Combing MRI Perfusion and 18F-FDG PET/CT Metabolic Biomarkers Helps Predict Survival in Advanced Nasopharyngeal Carcinoma: A Prospective Multimodal Imaging Study
Abstract
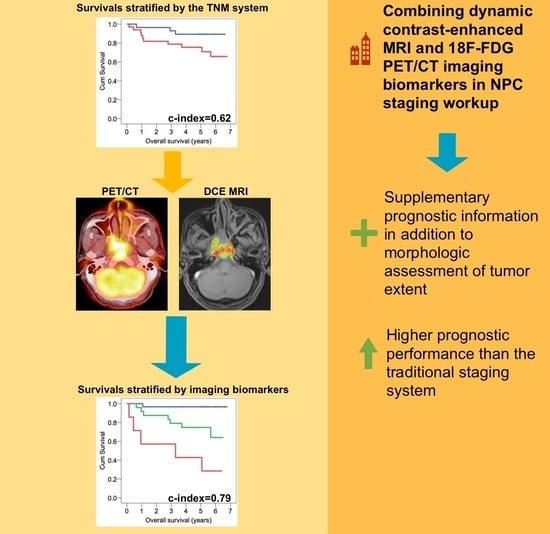
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Patients
2.2. Multimodal MRI
2.3. 18F-FDG PET/CT
2.4. PET Image Analysis
2.5. MR Image Analysis
2.6. Treatment and Follow-Up
2.7. Outcome Determination and Statistical Analysis
2.8. Performance and Validation of the Prognostic Model
3. Results
3.1. PET/CT and MRI Imaging Biomarkers for Predicting Survival Endpoints
3.2. Performance and Validation of the Prognostic Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.W.; Ma, B.B.; Ng, W.T.; Chan, A.T. Management of Nasopharyngeal Carcinoma: Current Practice and Future Perspective. J. Clin. Oncol. 2015, 33, 3356–3364. [Google Scholar] [CrossRef]
- Lin, J.C.; Jan, J.S.; Hsu, C.Y.; Liang, W.M.; Jiang, R.S.; Wang, W.Y. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: Positive effect on overall and progression-free survival. J. Clin. Oncol. 2003, 21, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Chua, M.L.; Wee, J.T.; Hui, E.P.; Chan, A.T. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Kamran, S.C.; Riaz, N.; Lee, N. Nasopharyngeal carcinoma. Surg. Oncol. Clin. N. Am. 2015, 24, 547–561. [Google Scholar] [CrossRef]
- Benz, M.R.; Vargas, H.A.; Sala, E. Functional MR Imaging Techniques in Oncology in the Era of Personalized Medicine. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bogowicz, M.; Vuong, D.; Huellner, M.W.; Pavic, M.; Andratschke, N.; Gabrys, H.S.; Guckenberger, M.; Tanadini-Lang, S. CT radiomics and PET radiomics: Ready for clinical implementation? Q J. Nucl. Med. Mol. Imaging 2019, 63, 355–370. [Google Scholar] [CrossRef]
- King, A.D.; Bhatia, K.S. Magnetic resonance imaging staging of nasopharyngeal carcinoma in the head and neck. World J. Radiol 2010, 2, 159–165. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zhang, Y.; Li, W.F.; Chen, L.; Mao, Y.P.; Shen, J.X.; Zhang, F.; Peng, H.; Liu, Q.; et al. Prognostic value of the primary lesion apparent diffusion coefficient (ADC) in nasopharyngeal carcinoma: A retrospective study of 541 cases. Sci. Rep. 2015, 5, 12242. [Google Scholar] [CrossRef]
- Zheng, D.; Yue, Q.; Ren, W.; Liu, M.; Zhang, X.; Lin, H.; Lai, G.; Chen, W.; Chan, Q.; Chen, Y. Early responses assessment of neoadjuvant chemotherapy in nasopharyngeal carcinoma by serial dynamic contrast-enhanced MR imaging. Magn. Reson. Imaging 2017, 35, 125–131. [Google Scholar] [CrossRef]
- Xie, P.; Yue, J.B.; Zhao, H.X.; Sun, X.D.; Kong, L.; Fu, Z.; Yu, J.M. Prognostic value of 18F-FDG PET-CT metabolic index for nasopharyngeal carcinoma. J. Cancer Res. Clin. Oncol. 2010, 136, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chang, K.P.; Chu, S.C.; Yen, T.C.; Wang, L.Y.; Chang, J.T.; Hsu, C.L.; Ng, S.H.; Liu, S.H.; Chan, S.C. Value of early evaluation of treatment response using 18F-FDG PET/CT parameters and the Epstein-Barr virus DNA load for prediction of outcome in patients with primary nasopha-ryngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 650–660. [Google Scholar] [CrossRef]
- Tofts, P.S.; Berkowitz, B.; Schnall, M.D. Quantitative analysis of dynamic Gd-DTPA enhancement in breast tumors using a permeability model. Magn. Reson. Med. 1995, 33, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Chen, P.T.; Chang, T.C.; Hsu, C.L.; Lin, C.Y.; Liao, C.T.; Chen, H. Concurrent chemoradiotherapy using biweekly cisplatin/tegafur plus uracil/leucovorin in stage III nasopharyngeal carcinoma. In Proceedings of the Abstracts of the 41st Annual Meeting of the American Society of Clinical Oncology (ASCO), Orlando, FL, USA, 13–17 May 2005. [Google Scholar]
- Kang, L.; Chen, W.; Petrick, N.A.; Gallas, B.D. Comparing two correlated C indices with right-censored survival outcome: A one-shot nonparametric approach. Stat. Med. 2015, 34, 685–703. [Google Scholar] [CrossRef] [Green Version]
- Walker-Samuel, S.; Leach, M.O.; Collins, D.J. Evaluation of response to treatment using DCE-MRI: The relationship between initial area under the gadolinium curve (IAUGC) and quantitative pharmacokinetic analysis. Phys. Med. Biol. 2006, 51, 3593–3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Kim, S.H.; Im, S.A.; Oh, D.Y.; Kim, T.Y.; Han, J.K. Multiparametric fully-integrated 18-FDG PET/MRI of advanced gastric cancer for prediction of chemotherapy response: A preliminary study. Eur. Radiol. 2016, 26, 2771–2778. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.S.; Chen, J.L.; Chen, J.Y.; Lee, Y.F.; Huang, J.Y.; Kuo, S.H.; Yen, R.F.; Chang, Y.C. Predicting tumor responses and patient survival in chemoradiotherapy-treated patients with non-small-cell lung cancer using dynamic contrast-enhanced integrated magnetic resonance-positron-emission tomography. Strahlenther. Onkol. 2019, 195, 707–718. [Google Scholar] [CrossRef]
- Jain, R.K. Transport of molecules in the tumor interstitium: A review. Cancer Res. 1987, 47, 3039–3051. [Google Scholar]
- Koh, T.S.; Ng, Q.S.; Thng, C.H.; Kwek, J.W.; Kozarski, R.; Goh, V. Primary colorectal cancer: Use of kinetic modeling of dynamic contrast-enhanced CT data to predict clinical outcome. Radiology 2013, 267, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.C.; Lin, C.Y.; Huang, B.S.; Tsang, N.M.; Fan, K.H.; Ku, Y.K.; Hsu, C.L.; Chan, S.C.; Huang, S.F.; Li, C.H.; et al. Pretreatment Dynamic Contrast-Enhanced MRI Improves Prediction of Early Distant Metastases in Patients With Nasopharyngeal Carcinoma. Medicine 2016, 95, e2567. [Google Scholar] [CrossRef]
- Chawla, S.; Kim, S.; Loevner, L.A.; Hwang, W.T.; Weinstein, G.; Chalian, A.; Quon, H.; Poptani, H. Prediction of disease-free survival in patients with squamous cell carcinomas of the head and neck using dynamic contrast-enhanced MR imaging. AJNR Am. J. Neuroradiol. 2011, 32, 778–784. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.H.; Liao, C.T.; Lin, C.Y.; Chan, S.C.; Lin, Y.C.; Yen, T.C.; Chang, J.T.; Ko, S.F.; Fan, K.H.; Wang, H.M.; et al. Dynamic contrast-enhanced MRI, diffusion-weighted MRI and 18F-FDG PET/CT for the prediction of survival in oropharyngeal or hypopharyngeal squamous cell carcinoma treated with chemoradiation. Eur. Radiol 2016, 26, 4162–4172. [Google Scholar] [CrossRef]
- Yan, D.F.; Zhang, W.B.; Ke, S.B.; Zhao, F.; Yan, S.X.; Wang, Q.D.; Teng, L.S. The prognostic value of pretreatment tumor apparent diffusion coefficient values in nasopharyngeal carcinoma. BMC Cancer 2017, 17, 678. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.C.; Chang, J.T.; Lin, C.Y.; Ng, S.H.; Wang, H.M.; Liao, C.T.; Chang, C.J.; Lin, S.Y.; Yen, T.C. Clinical utility of 18F-FDG PET parameters in patients with advanced nasopharyngeal carcinoma: Predictive role for different survival endpoints and impact on prognostic stratification. Nucl. Med. Commun. 2011, 32, 989–996. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Cheng, W.; Zhu, C.; Chen, L.; Xia, F.; Wang, M.; Yang, F.; Ma, X. Prognostic value of maximum standard uptake value, metabolic tumor volume, and total lesion glycolysis of positron emission tomography/computed tomography in patients with nasopharyngeal carcinoma: A systematic review and meta-analysis. Medicine 2017, 96, e8084. [Google Scholar] [CrossRef]
- Chang, K.P.; Tsang, N.M.; Liao, C.T.; Hsu, C.L.; Chung, M.J.; Lo, C.W.; Chan, S.C.; Ng, S.H.; Wang, H.M.; Yen, T.C. Prognostic Significance of 18F-FDG PET Parameters and Plasma Epstein-Barr Virus DNA Load in Patients with Nasopharyngeal Carcinoma. J. Nucl. Med. 2012, 53, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Mohan, S.; Natarajan, M. Radiation-Triggered NF-kappaB Activation is Responsible for the Angiogenic Signaling Pathway and Neovascularization for Breast Cancer Cell Proliferation and Growth. Breast Cancer 2012, 6, 125–135. [Google Scholar] [CrossRef]
- Lee, N.Y.; Zhang, Q.; Pfister, D.G.; Kim, J.; Garden, A.S.; Mechalakos, J.; Hu, K.; Le, Q.T.; Colevas, A.D.; Glisson, B.S.; et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): A phase 2 multi-institutional trial. Lancet Oncol. 2012, 13, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Tsao, S.W.; Tsang, C.M.; Lo, K.W. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef]
- Chan, S.C.; Ng, S.H.; Yeh, C.H.; Chang, K.P. Multiparametric PET/MRI in nasopharyngeal carcinoma: Correlations between MRI functional parameters and 18F-FDG PET imaging biomarkers and their predictive value for treatment failure. Tzu. Chi. Med. J. 2020, 33, 61–69. [Google Scholar] [CrossRef]
- Lee, V.H.; Kwong, D.L.; Leung, T.W.; Choi, C.W.; Lam, K.O.; Sze, C.K.; Ho, P.; Chan, W.L.; Wong, L.S.; Leun, D. Post-radiation plasma Epstein-Barr virus DNA and local clinical remission after radical intensity-modulated radiation therapy for nasopharyngeal carcinoma. Clin. Oncol. Coll. Radiol. 2016, 28, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Codecà, C.; Bertuzzi, C.; Broggio, B.; Crepaldi, F.; Luciani, A.; Floriani, I.; Ansarin, M.; Chiesa, F.; Alterio, D.; et al. Role of plasma EBV DNA levels in predicting recurrence of nasopharyngeal carcinoma in a Western population. BMC Cancer 2012, 12, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argirion, I.; Zarins, K.R.; Ruterbusch, J.J.; Vatanasapt, P.; Sriplung, H.; Seymour, E.K.; Rozek, L.S. Increasing incidence of Epstein-Barr virus-related nasopharyngeal carcinoma in the United States. Cancer 2020, 126, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Variable | Number of Patients (%) |
|---|---|
| Age (years), median ± SD | 46 ± 13 |
| Sex | |
| Male | 50 (82) |
| Female | 11 (18) |
| Histology, n (%) | |
| Non-keratinizing carcinoma | 3 (5) |
| Undifferentiated carcinoma | 58 (95) |
| Overall stage | |
| III | 28 (46) |
| IVa | 21 (34) |
| IVb | 12 (20) |
| T classification | |
| T1 | 13 (21) |
| T2 | 2 (3) |
| T3 | 19 (31) |
| T4 | 27 (45) |
| N classification | |
| N0 | 3 (5) |
| N1 | 20 (33) |
| N2 | 26 (42) |
| N3 | 12 (20) |
| EBV copy number (mL−1) | |
| Mean ± SD | 18,486 ± 65,394 |
| Parameter | Patient No. | OS | RFS | ||
|---|---|---|---|---|---|
| 5-Year OS (Event No.) | p Value | 5-Year RFS (Event No.) | p Value | ||
| Age (years) | 0.073 | 0.641 | |||
| ≤46 | 32 | 82.4 (4) | 41.8 (10) | ||
| >46 | 29 | 67.1 (9) | 63.4 (10) | ||
| Sex | 0.793 | 0.107 | |||
| Male | 50 | 78.6 (11) | 39.1 (19) | ||
| Female | 11 | 81.8 (2) | 90.9 (1) | ||
| Tumor stage | 0.074 | 0.247 | |||
| III | 28 | 89.3 (3) | 33.7 (7) | ||
| IVa-b | 33 | 70.8 (10) | 59.0 (13) | ||
| T classification | 0.007 | 0.017 | |||
| T1–3 | 34 | 93.3 (1) | 49.6 (7) | ||
| T4 | 27 | 74.6 (12) | 49.9 (13) | ||
| N classification | 0.581 | 0.685 | |||
| N 0–1 | 23 | 82.1 (4) | 31.2 (9) | ||
| N 2–3 | 38 | 77.9 (9) | 65.5 (11) | ||
| Histology | 0.408 | 0.890 | |||
| Non-keratinizing carcinoma | 3 | 100 (0) | 66.7 (1) | ||
| Undifferentiated carcinoma | 58 | 74.7 (13) | 47.7 (19) | ||
| EBV DNA load (copies/mL) | 0.331 | 0.022 | |||
| ≤2750 | 31 | 83.6 (5) | 55.5 (6) | ||
| >2750 | 30 | 75.3 (8) | 44.7 (14) | ||
| Imaging biomarker | |||||
| TLG (g/mL × mL) | 0.0001 | 0.0002 | |||
| ≤170 | 43 | 87.7 (4) | 56.6 (6) | ||
| >170 | 18 | 46.3 (9) | 36.5 (14) | ||
| SUVmax (g/mL) | 0.419 | 0.505 | |||
| ≤11 | 32 | 71.2 (8) | 70.1 (9) | ||
| >11 | 29 | 81.3 (5) | 27.0 (11) | ||
| ADCmin (10−6 mm2/s) | 0.191 | 0.01 | |||
| ≤200 | 16 | 62.5 (5) | 38.5 (9) | ||
| >200 | 45 | 79.9 (8) | 48.9 (11) | ||
| Ktrans (10−3 min−1) | 0.015 | 0.916 | |||
| ≤680 | 16 | 60.2 (7) | 64.8 (5) | ||
| >680 | 45 | 86.7 (6) | 47.8 (15) | ||
| Kep (10−3 min−1) | 0.041 | 0.081 | |||
| ≤950 | 37 | 75.4 (11) | 0 (15) | ||
| >950 | 24 | 91.7 (2) | 76.0 (5) | ||
| Ve (10−3) | 0.224 | 0.049 | |||
| ≤140 | 37 | 80.7 (6) | 55.4 (9) | ||
| >140 | 24 | 68.8 (7) | 49.8 (11) | ||
| iAUC | 0.013 | 0.952 | |||
| ≤650 | 20 | 53.3 (8) | 66.1 (6) | ||
| >650 | 41 | 87.8 (5) | 47.3 (14) | ||
| TLG/iAUC | <0.0001 | 0.004 | |||
| ≤0.26 | 47 | 86.8 (5) | 52.6 (12) | ||
| >0.26 | 14 | 33.3 (8) | 42.9 (8) | ||
| Risk Factor | Cox-Regression Analysis with TLG and iAUC | Cox-Regression Analysis with TLG/iAUC | ||||||
|---|---|---|---|---|---|---|---|---|
| OS | RFS | OS | RFS | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| T classification | - | ns | - | ns | - | ns | - | ns |
| EBV DNA load | - | N/A | 2.65 (1.01-6.97) | 0.049 | - | N/A | 3.05 (1.14–8.18) | 0.027 |
| TLG | 6.70 (2.05–12.87) | 0.002 | 4.41 (1.76–11.09) | 0.002 | - | N/A | - | N/A |
| ADCmin | - | N/A | - | ns | - | N/A | - | ns |
| Ktrans | - | ns | - | N/A | - | ns | - | N/A |
| Kep | - | ns | - | N/A | - | ns | - | N/A |
| Ve | - | N/A | - | ns | - | N/A | 2.85 (1.13–7.18) | 0.027 |
| iAUC | 0.30 (0.10–0.92) | 0.036 | - | N/A | - | N/A | - | N/A |
| TLG/iAUC | - | N/A | - | N/A | 6.13 (1.91–19.68) | 0.002 | 2.92 (1.14–7.43) | 0.025 |
| Model | OS | RFS | ||
|---|---|---|---|---|
| c-Index | 95% CI | c-Index | 95% CI | |
| TNM stage | 0.62 | 0.50–0.75 | 0.61 | 0.51–0.72 |
| TLG | 0.74 | 0.63–0.86 | 0.71 | 0.60–0.81 |
| iAUC | 0.65 | 0.51–0.78 | 0.52 | 0.40–0.63 |
| Prognostic model | ||||
| TLG + iAUC | 0.79 * | 0.68–0.90 | N/A | N/A |
| TLG/iAUC | 0.74 | 0.61–0.86 | N/A | N/A |
| EBV DNA + TLG | N/A | N/A | 0.74 | 0.63–0.84 |
| EBV DNA + Ve + TLG/iAUC | N/A | N/A | 0.76 ** | 0.67–0.85 |
| Model | Training | Bootstrapping Validation | ||||||
|---|---|---|---|---|---|---|---|---|
| OS | RFS | OS | RFS | |||||
| c-Index | 95% CI | c-Index | 95% CI | c-Index | 95% CI | c-Index | 95% CI | |
| TNM stage | 0.62 | 0.50–0.75 | 0.61 | 0.51–0.72 | 0.63 | 0.51–0.75 | 0.62 | 0.46–0.71 |
| Prognostic model | ||||||||
| TLG + iAUC | 0.79 | 0.68–0.90 | N/A | N/A | 0.80 | 0.66–0.90 | N/A | N/A |
| EBV DNA + Ve + TLG/iAUC | N/A | N/A | 0.76 | 0.67–0.85 | N/A | N/A | 0.76 | 0.66–0.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, S.-C.; Yeh, C.-H.; Chang, J.T.-C.; Chang, K.-P.; Wang, J.-H.; Ng, S.-H. Combing MRI Perfusion and 18F-FDG PET/CT Metabolic Biomarkers Helps Predict Survival in Advanced Nasopharyngeal Carcinoma: A Prospective Multimodal Imaging Study. Cancers 2021, 13, 1550. https://doi.org/10.3390/cancers13071550
Chan S-C, Yeh C-H, Chang JT-C, Chang K-P, Wang J-H, Ng S-H. Combing MRI Perfusion and 18F-FDG PET/CT Metabolic Biomarkers Helps Predict Survival in Advanced Nasopharyngeal Carcinoma: A Prospective Multimodal Imaging Study. Cancers. 2021; 13(7):1550. https://doi.org/10.3390/cancers13071550
Chicago/Turabian StyleChan, Sheng-Chieh, Chih-Hua Yeh, Joseph Tung-Chieh Chang, Kai-Ping Chang, Jen-Hung Wang, and Shu-Hang Ng. 2021. "Combing MRI Perfusion and 18F-FDG PET/CT Metabolic Biomarkers Helps Predict Survival in Advanced Nasopharyngeal Carcinoma: A Prospective Multimodal Imaging Study" Cancers 13, no. 7: 1550. https://doi.org/10.3390/cancers13071550