In Utero Exposure to Hormonal Contraception and Mortality in Offspring with and without Cancer: A Nationwide Cohort Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Validity and Completeness
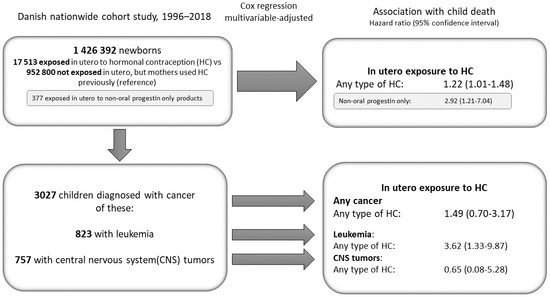
2.4. Study Populations
2.5. Measures and Variables
2.5.1. Exposure
2.5.2. Other Variables
2.5.3. Outcome
2.5.4. Other Measurements
2.6. Statistical Analysis
3. Results
3.1. Long-Term Child Mortality
3.2. Case Fatality
3.3. Types of Hormonal Contraception
3.4. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Contraceptive Use by Method 2019: Data Booklet; United Nations: New York, NY, USA, 2019; ISBN 978-92-1-004652-7. [Google Scholar]
- Kristensen, S.I.; Lidegaard, Ø. Hormonal Contraceptive Use in Denmark 2010–2019. Dan. Med. J. 2021, 68, A08200599. [Google Scholar]
- Teal, S.; Edelman, A. Contraception Selection, Effectiveness, and Adverse Effects: A Review. JAMA 2021, 326, 2507. [Google Scholar] [CrossRef]
- Vargesson, N. Thalidomide-induced Teratogenesis: History and Mechanisms. Birth Defect. Res. C 2015, 105, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.; Invernizzi, N. A Tragédia Da Talidomida: A Luta Pelos Direitos Das Vítimas e Por Melhor Regulação de Medicamentos. História Ciências Saúde-Manguinhos 2017, 24, 603–622. [Google Scholar] [CrossRef] [PubMed]
- Al Jishi, T.; Sergi, C. Current Perspective of Diethylstilbestrol (DES) Exposure in Mothers and Offspring. Reprod. Toxicol. 2017, 71, 71–77. [Google Scholar] [CrossRef]
- Herbst, A.L.; Ulfelder, H.; Poskanzer, D.C. Adenocarcinoma of the Vagina: Association of Maternal Stilbestrol Therapy with Tumor Appearance in Young Women. N. Engl. J. Med. 1971, 284, 878–881. [Google Scholar] [CrossRef]
- Giusti, R.M.; Iwamoto, K.; Hatch, E.E. Diethylstilbestrol Revisited: A Review of the Long-Term Health Effects. Ann. Intern. Med. 1995, 122, 778–788. [Google Scholar] [CrossRef]
- Palmer, J.R.; Wise, L.A.; Hatch, E.E.; Troisi, R.; Titus-Ernstoff, L.; Strohsnitter, W.; Kaufman, R.; Herbst, A.L.; Noller, K.L.; Hyer, M.; et al. Prenatal Diethylstilbestrol Exposure and Risk of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Troisi, R.; Hatch, E.E.; Titus, L.; Strohsnitter, W.; Gail, M.H.; Huo, D.; Adam, E.; Robboy, S.J.; Hyer, M.; Hoover, R.N.; et al. Prenatal Diethylstilbestrol Exposure and Cancer Risk in Women. Environ. Mol. Mutagen. 2019, 60, 395–403. [Google Scholar] [CrossRef]
- Verloop, J.; van Leeuwen, F.E.; Helmerhorst, T.J.M.; van Boven, H.H.; Rookus, M.A. Cancer Risk in DES Daughters. Cancer Causes Control 2010, 21, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Anderson, D.; Palmer, J.R.; Herbst, A.L. Incidence Rates and Risks of Diethylstilbestrol-Related Clear-Cell Adenocarcinoma of the Vagina and Cervix: Update after 40-Year Follow-Up. Gynecol. Oncol. 2017, 146, 566–571. [Google Scholar] [CrossRef]
- Strohsnitter, W.C.; Hyer, M.; Bertrand, K.A.; Cheville, A.L.; Palmer, J.R.; Hatch, E.E.; Aagaard, K.M.; Titus, L.; Romero, I.L.; Huo, D.; et al. Prenatal Diethylstilbestrol Exposure and Cancer Risk in Males. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.N.; Hyer, M.; Pfeiffer, R.M.; Adam, E.; Bond, B.; Cheville, A.L.; Colton, T.; Hartge, P.; Hatch, E.E.; Herbst, A.L.; et al. Adverse Health Outcomes in Women Exposed in Utero to Diethylstilbestrol. N. Engl. J. Med. 2011, 365, 1304–1314. [Google Scholar] [CrossRef]
- Troisi, R.; Hyer, M.; Titus, L.; Palmer, J.R.; Hatch, E.E.; Huo, D.; Aagaard, K.M.; Strohsnitter, W.C.; Hoover, R.N. Prenatal Diethylstilbestrol Exposure and Risk of Diabetes, Gallbladder Disease, and Pancreatic Disorders and Malignancies. J. Dev. Orig. Health Dis. 2021, 12, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Hargreave, M.; Mørch, L.S.; Andersen, K.K.; Winther, J.F.; Schmiegelow, K.; Kjaer, S.K. Maternal Use of Hormonal Contraception and Risk of Childhood Leukaemia: A Nationwide, Population-Based Cohort Study. Lancet Oncol. 2018, 19, 1307–1314. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; Bouzbid, S.; et al. International Incidence of Childhood Cancer, 2001–2010: A Population-Based Registry Study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Steliarova-Foucher, E.; Stiller, C.; Kaatsch, P.; Berrino, F.; Coebergh, J.-W.; Lacour, B.; Perkin, M. Geographical Patterns and Time Trends of Cancer Incidence and Survival among Children and Adolescents in Europe since the 1970s (the ACCIS Project): An Epidemiological Study. Lancet 2004, 364, 2097–2105. [Google Scholar] [CrossRef]
- Grabas, M.R.; Kjaer, S.K.; Frederiksen, M.H.; Winther, J.F.; Erdmann, F.; Dehlendorff, C.; Hargreave, M. Incidence and Time Trends of Childhood Cancer in Denmark, 1943–2014. Acta Oncol. 2020, 59, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Metayer, C.; Dahl, G.; Wiemels, J.; Miller, M. Childhood Leukemia: A Preventable Disease. Pediatrics 2016, 138, S45–S55. [Google Scholar] [CrossRef]
- Gatta, G.; Corazziari, I.; Magnani, C.; Peris-Bonet, R.; Roazzi, P.; Stiller, C. Childhood Cancer Survival in Europe. Ann. Oncol. 2003, 14, v119–v127. [Google Scholar] [CrossRef]
- Pedersen, C.B. The Danish Civil Registration System. Scand. J. Public Health 2011, 39, 22–25. [Google Scholar] [CrossRef]
- Schmidt, M.; Pedersen, L.; Sørensen, H.T. The Danish Civil Registration System as a Tool in Epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef]
- Kildemoes, H.W.; Sørensen, H.T.; Hallas, J. The Danish National Prescription Registry. Scand. J. Public Health 2011, 39, 38–41. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Olsen, J. The Danish Medical Birth Registry. Dan. Med. Bull. 1998, 45, 320–323. [Google Scholar] [PubMed]
- Jensen, V.M.; Rasmussen, A.W. Danish Education Registers. Scand. J. Public Health 2011, 39, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Gjerstorff, M.L. The Danish Cancer Registry. Scand. J. Public Health 2011, 39, 42–45. [Google Scholar] [CrossRef] [PubMed]
- ICCC Recode ICD-O-3/IARC 2017 Table—SEER Recodes. Available online: https://seer.cancer.gov/iccc/iccc-iarc-2017.html (accessed on 16 May 2022).
- Pedersen, C.B.; Gøtzsche, H.; Møller, J.O.; Mortensen, P.B. The Danish Civil Registration System. A Cohort of Eight Million Persons. Dan. Med. Bull. 2006, 53, 441–449. [Google Scholar]
- Kristensen, J.; Langhoff-Roos, J.; Skovgaard, L.T.; Kristensen, F.B. Validation of the Danish Birth Registration. J. Clin. Epidemiol. 1996, 49, 893–897. [Google Scholar] [CrossRef]
- Storm, H.H.; Michelsen, E.V.; Clemmensen, I.H.; Pihl, J. The Danish Cancer Registry—History, Content, Quality and Use. Dan. Med. Bull. 1997, 44, 535–539. [Google Scholar]
- Tiemeier, H. Are Women Using Hormonal Contraceptives the Risk-Takers? Eur. J. Epidemiol. 2020, 35, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.L. A Note on Robust Variance Estimation for Cluster-Correlated Data. Biometrics 2000, 56, 645–646. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268. [Google Scholar] [CrossRef]
- Britton, L.E.; Alspaugh, A.; Greene, M.Z.; McLemore, M.R. CE: An Evidence-Based Update on Contraception. AJN Am. J. Nurs. 2020, 120, 22–33. [Google Scholar] [CrossRef]
- Khamsi, R. Fetuses Suffer from Extra Oestrogen Exposure. Nature 2005, news050502-1. [Google Scholar] [CrossRef]
- Siemienowicz, K.J.; Wang, Y.; Marečková, M.; Nio-Kobayashi, J.; Fowler, P.A.; Rae, M.T.; Duncan, W.C. Early Pregnancy Maternal Progesterone Administration Alters Pituitary and Testis Function and Steroid Profile in Male Fetuses. Sci. Rep. 2020, 10, 21920. [Google Scholar] [CrossRef] [PubMed]
- Minkin, S. Depo-Provera: A Critical Analysis. Women Health 1981, 5, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Gray, R.H.; Pardthaisong, T. In Utero Exposure to Steroid Contraceptives and Survival During Infancy. Am. J. Epidemiol. 1991, 134, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Pardthaisong, T.; Gray, R.H.; McDaniel, E.B.; Chandacham, A. Steroid Contraceptive Use and Pregnancy Outcome. Teratology 1988, 38, 51–58. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (Ed.) Sex Hormones. 2: Sex Hormones [II]: [Views and Expert Opinions of an IARC Working Group]. In IARC Working Group on the Evaluation of the Carcinogenic Risk of Chemicals to Humans; IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans; IARC: Lyon, France, 1979; ISBN 978-92-832-1221-8. [Google Scholar]
- Harris, R.M.; Waring, R.H. Diethylstilboestrol—A Long-Term Legacy. Maturitas 2012, 72, 108–112. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Lundberg, F.E.; Ekberg, S.; Johansson, A.L.V.; Ludvigsson, J.F.; Almqvist, C.; Cnattingius, S.; Iliadou, A.N. Mortality from Infancy to Adolescence in Singleton Children Conceived from Assisted Reproductive Techniques versus Naturally Conceived Singletons in Sweden. Fertil. Steril. 2020, 113, 524–532. [Google Scholar] [CrossRef]
- Hargreave, M.; Mørch, L.S.; Winther, J.F.; Schmiegelow, K.; Kjaer, S.K. Association Between Maternal Hormonal Contraception Use and Central Nervous System Tumors in Children. JAMA 2022, 327, 59. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, C.H.; Kjaer, S.K.; Jezek, A.H.; Verhulst, F.C.; Pagsberg, A.K.; Kamper-Jørgensen, M.; Mørch, L.S.; Hargreave, M. Maternal Use of Hormonal Contraception and Risk of Childhood ADHD: A Nationwide Population-Based Cohort Study. Eur. J. Epidemiol. 2020, 35, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Halane, H.I.M.; Hargreave, M.; Kjaer, S.K.; Christensen, J.; Mørch, L.S. Maternal Use of Hormonal Contraception and Epilepsy in Offspring. Hum. Reprod. 2021, 36, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Young, J. ADHD Is a Risk Factor for Premature Death, Danish Study Shows. BMJ 2015, 350, h1094. [Google Scholar] [CrossRef]
- Green, A.L.; Furutani, E.; Ribeiro, K.B.; Rodriguez Galindo, C. Death within 1 Month of Diagnosis in Childhood Cancer: An Analysis of Risk Factors and Scope of the Problem. J. Clin. Oncol. 2017, 35, 1320–1327. [Google Scholar] [CrossRef]
- Sethi, D.; World Health Organization (Eds.) European Report on Child Injury Prevention; WHO Regional Office for Europe: Copenhagen, Denmark, 2008; ISBN 978-92-890-4295-6. [Google Scholar]
- Merrill, R.; Lyon, J.; Baker, R.; Gren, L. Attention Deficit Hyperactivity Disorder and Increased Risk of Injury. Adv. Med. Sci. 2009, 54, 20. [Google Scholar] [CrossRef]
- Balaj, M.; York, H.W.; Sripada, K.; Besnier, E.; Vonen, H.D.; Aravkin, A.; Friedman, J.; Griswold, M.; Jensen, M.R.; Mohammad, T.; et al. Parental Education and Inequalities in Child Mortality: A Global Systematic Review and Meta-Analysis. Lancet 2021, 398, 608–620. [Google Scholar] [CrossRef]
- Schramm, S.; Møller, S.P.; Tolstrup, J.S.; Laursen, B. Effects of Individual and Parental Educational Levels on Multimorbidity Classes: A Register-Based Longitudinal Study in a Danish Population. BMJ Open 2022, 12, e053274. [Google Scholar] [CrossRef]

| Characteristics | No. (%) | |||
|---|---|---|---|---|
| During Pregnancy | Recent Use | Previous Use | No Use | |
| Number of children | 17,513 (1.2) a | 154,381 (10.8) b | 952,800 (66.8) c | 301,698 (21.2) |
| Child characteristics | ||||
| Year of birth | ||||
| 1996–1999 | 4081 (23.3) | 26,532 (17.2) | 140,434 (14.7) | 152,919 (50.7) |
| 2000–2004 | 4075 (23.3) | 31,500 (20.4) | 223,118 (23.4) | 60,316 (20.0) |
| 2005–2009 | 4070 (23.2) | 35,194 (22.8) | 244,180 (25.6) | 33,190 (11.0) |
| 2010–2014 | 3502 (20.0) | 34,905 (22.6) | 214,726 (22.5) | 31,521 (10.5) |
| 2015–2018 | 1785 (10.2) | 26,250 (17.0) | 130,342 (13.7) | 23,752 (7.9) |
| Median (IQR) | 2006 (2001–2012) | 2008 (2003–2014) | 2008 (2003–2013) | 2000 (1997–2007) |
| Sex | ||||
| Male | 9126 (52.1) | 79,427 (51.5) | 487,390 (51.2) | 154,746 (51.3) |
| Female | 8362 (47.8) | 74,698 (48.4) | 463,728 (48.7) | 146,055 (48.4) |
| Missing | 25 (0.1) | 256 (0.2) | 1673 (0.2) | 897 (0.3) |
| Birth order | ||||
| First | 7954 (45.4) | 68,250 (44.2) | 433,755 (45.5) | 111,685 (37.0) |
| Second or higher | 9553 (54.6) | 86,100 (55.8) | 518,861 (54.5) | 189,739 (62.9) |
| Missing | 6 (0.03) | 31 (0.02) | 184 (0.02) | 274 (0.09) |
| Parental characteristics | ||||
| Origin (mother) | ||||
| Danish or descendant d | 15,500 (88.5) | 142,316 (92.2) | 883,169 (92.7) | 187,473 (62.1) |
| Immigrant | 2013 (11.5) | 12,065 (7.8) | 69,631 (7.3) | 114,208 (37.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 17 (0.01) |
| BMI $ (mother) | ||||
| <25 | 6281 (35.9) | 66,015 (42.8) | 411,330 (43.2) | 64,619 (21.4) |
| 25–30 | 2563 (14.6) | 26,333 (17.1) | 157,974 (16.6) | 23,694 (7.9) |
| 31–35 | 1048 (6.0) | 9038 (5.9) | 58,293 (6.1) | 8066 (2.7) |
| >35 | 571 (3.3) | 4587 (3) | 32,594 (3.4) | 3990 (1.3) |
| Missing | 7050 (40.3) | 48,408 (31.4) | 292,609 (30.7) | 201,329 (66.7) |
| Maternal education e | ||||
| Basic | 6808 (38.9) | 34,668 (22.5) | 166,285 (17.5) | 68,800 (22.8) |
| Vocational | 6986 (39.9) | 65,345 (42.3) | 394,412 (41.4) | 101,612 (33.7) |
| Higher | 3136 (17.9) | 51,767 (33.5) | 380,941 (40.0) | 81,629 (27.1) |
| Missing | 583 (3.3) | 2601 (1.7) | 11,162 (1.2) | 49,657 (16.5) |
| Paternal education e | ||||
| Basic | 5715 (32.6) | 32,933 (21.3) | 171,800 (18.0) | 64,844 (21.5) |
| Vocational | 8043 (45.9) | 74,904 (48.5) | 448,764 (47.1) | 117,200 (38.9) |
| Higher | 2793 (16.0) | 41,604 (27.0) | 299,286 (31.4) | 80,600 (26.7) |
| Missing | 962 (5.5) | 4940 (3.2) | 32,950 (3.5) | 39,054 (12.9) |
| Maternal age at birth (y) | ||||
| <28 | 8781 (50.1) | 60,522 (39.2) | 282,079 (29.6) | 84,850 (28.1) |
| 28–31 | 4261 (24.3) | 47,993 (31.1) | 320,411 (33.6) | 83,774 (27.8) |
| 32–35 | 2841 (16.2) | 31,160 (20.2) | 229,328 (24.1) | 76,828 (25.5) |
| >35 | 1630 (9.3) | 14,706 (9.5) | 120,982 (12.7) | 56,246 (18.6) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Median (IQR) | 27 (23–32) | 29 (26–32) | 30 (27–33) | 31 (27–34) |
| Paternal age at birth (y) | ||||
| <28 | 5949 (34.0) | 37,332 (24.2) | 164,177 (17.2) | 43,477 (14.4) |
| 28–31 | 4109 (23.5) | 44,665 (28.9) | 273,430 (28.7) | 67,561 (22.4) |
| 32–35 | 3352 (19.1) | 37,605 (24.4) | 261,277 (27.4) | 77,958 (25.8) |
| >35 | 3779 (21.6) | 33,552 (21.7) | 242,786 (25.5) | 105,933 (35.1) |
| Missing | 324 (1.9) | 1227 (0.8) | 10,990 (1.2) | 6769 (2.2) |
| Median (IQR) | 30 (26–35) | 31 (28–35) | 32 (29–36) | 33 (30–38) |
| Maternal smoking f,* | 4577 (26.1) | 25,027 (16.2) | 137,865 (14.5) | 40,195 (13.3) |
| Missing | 1405 (8.0) | 9361 (6.1) | 49,933 (5.2) | 48,395 (16.0) |
| Maternal infertility g | 505 (2.9) | 7589 (4.9) | 85,916 (9) | 29,323 (9.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Number of Children | Person-Years of Follow-Up | Long-Term Mortality | ||||
|---|---|---|---|---|---|---|
| Number of Deaths | Mortality Rate per 100,000 (95% CI) $ | HR (95% CI) * | p-Value | |||
| Previous use | 952,800 | 9,908,070 | 4420 | 66.3 (63.5–69.1) | 1 (reference) | |
| Recent use | 154,381 | 1,578,630 | 748 | 67.1 (62.5–73.2) | 1.02 (0.94–1.11) | 0.613 |
| During pregnancy | 17,513 | 204,490 | 114 | 80.1 (65.4–96.6) | 1.22 (1.01–1.48) | 0.040 |
| Number of Children | Person-Years of Follow-Up | Long-Term Mortality | ||||
|---|---|---|---|---|---|---|
| Number of Deaths | Mortality Rate per 100,000 (95% CI) $ | HR (95% CI) * | p-Value | |||
| Any type | ||||||
| Previous use | 952,800 | 9,908,070 | 4420 | 66.3 (63.5–69.1) | 1 (reference) | … |
| Combined contraceptives | ||||||
| Oral contraception | ||||||
| Recent use | 111,608 | 1,314,780 | 585 | 68.2 (62.1–74.4) | 1.04 (0.95–1.13) | 0.424 |
| During pregnancy | 14,746 | 176,610 | 97 | 80.4 (63.5–97.3) | 1.22 (0.99–1.50) | 0.064 |
| Non-oral contraceptives | ||||||
| Recent use | 3138 | 23,210 | 12 | 61.3 (23.7–98.8) | 0.93 (0.51–1.71) | 0.815 |
| During pregnancy | 501 | 3720 | <5 £ | 30.8 (-29.6–912) | 0.47 (0.66–3.32) | 0.447 |
| Progestin-only contraceptives | ||||||
| Oral contraception | ||||||
| Recent use | 7636 | 63,770 | 27 | 55.4 (34.4–76.4) | 0.82 (0.56–1.19) | 0.296 |
| During pregnancy | 1405 | 12,950 | 7 | 72.6 (18.6–126.5) | 1.08 (0.51–2.26) | 0.845 |
| Non-oral contraceptives | ||||||
| Recent use | 33,547 | 176,880 | 132 | 72.4 (60.0–84.8) | 1.04 (0.87–1.24) | 0.671 |
| During pregnancy | 377 | 2520 | 5 | 203.9 (23.9–384.0) | 2.92 (1.21–7.04) | 0.017 |
| Emergency contraceptives | ||||||
| Recent use | 1203 | 20,650 | 6 | 50.5 (10.0–91.1) | 0.77 (0.34–1.71) | 0.515 |
| During pregnancy | 591 | 10,240 | 5 | 87.2 (10.5–163.8) | 1.31 (0.55–3.15) | 0.544 |
| Number of Children | Person-Years of Follow-Up | Long-Term Mortality | ||||
|---|---|---|---|---|---|---|
| Any cancer | Number of Deaths | Mortality Rate per 1000 (95% CI) $ | HR (95% CI) *,¤ | p-Value | ||
| Previous use | 1864 | 260 | 217 | 19.7 (16.7–22.7) | 1 (reference) | … |
| Recent use | 304 | 11,590 | 42 | 24.6 (16.6–32.7) | 1.18 (0.85–1.64) | 0.325 |
| During pregnancy | 43 | 1840 | 7 | 30.7 (6.2–552.9) | 1.49 (0.70–3.17) | 0.299 |
| Leukemia | ||||||
| Previous use | 548 | 80 | 51 | 16.2 (9.7–22.6) | 1 (reference) | … |
| Recent use | 86 | 3980 | 12 | 26.0 (8.8–43.2) | 1.35 (0.69–2.63) | 0.379 |
| During pregnancy | 13 | 620 | <5 £ | 65.1 (−15.1–145.3) | 3.62 (1.33–9.87) | 0.012 |
| CNS tumors | ||||||
| Previous use | 446 | 70 | 82 | 37.0 (27.5–46.5) | 1 (reference) | … |
| Recent use | 81 | 2350 | 12 | 31.0 (12.3–49.6) | 0.83 (0.45–1.54) | 0.555 |
| During pregnancy | 9 | 410 | <5 £ | 17.8 (−18.9–54.4) | 0.65 (0.08–5.28) | 0.688 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mørch, L.S.; Gamborg, M.; Hemmingsen, C.H.; Skovlund, C.W.; Kjær, S.K.; Hargreave, M. In Utero Exposure to Hormonal Contraception and Mortality in Offspring with and without Cancer: A Nationwide Cohort Study. Cancers 2023, 15, 3163. https://doi.org/10.3390/cancers15123163
Mørch LS, Gamborg M, Hemmingsen CH, Skovlund CW, Kjær SK, Hargreave M. In Utero Exposure to Hormonal Contraception and Mortality in Offspring with and without Cancer: A Nationwide Cohort Study. Cancers. 2023; 15(12):3163. https://doi.org/10.3390/cancers15123163
Chicago/Turabian StyleMørch, Lina Steinrud, Mads Gamborg, Caroline Hallas Hemmingsen, Charlotte Wessel Skovlund, Susanne Krüger Kjær, and Marie Hargreave. 2023. "In Utero Exposure to Hormonal Contraception and Mortality in Offspring with and without Cancer: A Nationwide Cohort Study" Cancers 15, no. 12: 3163. https://doi.org/10.3390/cancers15123163