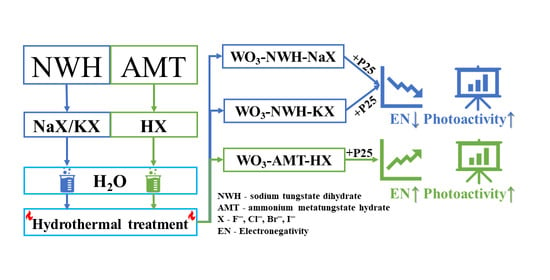
Synthesis Design of Electronegativity Dependent WO3 and WO3∙0.33H2O Materials for a Better Understanding of TiO2/WO3 Composites’ Photocatalytic Activity
Abstract
:1. Introduction
2. Results
2.1. The Morphology of the Semiconductors
2.2. Optical Properties of the WO3 Semiconductors and TiO2/WO3 Composite Systems
2.3. Crystal Phase and Crystallite Mean Size Determination
2.4. Raman Spectroscopy
2.5. X-ray Photoelectron Spectroscopy
2.6. Photocatalytic Activity and Kinetic Study
3. Correlations between Structural and Optical Parameters between Structural, Optical, Electronegativity, and the Assessed Photocatalytic Performance
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of WO3 and WO3∙0.33H2O Semiconductors
4.3. Preparation of WO3-TiO2 Semiconductor Nanocomposites
4.4. Methods and Instrumentation, Kinetic Study and Assessment of Photocatalytic Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw Hill: New York, NY, USA, 2003; Volume 40, ISBN 0070494398. [Google Scholar]
- Urasinska-Wojcik, B.; Vincent, T.A.; Chowdhury, M.; Gardner, J.W. Ultrasensitive WO3 gas sensors for NO2 detection in air and low oxygen environment. Sens. Actuators B Chem. 2017, 239, 1051–1059. [Google Scholar] [CrossRef]
- Dong, C.; Zhao, R.; Yao, L.; Ran, Y.; Zhang, X.; Wang, Y. A review on WO3 based gas sensors: Morphology control and enhanced sensing properties. J. Alloy. Compd. 2020, 820, 153194. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, X.; Li, C.; Men, G.; Han, D.; Gu, F. Humidity-Sensing Performance of 3DOM WO3 with Controllable Structural Modification. ACS Appl. Mater. Interfaces 2018, 10, 3776–3783. [Google Scholar] [CrossRef]
- Bogati, S.; Georg, A.; Graf, W. Photoelectrochromic devices based on sputtered WO3 and TiO2 films. Sol. Energy Mater. Sol. Cells 2017, 163, 170–177. [Google Scholar] [CrossRef]
- Bae, J.; Kim, H.; Moon, H.C.; Kim, S.H. Low-voltage, simple WO3-based electrochromic devices by directly incorporating an anodic species into the electrolyte. J. Mater. Chem. C 2016, 4, 10887–10892. [Google Scholar] [CrossRef]
- Cossari, P.; Pugliese, M.; Simari, C.; Mezzi, A.; Maiorano, V.; Nicotera, I.; Gigli, G. Simplified All-Solid-State WO3 Based Electrochromic Devices on Single Substrate: Toward Large Area, Low Voltage, High Contrast, and Fast Switching Dynamics. Adv. Mater. Interfaces 2020, 7. [Google Scholar] [CrossRef]
- Yun, T.Y.; Li, X.; Bae, J.; Kim, S.H.; Moon, H.C. Non-volatile, Li-doped ion gel electrolytes for flexible WO3-based electrochromic devices. Mater. Des. 2019, 162, 45–51. [Google Scholar] [CrossRef]
- Bazarjani, M.S.; Hojamberdiev, M.; Morita, K.; Zhu, G.; Cherkashinin, G.; Fasel, C.; Herrmann, T.; Breitzke, H.; Gurlo, A.; Riedel, R. Visible Light Photocatalysis with c-WO3–x/WO3×H2O Nanoheterostructures In Situ Formed in Mesoporous Polycarbosilane-Siloxane Polymer. J. Am. Chem. Soc. 2013, 135, 4467–4475. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, F.; Lin, H.; Xie, Y.; Tong, N.; Lin, J.; Zhang, X.; Zhang, Z.; Wang, X. Surface oxygen vacancy and defect engineering of WO3 for improved visible light photocatalytic performance. Catal. Sci. Technol. 2018, 8, 4399–4406. [Google Scholar] [CrossRef]
- Shang, J.; Xiao, Z.; Yu, L.; Aprea, P.; Hao, S. An insight on the role of PVP in the synthesis of monoclinic WO3 with efficiently photocatalytic activity. Nanotechnology 2019, 31, 125603. [Google Scholar] [CrossRef]
- Baia, L.; Orbán, E.; Fodor, S.; Hampel, B.; Kedves, E.Z.; Saszet, K.; Székely, I.; Karácsonyi, É.; Réti, B.; Berki, P.; et al. Preparation of TiO2/WO3 composite photocatalysts by the adjustment of the semiconductors’ surface charge. Mater. Sci. Semicond. Process. 2016, 42, 66–71. [Google Scholar] [CrossRef]
- Kokorin, A.I.; Sviridova, T.V.; Konstantinova, E.A.; Sviridov, D.V.; Bahnemann, D.W. Dynamics of Photogenerated Charge Carriers in TiO2/MoO3, TiO2/WO3 and TiO2/V2O5 Photocatalysts with Mosaic Structure. Catalysts 2020, 10, 1022. [Google Scholar] [CrossRef]
- Fernández-Domene, R.; Sánchez-Tovar, R.; Lucas-Granados, B.; Roselló-Márquez, G.; Garcia-Anton, J. A simple method to fabricate high-performance nanostructured WO3 photocatalysts with adjusted morphology in the presence of complexing agents. Mater. Des. 2017, 116, 160–170. [Google Scholar] [CrossRef]
- Chen, P.; Qin, M.; Chen, Z.; Jia, B.; Zhao, S.; Wan, Q.; Qu, X. A novel approach to synthesize the amorphous carbon-coated WO3 with defects and excellent photocatalytic properties. Mater. Des. 2016, 106, 22–29. [Google Scholar] [CrossRef]
- Wu, C.-M.; Naseem, S.; Chou, M.-H.; Wang, J.-H.; Jian, Y.-Q. Recent Advances in Tungsten-Oxide-Based Materials and Their Applications. Front. Mater. 2019, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Hu, S.; Jiang, W.; Zhang, J.; Xu, K.; Wang, Z. In situ construction of WO3 nanoparticles decorated Bi2MoO6 microspheres for boosting photocatalytic degradation of refractory pollutants. J. Colloid Interface Sci. 2019, 556, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhao, G.; Du, W.; Tian, Q. Synthesis and excellent acetone sensing properties of porous WO3 nanofibers. Vacuum 2016, 124, 32–39. [Google Scholar] [CrossRef]
- Jeong, K.; Lee, J.; Byun, I.; Seong, M.-J.; Park, J.; Nam, H.-S.; Lee, J. Pulsed laser chemical vapor deposition of a mixture of W, WO2, and WO3 from W(CO)6 at atmospheric pressure. Thin Solid Films 2017, 626, 145–153. [Google Scholar] [CrossRef]
- Ito, A.; Morishita, Y. Selective self-oriented growth of (2 0 0), (0 0 2), and (0 2 0) δ-WO3 films via metal-organic chemical vapor deposition. Mater. Lett. 2020, 258, 126817. [Google Scholar] [CrossRef]
- Verma, M.; Singh, K.P.; Kumar, A. Reactive magnetron sputtering based synthesis of WO3 nanoparticles and their use for the photocatalytic degradation of dyes. Solid State Sci. 2020, 99, 105847. [Google Scholar] [CrossRef]
- Fan, G.; Chen, D.; Li, T.; Yi, S.; Ji, H.; Wang, Y.; Zhang, Z.; Shao, G.; Fan, B.; Wang, H.; et al. Enhanced room-temperature ammonia-sensing properties of polyaniline-modified WO3 nanoplates derived via ultrasonic spray process. Sens. Actuators B Chem. 2020, 312, 127892. [Google Scholar] [CrossRef]
- Govender, M.; Shikwambana, L.; Mwakikunga, B.W.; Sideras-Haddad, E.; Erasmus, R.M.; Forbes, A. Formation of tungsten oxide nanostructures by laser pyrolysis: Stars, fibres and spheres. Nanoscale Res. Lett. 2011, 6, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.-M.; Nah, Y.-C.; Nam, C. Effects of Hydrothermal Treatment Duration on Morphology of WO3 Nanostructures. J. Nanosci. Nanotechnol. 2017, 17, 7719–7722. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Y.; Yao, Q.; Wang, W.; Wang, H.; Wang, D.; Liu, X.; Xu, J. Facile synthesis of WO3 nanocuboids from tungsten trioxide powder and hydrogen peroxide. Mater. Lett. 2019, 236, 197–200. [Google Scholar] [CrossRef]
- Li, N.; Chang, T.; Gao, H.; Gao, X.; Ge, L. Morphology-controlled WO3-x homojunction: Hydrothermal synthesis, adsorption properties, and visible-light-driven photocatalytic and chromic properties. Nanotechnology 2019, 30, 415601. [Google Scholar] [CrossRef]
- Liu, D.; Ren, X.; Li, Y.; Tang, Z.; Zhang, Z. Nanowires-assembled WO3 nanomesh for fast detection of ppb-level NO2 at low temperature. J. Adv. Ceram. 2020, 9, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Zeb, S.; Peng, X.; Yuan, G.; Zhao, X.; Qin, C.; Sun, G.; Nie, Y.; Cui, Y.; Jiang, X. Controllable synthesis of ultrathin WO3 nanotubes and nanowires with excellent gas sensing performance. Sens. Actuators B Chem. 2020, 305, 127435. [Google Scholar] [CrossRef]
- Lu, N.; Gao, X.; Yang, C.; Xiao, F.; Wang, J.; Su, X. Enhanced formic acid gas-sensing property of WO3 nanorod bundles via hydrothermal method. Sens. Actuators B Chem. 2016, 223, 743–749. [Google Scholar] [CrossRef]
- Ryu, S.-M.; Nam, C. Adsorption Characteristics of Methylene Blue on WO3 Nanorods Prepared by Microwave-Assisted Hydrothermal Methods. Phys. Status Solidi A 2018, 215, 1700996. [Google Scholar] [CrossRef]
- Cao, S.; Zhao, C.; Han, T.; Peng, L. Hydrothermal synthesis, characterization and gas sensing properties of the WO3 nanofibers. Mater. Lett. 2016, 169, 17–20. [Google Scholar] [CrossRef]
- Yao, S.; Zheng, X.; Zhang, X.; Xiao, H.; Qu, F.; Wu, X. Facile synthesis of flexible WO3 nanofibers as supercapacitor electrodes. Mater. Lett. 2017, 186, 94–97. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, L.; Ren, Z.; Yang, Y.; Xiao, Y.; Li, Z. Nanostructured WO3 films synthesized on mica substrate with novel photochromic properties. J. Alloy. Compd. 2016, 657, 450–456. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, S.; Chen, Y.; Gaskov, A. Facile morphology control of WO3 nanostructure arrays with enhanced photoelectrochemical performance. Appl. Surf. Sci. 2017, 403, 274–281. [Google Scholar] [CrossRef]
- Adhikari, S.; Chandra, K.S.; Kim, D.-H.; Madras, G.; Sarkar, D. Understanding the morphological effects of WO3 photocatalysts for the degradation of organic pollutants. Adv. Powder Technol. 2018, 29, 1591–1600. [Google Scholar] [CrossRef]
- Ghasemi, L.; Jafari, H. Morphological Characterization of Tungsten Trioxide Nanopowders Synthesized by Sol-Gel Modified Pechini’s Method. Mater. Res. 2017, 20, 1713–1721. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.B.; Nabi, G.; Khalid, N.R.; Khan, W.S. Synthesis of Nanostructured Based WO3 Materials for Photocatalytic Applications. J. Inorg. Organomet. Polym. Mater. 2017, 28, 777–782. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, Y.; Li, G.; Ma, Z.; Wang, X.; Cheng, Z.; Xu, J. Highly sensitive BTEX sensors based on hexagonal WO3 nanosheets. Sensors Actuators B: Chem. 2019, 293, 23–30. [Google Scholar] [CrossRef]
- Fan, Y.; Xi, X.; Liu, Y.; Nie, Z.; Zhang, Q.; Zhao, L. Growth mechanism of immobilized WO3 nanostructures in different solvents and their visible-light photocatalytic performance. J. Phys. Chem. Solids 2020, 140, 109380. [Google Scholar] [CrossRef]
- Wang, C.; Ding, M.; Kou, X.; Guo, L.; Feng, C.; Li, X.; Zhang, H.; Sun, P.; Sun, Y.; Lu, G. Detection of nitrogen dioxide down to ppb levels using flower-like tungsten oxide nanostructures under different annealing temperatures. J. Colloid Interface Sci. 2016, 483, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Pudukudy, M.; Jia, Q. Facile chemical synthesis of nanosheets self-assembled hierarchical H2WO4 microspheres and their morphology-controlled thermal decomposition into WO3 microspheres. J. Mater. Sci. 2019, 54, 13914–13937. [Google Scholar] [CrossRef]
- Kožakova, Z.; Mrlik, M.; Sedlačik, M.; Pavlinek, V.; Kuritka, I. Preparation of TiO2 powder by microwave-assisted molten-salt synthesis. In Proceedings of the NANOCON 2011, 3rd International Conference, Brno, Czech Republic, 21–23 September 2011; pp. 345–351. [Google Scholar]
- Putri, I.E.; Budiarti, H.A.; Sawitri, D.; Risanti, D.D. On the Role of NaCl Addition to Phase Transformation of TiO2 from TiCl3. Adv. Mater. Res. 2015, 1112, 313–316. [Google Scholar] [CrossRef]
- Yin, H.; Wada, Y.; Kitamura, T.; Sumida, T.; Hasegawa, Y.; Yanagida, S. Novel synthesis of phase-pure nano-particulate anatase and rutile TiO2 using TiCl4 aqueous solutions. J. Mater. Chem. 2001, 12, 378–383. [Google Scholar] [CrossRef]
- Kaur, M.; Kalia, A. Role of salt precursors for the synthesis of zinc oxide nanoparticles and in imparting variable antimicrobial activity. J. Appl. Nat. Sci. 2016, 8, 1039–1048. [Google Scholar] [CrossRef]
- Pourrahimi, A.M.; Liu, D.; Pallon, L.K.H.; Andersson, R.L.; Abad, A.M.; Lagarón, J.-M.; Hedenqvist, M.S.; Ström, V.; Gedde, U.W.; Olsson, R.T. Water-based synthesis and cleaning methods for high purity ZnO nanoparticles—Comparing acetate, chloride, sulphate and nitrate zinc salt precursors. RSC Adv. 2014, 4, 35568–35577. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.-Z.; Zhang, M.-L. Preparation and electrochemical properties of nickel oxide by molten-salt synthesis. Mater. Lett. 2007, 61, 3967–3969. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Ham, D.J.; Hong, S.J.; Thongtem, S.; Lee, J.S. Synthesis of hexagonal WO3nanowires by microwave-assisted hydrothermal method and their electrocatalytic activities for hydrogen evolution reaction. J. Mater. Chem. 2010, 20, 1683–1690. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Lam, S.; Zeng, Q.; Amal, R.; Yu, A. Effect of Cation Intercalation on the Growth of Hexagonal WO3Nanorods. J. Phys. Chem. C 2012, 116, 11722–11727. [Google Scholar] [CrossRef]
- Uresti, D.B.H.; Martínez, D.S.; la Cruz, A.M.-D.; Sepulveda-Guzman, S.; Torres-Martínez, L.M. Characterization and photocatalytic properties of hexagonal and monoclinic WO3 prepared via microwave-assisted hydrothermal synthesis. Ceram. Int. 2014, 40, 4767–4775. [Google Scholar] [CrossRef]
- Kang, M.; Liang, J.; Wang, F.; Chen, X.; Lu, Y.; Zhang, J. Structural design of hexagonal/monoclinic WO3 phase junction for photocatalytic degradation. Mater. Res. Bull. 2020, 121, 110614. [Google Scholar] [CrossRef]
- Wang, J.-C.; Shi, W.; Sun, X.-Q.; Wu, F.-Y.; Li, Y.; Hou, Y. Enhanced Photo-Assisted Acetone Gas Sensor and Efficient Photocatalytic Degradation Using Fe-Doped Hexagonal and Monoclinic WO3 Phase−Junction. Nanomaterials 2020, 10, 398. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Schoonen, M.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Ali, H.; Guler, A.; Masar, M.; Urbanek, P.; Urbanek, M.; Skoda, D.; Suly, P.; Machovsky, M.; Galusek, D.; Kuritka, I. Solid-State Synthesis of Direct Z-Scheme Cu2O/WO3 Nanocomposites with Enhanced Visible-Light Photocatalytic Performance. Catalysts 2021, 11, 293. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Yang, T.; Guo, X.; Wu, S.; Wang, S. Synthesis of Pt nanoparticles functionalized WO3 nanorods and their gas sensing properties. Sens. Actuators B Chem. 2011, 156, 918–923. [Google Scholar] [CrossRef]
- Xiang, Q.; Meng, G.F.; Zhao, H.B.; Zhang, Y.; Li, H.; Ma, W.J.; Xu, J.Q. Au Nanoparticle Modified WO3 Nanorods with Their Enhanced Properties for Photocatalysis and Gas Sensing. J. Phys. Chem. C 2010, 114, 2049–2055. [Google Scholar] [CrossRef]
- Pfeifer, J.; Guifang, C.; Tekula-Buxbaum, P.; Kiss, B.; Farkas-Jahnke, M.; Vadasdi, K. A reinvestigation of the preparation of tungsten oxide hydrate WO3, 1/3H2O. J. Solid State Chem. 1995, 119, 90–97. [Google Scholar] [CrossRef]
- Mattoni, G.; Filippetti, A.; Manca, N.; Zubko, P.; Caviglia, A.D. Charge doping and large lattice expansion in oxygen-deficient heteroepitaxial WO3. Phys. Rev. Mater. 2018, 2, 053402. [Google Scholar] [CrossRef] [Green Version]
- Lethy, K.; Beena, D.; Kumar, R.; Pillai, V.M.; Ganesan, V.; Sathe, V. Structural, optical and morphological studies on laser ablated nanostructured WO3 thin films. Appl. Surf. Sci. 2008, 254, 2369–2376. [Google Scholar] [CrossRef]
- Li, J.; Guo, H.; Feng, X. Nanorod-Assembled WO3·0.33H2O Microstructures with Improved Photocatalytic Property. Chin. J. Appl. Chem. 2017, 34, 60–70. [Google Scholar]
- Kumar, N.; Kumbhat, S. Concise Concepts of Nanoscience and Nanomaterials; Scientific Publishers: Singapore, 2018; ISBN 9789388172066. [Google Scholar]
- Lee, S.-H.; Cheong, H.M.; Liu, P.; Smith, D.; Tracy, C.; Mascarenhas, A.; Pitts, J.R.; Deb, S.K. Raman spectroscopic studies of gasochromic a-WO3 thin films. Electrochimica Acta 2001, 46, 1995–1999. [Google Scholar] [CrossRef]
- Singh, D.M.D.J.; Pradeep, T.; Thirumoorthy, K.; Balasubramanian, K. Closed-Cage Tungsten Oxide Clusters in the Gas Phase. J. Phys. Chem. A 2010, 114, 5445–5452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankova, N.; Atanasov, P.; Stanimirova, T.; Dikovska, A.O.; Eason, R. Thin (001) tungsten trioxide films grown by laser deposition. Appl. Surf. Sci. 2005, 247, 401–405. [Google Scholar] [CrossRef]
- De Wijs, G.; De Groot, R. Amorphous WO3: A first-principles approach. Electrochimica Acta 2001, 46, 1989–1993. [Google Scholar] [CrossRef]
- Yan, H.; Tian, C.; Sun, L.; Wang, B.; Wang, L.; Yin, J.; Wu, A.; Fu, H. Small-sized and high-dispersed WN from [SiO4(W3O9)4]4− clusters loading on GO-derived graphene as promising carriers for methanol electro-oxidation. Energy Environ. Sci. 2014, 7, 1939–1949. [Google Scholar] [CrossRef]
- Garcia-Sanchez, R.F.; Ahmido, T.; Casimir, D.; Baliga, S.; Misra, P. Thermal Effects Associated with the Raman Spectroscopy of WO3Gas-Sensor Materials. J. Phys. Chem. A 2013, 117, 13825–13831. [Google Scholar] [CrossRef] [PubMed]
- Tägtström, P.; Jansson, U. Chemical vapour deposition of epitaxial WO3 films. Thin Solid Films 1999, 352, 107–113. [Google Scholar] [CrossRef]
- Daniel, M.; Desbat, B.; Lassegues, J.; Gerand, B.; Figlarz, M. Infrared and Raman study of WO3 tungsten trioxides and WO3, xH2O tungsten trioxide tydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Selvakumar, D.; Nagaraju, P.; Jayavel, R. Graphene-metal oxide based nanocomposites for supercapacitor applications. TechConnect Briefs 2018, 1, 70–73. [Google Scholar]
- Cremonesi, A.; Bersani, D.; Lottici, P.P.; Djaoued, Y.; Ashrit, P. WO3 thin films by sol–gel for electrochromic applications. J. Non-Crystalline Solids 2004, 345-346, 500–504. [Google Scholar] [CrossRef]
- Wolcott, A.; Kuykendall, T.R.; Chen, W.; Chen, A.S.; Zhang, J.Z. Synthesis and Characterization of Ultrathin WO3Nanodisks Utilizing Long-Chain Poly(ethylene glycol). J. Phys. Chem. B 2006, 110, 25288–25296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, A.; Akgul, F.A.; Chen, Q.; Erat, S.; Huang, T.-W.; Jabeen, N.; Liu, Z.; Mun, B.S.; Mao, S.S.; Zhang, X. Observation of Substrate Orientation-Dependent Oxygen Defect Filling in Thin WO3−δ/TiO2 Pulsed Laser-Deposited Films with in Situ XPS at High Oxygen Pressure and Temperature. Chem. Mater. 2012, 24, 3473–3480. [Google Scholar] [CrossRef]
- Shpak, A.; Korduban, A.; Medvedskij, M.; Kandyba, V. XPS studies of active elements surface of gas sensors based on WO3−x nanoparticles. J. Electron Spectrosc. Relat. Phenom. 2007, 156-158, 172–175. [Google Scholar] [CrossRef]
- Leghari, S.A.K.; Sajjad, S.; Chen, F.; Zhang, J. WO3/TiO2 composite with morphology change via hydrothermal template-free route as an efficient visible light photocatalyst. Chem. Eng. J. 2011, 166, 906–915. [Google Scholar] [CrossRef]
- Paula, L.F.; Hofer, M.; Lacerda, V.P.B.; Bahnemann, D.W.; Patrocinio, A.O.T. Unraveling the photocatalytic properties of TiO2/WO3 mixed oxides. Photochem. Photobiol. Sci. 2019, 18, 2469–2483. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, S.; He, J.H.; Pan, F.; Lee, X.; Chen, W.; Wu, K.; Xu, G.Q. Enhancement of the photocatalytic efficiency of WO3 nanoparticles via hydrogen plasma treatment. Mater. Res. Express 2014, 1. [Google Scholar] [CrossRef]
- Yang, F.; Jia, J.; Mi, R.; Liu, X.; Fu, Z.; Wang, C.; Liu, X.; Tang, Y. Fabrication of WO3·2H2O/BC Hybrids by the Radiation Method for Enhanced Performance Supercapacitors. Front. Chem. 2018, 6, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, A.K.; Das, A.K.; Pradhan, D. High Performance Solid-State Asymmetric Supercapacitor using Green Synthesized Graphene–WO3 Nanowires Nanocomposite. ACS Sustain. Chem. Eng. 2017, 5, 10128–10138. [Google Scholar] [CrossRef]
- Cole, B.; Marsen, B.; Miller, E.; Yan, Y.; To, B.; Jones, K.; Al-Jassim, M. Evaluation of Nitrogen Doping of Tungsten Oxide for Photoelectrochemical Water Splitting. J. Phys. Chem. C 2008, 112, 5213–5220. [Google Scholar] [CrossRef]
- Solarska, R.; Alexander, B.; Braun, A.; Jurczakowski, R.; Fortunato, G.; Stiefel, M.; Graule, T.; Augustynski, J. Tailoring the morphology of WO3 films with substitutional cation doping: Effect on the photoelectrochemical properties. Electrochimica Acta 2010, 55, 7780–7787. [Google Scholar] [CrossRef]
- Székely, I.; Baia, M.; Magyari, K.; Boga, B.; Pap, Z. The effect of the pH adjustment upon the WO3-WO3·0.33H2O-TiO2 ternary composite systems’ photocatalytic activity. Appl. Surf. Sci. 2019, 490, 469–480. [Google Scholar] [CrossRef]
- Nie, C.; Dong, J.; Sun, P.; Yan, C.; Wu, H.; Wang, B. An efficient strategy for full mineralization of an azo dye in wastewater: A synergistic combination of solar thermo- and electrochemistry plus photocatalysis. RSC Adv. 2017, 7, 36246–36255. [Google Scholar] [CrossRef] [Green Version]
- Karácsonyi, É.; Baia, L.; Dombi, A.; Danciu, V.; Mogyorósi, K.; Pop, L.; Kovács, G.; Coşoveanu, V.; Vulpoi, A.; Simon, S.; et al. The photocatalytic activity of TiO2/WO3/noble metal (Au or Pt) nanoarchitectures obtained by selective photodeposition. Catal. Today 2013, 208, 19–27. [Google Scholar] [CrossRef]
- Székely, I.; Kovács, G.; Baia, L.; Danciu, V.; Pap, Z. Synthesis of Shape-Tailored WO3 Micro-/Nanocrystals and the Photocatalytic Activity of WO3/TiO2 Composites. Materials 2016, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boga, B.; Székely, I.; Pap, Z.; Baia, L.; Baia, M. Detailed Spectroscopic and Structural Analysis of TiO2/WO3 Composite Semiconductors. J. Spectrosc. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kedves, E.-Z.; Székely, I.; Baia, L.; Baia, M.; Csavdári, A.; Pap, Z. The Comparison of the Photocatalytic Performance Shown by TiO2 and TiO2/WO3 Composites—A Parametric and Kinetic Study. J. Nanosci. Nanotechnol. 2019, 19, 356–365. [Google Scholar] [CrossRef]
- Matthews, F. Specimen preparation. Mech. Test. Adv. Fibre Compos. 2000, 138, 36–42. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, K. The Kubelka-Munk Theory of Reflectance. Zeit. Für Tekn. Phys. 1931, 12, 539. [Google Scholar]
- Veréb, G.; Manczinger, L.; Bozsó, G.; Sienkiewicz, A.; Forró, L.; Mogyorósi, K.; Hernádi, K.; Dombi, A. Comparison of the photocatalytic efficiencies of bare and doped rutile and anatase TiO2 photocatalysts under visible light for phenol degradation and E. coli inactivation. Appl. Catal. B Environ. 2013, 129, 566–574. [Google Scholar] [CrossRef]












| WO3 | TiO2/WO3 | ||
|---|---|---|---|
| Sample | Band Gap Value (eV) | Sample | Band Gap Value (eV) |
| WO3-NWH-NaF | 2.95 ↑ | WO3-NWH-NaF+P25 | 2.98 |
| WO3-NWH-NaCl | 2.69 ↓ | WO3-NWH-NaCl+P25 | 2.97 |
| WO3-NWH-NaBr | 2.84 | WO3-NWH-NaBr+P25 | 2.99 |
| WO3-NWH-NaI | 2.78 | WO3-NWH-NaI+P25 | 3.04 |
| WO3-NWH-KCl | 2.64 | WO3-NWH-KCl+P25 | 3.00 |
| WO3-NWH-KBr | 2.66 ↑ | WO3-NWH-KBr+P25 | 3.02 |
| WO3-NWH-KI | 2.54 ↓ | WO3-NWH-KI+P25 | 3.03 |
| WO3-AMT-HF | 2.94 ↑ | WO3-AMT-HF+P25 | 3.01 |
| WO3-AMT-HCl | 2.25 ↓ | WO3-AMT-HCl+P25 | 3.10 |
| WO3-AMT-HBr | 2.53 | WO3-AMT-HBr+P25 | 2.78 |
| WO3-AMT-HI | 2.77 | WO3-AMT-HI+P25 | 2.87 |
| Sample | Eg (eV) | ECB (eV) | EVB (eV) |
|---|---|---|---|
| WO3-NWH-NaF | 2.95 | 0.62 | 3.57 |
| WO3-NWH-NaCl | 2.69 | 0.75 | 3.44 |
| WO3-NWH-NaBr | 2.84 | 0.67 | 3.51 |
| WO3-NWH-Nal | 2.78 | 0.70 | 3.48 |
| WO3-NWH-KCl | 2.64 | 0.77 | 3.41 |
| WO3-NWH-KBr | 2.66 | 0.76 | 3.42 |
| WO3-NWH-Kl | 2.54 | 0.82 | 3.36 |
| WO3-AMT-HF | 2.94 | 0.62 | 3.56 |
| WO3-AMT-HCl | 2.25 | 0.97 | 3.22 |
| WO3-AMT-HBr | 2.53 | 0.83 | 3.36 |
| WO3-AMT-HI | 2.77 | 0.71 | 3.48 |
| Sample | Crystallite Mean Size (nm) | Lattice Strain |
|---|---|---|
| WO3-NWH-NaF | 34.2 | 0.0044 |
| WO3-NWH-NaCl | 35.7 | 0.0042 |
| WO3-NWH-NaBr | 20.4 | 0.0074 |
| WO3-NWH-NaI | 18.2 | 0.0082 |
| WO3-NWH-KCl | 6.1 | 0.0245 |
| WO3-NWH-KBr | 8.4 | 0.0179 |
| WO3-NWH-KI | 9.8 | 0.0153 |
| WO3-AMT-HF | 12.4 | 0.0121 |
| WO3-AMT-HCl | 17.9 | 0.0085 |
| WO3-AMT-HBr | 32.9 | 0.0061 |
| WO3-AMT-HI | 20.9 | 0.0072 |
| Sample | Reaction Order (n) | Rate Apparent Constant (kapp) (µM∙min−1) | R2 | Conversion (X) (%) |
|---|---|---|---|---|
| WO3-NWH-NaF+P25 | 0 | 0.2713 | 0.993 | 27.5 |
| WO3-NWH-NaCl+P25 | 0.6514 | 0.986 | 57.7 | |
| WO3-NWH-NaBr+P25 | 0.6675 | 0.998 | 63.7 | |
| WO3-NWH-NaI+P25 | 0.7297 | 0.990 | 67.4 | |
| WO3-NWH-KCl+P25 | 0.4697 | 0.991 | 44.2 | |
| WO3-NWH-KBr+P25 | 0.5526 | 0.990 | 51.5 | |
| WO3-NWH-KI+P25 | 0.7534 | 0.988 | 69.3 | |
| WO3-AMT-HF+P25 | 0.9195 | 0.996 | 87.7 | |
| WO3-AMT-HCl+P25 | 0.8603 | 0.996 | 84.6 | |
| WO3-AMT-HBr+P25 | 0.5856 | 0.989 | 53.4 | |
| WO3-AMT-HI+P25 | 0.4477 | 0.999 | 42.9 | |
| Sample | Reaction Order(n) | Rate Apparent Constant (kapp) (µM0.5∙s)−1 | R2 | Conversion (X) (%) |
| P25 | 0.5 | 0.0542 | 0.999 | 82.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Székely, I.; Kedves, E.-Z.; Pap, Z.; Baia, M. Synthesis Design of Electronegativity Dependent WO3 and WO3∙0.33H2O Materials for a Better Understanding of TiO2/WO3 Composites’ Photocatalytic Activity. Catalysts 2021, 11, 779. https://doi.org/10.3390/catal11070779
Székely I, Kedves E-Z, Pap Z, Baia M. Synthesis Design of Electronegativity Dependent WO3 and WO3∙0.33H2O Materials for a Better Understanding of TiO2/WO3 Composites’ Photocatalytic Activity. Catalysts. 2021; 11(7):779. https://doi.org/10.3390/catal11070779
Chicago/Turabian StyleSzékely, István, Endre-Zsolt Kedves, Zsolt Pap, and Monica Baia. 2021. "Synthesis Design of Electronegativity Dependent WO3 and WO3∙0.33H2O Materials for a Better Understanding of TiO2/WO3 Composites’ Photocatalytic Activity" Catalysts 11, no. 7: 779. https://doi.org/10.3390/catal11070779