13 | Ph | CAL-B | acetonitrile | 22 | 15 | 26 | 1.26 | 28 |
| 11 | 13 | Ph | CAL-B | CHCl3 | 12 | 15 | 16 | −1.50 | 33 |
| 12 | 13 | Ph | CAL-B | CH2Cl2 | 29 | 15 | 5 | −1.40 | 31 |
| 13 | 13 | Ph | PFL | CHCl3 | 12 | 15 | 6 | −2.26 | 49 |
| 14 | 13 | Ph | LPL | toluene | 28 | 15 | <5 | - | - |
| 15 | 13 | Ph | CR | toluene | 33 | 15 | 11 | 2.54 | 56 |
| 16 | 13 | Ph | PS | CHCl3 | 29 | 15 | 10 | - | - |
| 17 | 13 | Ph | - | Toluene | 24 | - | - | - | - |
| Entry | Diol | R | Enzyme (a) | Solvent + Pyridine | Time | Monoacetate | |||
|---|---|---|---|---|---|---|---|---|---|
| [Days] | Symbol | Yield | [α]D (c) | Ee | |||||
| [%] | [%] (d) | ||||||||
| 1 | 22 | Me | CAL-B | TBME (b) | 4 | 24 | 60 | 4.7 | 72 (e) |
| 2 | 22 | Me | CAL-B | toluene | 4 | 24 | 80 | 3.25 | 50 (e) |
| 3 | 22 | Me | CAL-B | acetone | 4 | 24 | 70 | 1.65 | 25 (e) |
| 4 | 22 | Me | CAL-B | CH2Cl2 | 8 | 24 | 49 | 0 | 0 |
| 5 | 22 | Me | CR | TBME | 11 | 24 | 55 | 0 | 0 |
| 6 | 22 | Me | PFL | TBME | 11 | 24 | 60 | 0 | 0 |
| 7 | 23 | Ph | PFL | TBME | 38 | 25 | 60 | −7.57 | 77 |
| 8 | 23 | Ph | PFL | Et2O | 38 | 25 | 55 | −4.44 | 45 |
| 9 | 23 | Ph | PFL | toluene | 38 | 25 | 66 | −1.56 | 16 |
| 10 | 23 | Ph | PFL | CH2Cl2 | 38 | 25 | 44 | −1.62 | 16 |
| 11 | 23 | Ph | CAL-B | Et2O | 38 | 25 | 44 | 3.25 | 54 |
| 12 | 23 | Ph | CAL-B | i-Pr2O | 38 | 25 | 37 | 1.1 | 0 |
| 13 | 23 | Ph | CAL-B | acetone | 38 | 25 | 14 | 2.25 | 23 |
| 14 | 23 | Ph | CAL-B | toluene | 26 | only substrate | |||
| 15 | 23 | Ph | CAL-B | TBME | 16 | 25 | 10 | 2.2 | 23 |
| 16 | 23 | Ph | CR | TBME | 38 | 25 | 23 | 1.8 | 18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madalińska, L.; Kiełbasiński, P.; Kwiatkowska, M. Enzymatic Desymmetrisation of Prochiral Phosphines and Phosphine P-Sulfides as a Route to P-Chiral Catalysts. Catalysts 2022, 12, 171. https://doi.org/10.3390/catal12020171
Madalińska L, Kiełbasiński P, Kwiatkowska M. Enzymatic Desymmetrisation of Prochiral Phosphines and Phosphine P-Sulfides as a Route to P-Chiral Catalysts. Catalysts. 2022; 12(2):171. https://doi.org/10.3390/catal12020171
Chicago/Turabian StyleMadalińska, Lidia, Piotr Kiełbasiński, and Małgorzata Kwiatkowska. 2022. "Enzymatic Desymmetrisation of Prochiral Phosphines and Phosphine P-Sulfides as a Route to P-Chiral Catalysts" Catalysts 12, no. 2: 171. https://doi.org/10.3390/catal12020171