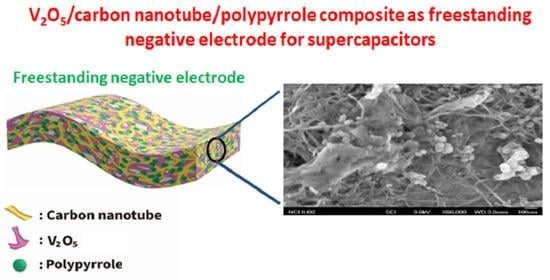
V2O5/Carbon Nanotube/Polypyrrole Based Freestanding Negative Electrodes for High-Performance Supercapacitors
Abstract
:1. Introduction
2. Results
2.1. Preparation of the V2O5/f-CNT/PPy Composite Film
2.2. Characterization of the V2O5/f-CNT/PPy Composite Films
2.3. Electrochemical Properties of the Freestanding Negative Electrodes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Vanadium Pentoxide Gel
3.3. Preparation of V2O5/f-CNTs Composite Film
3.4. Preparation of V2O5/f-CNT/PPy Composite Film
3.5. Characterizations
3.6. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Li, L.; Yan, J.M.; Zhang, X.B. Materials design and system construction for conventional and new-concept supercapacitors. Adv. Sci. 2017, 4, 1600382. [Google Scholar] [CrossRef]
- Cao, A.; Chen, Z.; Wang, Y.; Zhang, J.; Wang, Y.; Li, T.; Han, Y. Redox-active doped polypyrrole microspheres induced by phosphomolybdic acid as supercapacitor electrode materials. Synth. Met. 2019, 252, 135–141. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, C.; Liang, J.; Wu, W. Electrode materials and device architecture strategies for flexible supercapacitors in wearable energy storage. J. Mater. Chem. A 2021, 9, 8099–8128. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Lee, R.H. Facile, scalable, eco-friendly fabrication of high-performance flexible all-solid-state supercapacitors. Polymers 2018, 10, 1247. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhu, L.; Bai, Z.; Liang, G.; Liu, L.; Fang, D.; Xu, W. Conductive polypyrrole–bacterial cellulose nanocomposite membranes as flexible supercapacitor electrode. Org. Electron. 2013, 14, 3331–3338. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Li, B.; Du, W.; Huang, Q.; Zheng, M.; Xue, H.; Pang, H. Facile synthesis of polypyrrole nanowires for high-performance supercapacitor electrode materials. Prog. Nat. Sci. Mater. Int. 2016, 26, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Jyothibasu, J.P.; Wang, R.-H.; Ong, K.; Ong, J.H.L.; Lee, R.-H. Cellulose/carbon nanotube/MnO2 composite electrodes with high mass loadings for symmetric supercapacitors. Cellulose 2021, 28, 3549–3567. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Kuo, D.W.; Lee, R.H. Flexible and freestanding electrodes based on polypyrrole/carbon nanotube/cellulose composites for supercapacitor application. Cellulose 2019, 26, 4495–4513. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Vignesh, K.; Pillai, S. Electrode materials for supercapacitors: A review of recent advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- An, H.; Wang, Y.; Wang, X.; Zheng, L.; Wang, X.; Yi, L.; Bai, L.; Zhang, X. Polypyrrole/carbon aerogel composite materials for supercapacitor. J. Power Sources 2010, 195, 6964–6969. [Google Scholar] [CrossRef]
- Li, Z.; Cai, J.; Cizek, P.; Niu, H.; Du, Y.; Lin, T. A self-supported, flexible, binder-free pseudo-supercapacitor electrode material with high capacitance and cycling stability from hollow, capsular polypyrrole fibers. J. Mater. Chem. A 2015, 3, 16162–16167. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Liu, T.Y.; Xu, X.X.; Feng, D.Y.; Li, Y.; Liu, X.X. Pushing the cycling stability limit of polypyrrole for supercapacitors. Adv. Funct. Mater. 2015, 25, 4626–4632. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Liu, W.; Hou, Q.; Ni, Y. Lignocellulose-derived hydrogel/aerogel-based flexible quasi-solid-state supercapacitors with high-performance: A review. J. Mater. Chem. A 2021, 9, 14233–14264. [Google Scholar] [CrossRef]
- Khan, J.H.; Lin, J.; Young, C.; Matsagar, B.M.; Wu, K.C.W.; Dhepe, P.L.; Islam, M.T.; Rahman, M.; Shrestha, L.K.; Alshehri, S.M.; et al. High surface area nanoporous carbon derived from high quality jute from bangladesh. Mater. Chem. Phys. 2018, 216, 491–495. [Google Scholar] [CrossRef]
- Khan, J.H.; Marpaung, F.; Young, C.; Lin, J.; Islam, M.T.; Alsheri, S.M.; Ahamad, T.; Alhokbany, N.; Ariga, K.; Shrestha, L.K.; et al. Jute-derived microporous/mesoporous carbon with ultra-high surface area using a chemical activation process. Microporous Mesoporous Mater. 2019, 274, 251–256. [Google Scholar] [CrossRef] [Green Version]
- El-Mahdy, A.F.; Young, C.; Kim, J.; You, J.; Yamauchi, Y.; Kuo, S.W. Hollow microspherical and microtubular [3+3] carbazole-based covalent organic frameworks and their gas and energy storage applications. ACS Appl. Mater. Interfaces 2019, 11, 9343–9354. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, X.; Lu, W.; Yan, Y.; Zhu, J.; Chou, T.-W. MnO2 based sandwich structure electrode for supercapacitor with large voltage window and high mass loading. Chem. Eng. J. 2019, 368, 525–532. [Google Scholar] [CrossRef]
- Lao, Z.J.; Konstantinov, K.; Tournaire, Y.; Ng, S.H.; Wang, G.X.; Liu, H.K. Synthesis of vanadium pentoxide powders with enhanced surface-area for electrochemical capacitors. J. Power Sources 2006, 162, 1451–1454. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, X.; Wang, Y.; Li, C.; Wu, X. Fe2O3 nanoparticles decorated on graphene-carbon nanotubes conductive networks for boosting the energy density of all-solid-state asymmetric supercapacitor. ACS Sustain. Chem. Eng. 2019, 7, 9211–9219. [Google Scholar] [CrossRef]
- Meher, S.K.; Rao, G.R. Ultralayered Co3O4 for high-performance supercapacitor applications. J. Phys. Chem. C 2011, 115, 15646–15654. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Synthesis and electrochemical characterization of amorphous MnO2 electrochemical capacitor electrode material. J. Power Sources 2004, 132, 315–320. [Google Scholar] [CrossRef]
- Yao, D.; Ouyang, Y.; Jiao, X.; Ye, H.; Lei, W.; Xia, X.; Lu, L.; Hao, Q. Hierarchical NiO@NiCo2O4 core–shell nanosheet arrays on Ni foam for high-performance electrochemical supercapacitors. Ind. Eng. Chem. Res. 2018, 57, 6246–6256. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, G. Synthesis and enhanced intercalation properties of nanostructured vanadium oxides. Chem. Mater. 2006, 18, 2787–2804. [Google Scholar] [CrossRef]
- Ghosh, M.; Vijayakumar, V.; Soni, R.; Kurungot, S. A rationally designed self-standing V2O5 electrode for high voltage non-aqueous all-solid-state symmetric (2.0 V) and asymmetric (2.8 V) supercapacitors. Nanoscale 2018, 10, 8741–8751. [Google Scholar] [CrossRef]
- Chernova, N.A.; Roppolo, M.; Dillon, A.C.; Whittingham, M.S. Layered vanadium and molybdenum oxides: Batteries and electrochromics. J. Mater. Chem. 2009, 19, 2526–2552. [Google Scholar] [CrossRef]
- Velmurugan, R.; Premkumar, J.; Pitchai, R.; Ulaganathan, M.; Subramanian, B. Robust, flexible, and binder Free highly Crystalline V2O5 thin film electrodes and their superior supercapacitor performances. ACS Sustain. Chem. Eng. 2019, 7, 13115–13126. [Google Scholar] [CrossRef]
- Zhang, H.; Han, X.; Gan, R.; Guo, Z.; Ni, Y.; Zhang, L. A facile biotemplate-assisted synthesis of mesoporous V2O5 microtubules for high performance asymmetric supercapacitors. Appl. Surf. Sci. 2020, 511, 145527. [Google Scholar] [CrossRef]
- Panigrahi, K.; Howli, P.; Chattopadhyay, K.K. 3D network of V2O5 for flexible symmetric supercapacitor. Electrochim. Acta 2020, 337, 135701. [Google Scholar] [CrossRef]
- Kim, I.-H.; Kim, J.-H.; Cho, B.-W.; Lee, Y.-H.; Kim, K.-B. Synthesis and electrochemical characterization of vanadium oxide on carbon nanotube film substrate for pseudocapacitor applications. J. Electrochem. Soc. 2006, 153, A989. [Google Scholar] [CrossRef]
- Perera, S.D.; Patel, B.; Nijem, N.; Roodenko, K.; Seitz, O.; Ferraris, J.P.; Chabal, Y.J.; Balkus, K.J., Jr. Vanadium oxide nanowire–carbon nanotube binder-free flexible electrodes for supercapacitors. Adv. Energy Mater. 2011, 1, 936–945. [Google Scholar] [CrossRef]
- Mtz-Enriquez, A.I.; Gomez-Solis, C.; Oliva, A.I.; Zakhidov, A.; Martinez, P.M.; Garcia, C.R.; Herrera-Ramirez, A.; Oliva, J. Enhancing the voltage and discharge times of graphene supercapacitors depositing a CNT/V2O5 layer on their electrodes. Mater. Chem. Phys. 2020, 244, 122698. [Google Scholar] [CrossRef]
- Korkmaz, S.; Tezel, F.M.; Kariper, İ.A. Synthesis and characterization of GO/V2O5 thin film supercapacitor. Synth. Met. 2018, 242, 37–48. [Google Scholar] [CrossRef]
- Zheng, P.; Lv, X.; Shi, S.; Liu, Y.; Yang, L.; Ge, D. High-efficiency supercapacitors based on V2O5/rGONR network from hierarchical nanoribbon assemblies. J. Alloys Compd. 2019, 792, 468–473. [Google Scholar] [CrossRef]
- Bonso, J.S.; Rahy, A.; Perera, S.D.; Nour, N.; Seitz, O.; Chabal, Y.J.; Balkus, K.J., Jr.; Ferraris, J.P.; Yang, D.J. Exfoliated graphite nanoplatelets–V2O5 nanotube composite electrodes for supercapacitors. J. Power Sources 2012, 203, 227–232. [Google Scholar] [CrossRef]
- Choudhury, A.; Bonso, J.S.; Wunch, M.; Yang, K.S.; Ferraris, J.P.; Yang, D.J. In-situ synthesis of vanadium pentoxide nanofibre/exfoliated graphene nanohybrid and its supercapacitor applications. J. Power Sources 2015, 287, 283–290. [Google Scholar] [CrossRef]
- Lee, S.-M.; Park, Y.-J.; Lam, D.V.; Kim, J.-H.; Lee, K. Effects of annealing on electrochemical performance in graphene/V2O5 supercapacitor. Appl. Surf. Sci. 2020, 512, 145626. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, W.; Long, D.; Zhu, J.; Pezzotti, G. Porous V2O5 nanorods/reduced graphene oxide composites for high performance symmetric supercapacitors. Appl. Surf. Sci. 2019, 478, 383–392. [Google Scholar] [CrossRef]
- Sathiya, M.; Prakash, A.S.; Ramesha, K.; Tarascon, J.M.; Shukla, A.K. V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc. 2011, 133, 16291–16299. [Google Scholar] [CrossRef] [PubMed]
- Pande, S.A.; Pandit, B.; Sankapal, B.R. Vanadium oxide anchored MWCNTs nanostructure for superior symmetric electrochemical supercapacitors. Mater. Des. 2019, 182, 107972. [Google Scholar] [CrossRef]
- Chen, Z.; Augustyn, V.; Wen, J.; Zhang, Y.; Shen, M.; Dunn, B.; Lu, Y. High-Performance supercapacitors based on intertwined CNT/V2O5 nanowire nanocomposites. Adv. Mater. 2011, 23, 791–795. [Google Scholar] [CrossRef]
- Jiang, H.; Cai, X.; Qian, Y.; Zhang, C.; Zhou, L.; Liu, W.; Li, B.; Lai, L.; Huang, W. V2O5 embedded in vertically aligned carbon nanotube arrays as free-standing electrodes for flexible supercapacitors. J. Mater. Chem. A 2017, 5, 23727–23736. [Google Scholar] [CrossRef]
- Guo, C.X.; Yilmaz, G.; Chen, S.; Chen, S.; Lu, X. Hierarchical nanocomposite composed of layered V2O5/PEDOT/MnO2 nanosheets for high-performance asymmetric supercapacitors. Nano Energy 2015, 12, 76–87. [Google Scholar] [CrossRef]
- Qian, T.; Xu, N.; Zhou, J.; Yang, T.; Liu, X.; Shen, X.; Liang, J.; Yan, C. Interconnected three-dimensional V2O5/polypyrrole network nanostructures for high performance solid-state supercapacitors. J. Mater. Chem. A 2015, 3, 488–493. [Google Scholar] [CrossRef]
- Huguenin, F.; Ferreira, M.; Zucolotto, V.; Nart, F.C.; Torresi, R.M.; Oliveira, O.N. Molecular-level manipulation of V2O5/polyaniline layer-by-layer films to control electrochromogenic and electrochemical properties. Chem. Mater. 2004, 16, 2293–2299. [Google Scholar] [CrossRef]
- Bi, W.; Wu, Y.; Liu, C.; Wang, J.; Du, Y.; Gao, G.; Wu, G.; Cao, G. Gradient oxygen vacancies in V2O5/PEDOT nanocables for high-performance supercapacitors. ACS Appl. Energy Mater. 2019, 2, 668–677. [Google Scholar] [CrossRef]
- Bi, W.; Jahrman, E.; Seidler, G.; Wang, J.; Gao, G.; Wu, G.; Atif, M.; AlSalhi, M.; Cao, G. Tailoring energy and power density through controlling the concentration of oxygen vacancies in V2O5/PEDOT Nanocable-Based Supercapacitors. ACS Appl. Mater. Interfaces 2019, 11, 16647–16655. [Google Scholar] [CrossRef]
- Bi, W.; Huang, J.; Wang, M.; Jahrman, E.P.; Seidler, G.T.; Wang, J.; Wu, Y.; Gao, G.; Wu, G.; Cao, G. V2O5–Conductive polymer nanocables with built-in local electric field derived from interfacial oxygen vacancies for high energy density supercapacitors. J. Mater. Chem. A 2019, 7, 17966–17973. [Google Scholar] [CrossRef]
- Kónya, Z.; Vesselényi, I.; Kiss, J.; Farkas, A.; Oszkó, A.; Kiricsi, I. XPS study of multiwall carbon nanotube synthesis on Ni-, V-, and Ni, V-ZSM-5 catalysts. Appl. Catal. A Gen. 2004, 260, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Clark, M.; Zhang, Q.; Yu, D.; Liu, D.; Liu, J.; Cao, G. V2O5 nano-electrodes with high power and energy densities for thin film Li-ion batteries. Adv. Energy Mater. 2011, 1, 194–202. [Google Scholar] [CrossRef]
- Alonso, B.; Livage, J. Synthesis of vanadium oxide gels from peroxovanadic acid solutions: A 51V NMR study. J. Solid State Chem. 1999, 148, 16–19. [Google Scholar] [CrossRef]
- Roy, A.; Ray, A.; Sadhukhan, P.; Saha, S.; Das, S. Morphological behaviour, electronic bond formation and electrochemical performance study of V2O5-polyaniline composite and its application in asymmetric supercapacitor. Mater. Res. Bull. 2018, 107, 379–390. [Google Scholar] [CrossRef]
- Mjejri, I.; Gaudon, M.; Song, G.; Labrugère, C.; Rougier, A. Crystallized V2O5 as oxidized phase for unexpected multicolor electrochromism in V2O3 thick film. ACS Appl. Energy Mater. 2018, 1, 2721–2729. [Google Scholar] [CrossRef] [Green Version]
- Jyothibasu, J.P.; Lee, R.H. Green synthesis of polypyrrole tubes using curcumin template for excellent electrochemical performance in supercapacitors. J. Mater. Chem. A 2020, 8, 3186–3202. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.F.; Liu, Y.; Zhang, H.; Ren, Y.; Sun, C.J.; Lu, W.; Zhou, Y.; Stanciu, L.; Stach, E.A.; et al. Graphene-modified nanostructured vanadium pentoxide hybrids with extraordinary electrochemical performance for Li-ion batteries. Nat. Commun. 2015, 6, 6127. [Google Scholar] [CrossRef]
- You, M.; Zhang, W.; Yan, X.; Jiang, H.; Miao, J.; Li, Y.; Zhou, W.; Zhu, Y.; Cheng, X. V2O5 nanosheets assembled on 3D carbon fiber felt as a free-standing electrode for flexible asymmetric supercapacitor with remarkable energy density. Ceram. Int. 2021, 47, 3337–3345. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Wang, Z.Y.; Yang, L.X.; Yang, Z.G. Nitrogen-doped reduced graphene oxide intertwined with V2O3 nanoflakes as self-supported electrodes for flexible all-solid-state supercapacitors. RSC Adv. 2017, 7, 25732–25739. [Google Scholar] [CrossRef] [Green Version]
- Jyothibasu, J.P.; Chen, M.Z.; Lee, R.H. Polypyrrole/carbon nanotube freestanding electrode with excellent electrochemical properties for high-performance all-solid-state supercapacitors. ACS Omega 2020, 5, 6441–6451. [Google Scholar] [CrossRef] [Green Version]
- Saravanakumar, B.; Purushothaman, K.K.; Muralidharan, G. Interconnected V2O5 nanoporous network for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2012, 4, 4484–4490. [Google Scholar] [CrossRef]
- Wang, X.; Zuo, C.; Jia, L.; Liu, Q.; Guo, X.; Jing, X.; Wang, J. Synthesis of sandwich-like vanadium pentoxide/carbon nanotubes composites for high performance supercapacitor electrodes. J. Alloys Compd. 2017, 708, 134–140. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jyothibasu, J.P.; Chen, M.-Z.; Tien, Y.-C.; Kuo, C.-C.; Chen, E.-C.; Lin, Y.-C.; Chiang, T.-C.; Lee, R.-H. V2O5/Carbon Nanotube/Polypyrrole Based Freestanding Negative Electrodes for High-Performance Supercapacitors. Catalysts 2021, 11, 980. https://doi.org/10.3390/catal11080980
Jyothibasu JP, Chen M-Z, Tien Y-C, Kuo C-C, Chen E-C, Lin Y-C, Chiang T-C, Lee R-H. V2O5/Carbon Nanotube/Polypyrrole Based Freestanding Negative Electrodes for High-Performance Supercapacitors. Catalysts. 2021; 11(8):980. https://doi.org/10.3390/catal11080980
Chicago/Turabian StyleJyothibasu, Jincy Parayangattil, Ming-Zhu Chen, You-Ching Tien, Chi-Ching Kuo, Erh-Chiang Chen, Yi-Chun Lin, Tai-Chin Chiang, and Rong-Ho Lee. 2021. "V2O5/Carbon Nanotube/Polypyrrole Based Freestanding Negative Electrodes for High-Performance Supercapacitors" Catalysts 11, no. 8: 980. https://doi.org/10.3390/catal11080980