Complementary Targeting of Rb Phosphorylation and Growth in Cervical Cancer Cell Cultures and a Xenograft Mouse Model by SHetA2 and Palbociclib
Abstract
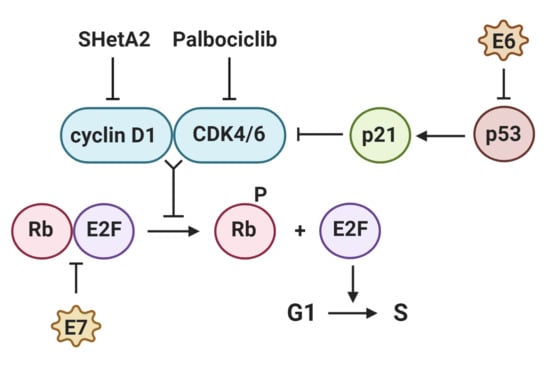
:1. Introduction
2. Results
2.1. SHetA2 and Palbociclib Act Synergistically in Cervical Cancer Cell Lines
2.2. SHetA2 and Palbociclib Regulation of Cyclin D1 and Phosphorylation of Rb
2.3. Additive Interaction of SHetA2 and Palbociclib In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Drugs
4.3. MTT- Cytotoxicity Assay and Drug Interaction Analysis
4.4. Western Blots
4.5. ELISA
4.6. Animal Model
4.7. Histochemical and Immunohistochemical Analysis of Xenograft Tumors
4.8. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALKP | alkaline phosphatase level test |
| AST | aspartate aminotransferase |
| BCA | bicinchoninic acid |
| BUN | blood urea nitrogen |
| CDK | cyclin dependent kinase |
| CI | combination index |
| CREA | creatinine |
| DRI | drug reduction index |
| DAB | 3, 3′-diaminobenzidine tetrahydrochloride |
| E6 | Early 6 |
| E7 | Early 7 |
| ECL | enhanced chemiluminescence |
| Flex-Het | flexible heteroarotinoid |
| GEE | generalized estimating equations |
| H&E | hematoxylin and eosin |
| HPV | human papilloma virus |
| HPV+ | HPV positive |
| HPV− | HPV negative |
| HRP | horse radish peroxidase |
| HSPA | heat shock protein A |
| IC50 | half maximal inhibitory concentration |
| NNK | nicotine-derived nitrosamine ketone |
| NOAEL | no observed adverse event level |
| PBS | phosphate buffered saline |
| PECAM-1 | platelet endothelial cell adhesion molecule-1 |
| phosph-RB | phosphorylated Rb |
| PVDF | polyvinylidene fluoride |
| RB | retinoblastoma |
| STR | short tandem repeats |
| TBST | Tris Buffered Saline with Tween |
| VEGF | vascular endothelial growth factor |
References
- Cibula, D.; Potter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie Meder, C.; Kohler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother. Oncol. 2018, 127, 404–416. [Google Scholar] [CrossRef] [PubMed]
- NCC Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Cervical Cancer. Available online: https://www.nccn.org/ (accessed on 2 December 2019).
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017, 390, 1654–1663. [Google Scholar] [CrossRef] [Green Version]
- Society, A.C. Cancer Facts & Figures 2019; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Senkomago, V.; Duran, D.; Loharikar, A.; Hyde, T.B.; Markowitz, L.E.; Unger, E.R.; Saraiya, M. CDC Activities for Improving Implementation of Human Papillomavirus Vaccination, Cervical Cancer Screening, and Surveillance Worldwide. Emerg. Infect. Dis. 2017, 23, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.K.; Balsitis, S.; Brake, T.; Lambert, P.F. Human papillomavirus E7 oncoprotein overrides the tumor suppressor activity of p21Cip1 in cervical carcinogenesis. Cancer Res. 2009, 69, 5656–5663. [Google Scholar] [CrossRef] [Green Version]
- Kato, J.; Matsushime, H.; Hiebert, S.W.; Ewen, M.E.; Sherr, C.J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993, 7, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Liao, S.; Xiao, S.; Chen, H.; Zhang, M.; Chen, Z.; Long, Y.; Gao, L.; He, J.; Ge, Y.; Yi, W.; et al. The receptor for activated protein kinase C promotes cell growth, invasion and migration in cervical cancer. Int. J. Oncol. 2017, 51, 1497–1507. [Google Scholar] [CrossRef] [Green Version]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30, 55–70. [Google Scholar] [CrossRef]
- Thomas, M.; Pim, D.; Banks, L. The role of the E6-p53 interaction in the molecular pathogenesis of HPV. Oncogene 1999, 18, 7690–7700. [Google Scholar] [CrossRef] [Green Version]
- Masamha, C.P.; Benbrook, D.M. Cyclin D1 degradation is sufficient to induce G1 cell cycle arrest despite constitutive expression of cyclin E2 in ovarian cancer cells. Cancer Res. 2009, 69, 6565–6572. [Google Scholar] [CrossRef] [Green Version]
- Benbrook, D.M.; Nammalwar, B.; Long, A.; Matsumoto, H.; Singh, A.; Bunce, R.A.; Berlin, K.D. SHetA2 interference with mortalin binding to p66shc and p53 identified using drug-conjugated magnetic microspheres. Invest. New Drugs 2014, 32, 412–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Hannafon, B.; Gill, L.; Kelly, W.; Benbrook, D. Flex-Hets differentially induce apoptosis in cancer over normal cells by directly targeting mitochondria. Mol. Cancer Ther. 2007, 6, 1814–1822. [Google Scholar] [CrossRef] [Green Version]
- Myers, T.; Chengedza, S.; Lightfoot, S.; Pan, Y.; Dedmond, D.; Cole, L.; Tang, Y.; Benbrook, D.M. Flexible heteroarotinoid (Flex-Het) SHetA2 inhibits angiogenesis in vitro and in vivo. Invest New Drugs 2009, 27, 304–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, F.; Lapidari, P.; Quaquarini, E.; Tagliaferri, B.; Sottotetti, F.; Palumbo, R. Palbociclib in metastatic breast cancer: Current evidence and real-life data. Drugs Context 2019, 8, 212579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabirov, K.K.; Kapetanovic, I.M.; Benbrook, D.M.; Dinger, N.; Mankovskaya, I.; Zakharov, A.; Detrisac, C.; Pereira, M.; Martin-Jimenez, T.; Onua, E.; et al. Oral toxicity and pharmacokinetic studies of SHetA2, a new chemopreventive agent, in rats and dogs. Drug Chem. Toxicol. 2013, 36, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Masamha, C.P.; Chengedza, S.; Berlin, K.D.; Lightfoot, S.; He, F.; Benbrook, D.M. Development of flexible-heteroarotinoids for kidney cancer. Mol. Cancer Ther. 2009, 8, 1227–1238. [Google Scholar] [CrossRef] [Green Version]
- Benbrook, D.M.; Janakiram, N.B.; Chandra, V.; Pathuri, G.; Madka, V.; Stratton, N.C.; Masamha, C.P.; Farnsworth, C.N.; Garcia-Contreras, L.; Hatipoglu, M.K.; et al. Development of a dietary formulation of the SHetA2 chemoprevention drug for mice. Invest. New Drugs 2018, 36, 561–570. [Google Scholar] [CrossRef]
- Varella, L.; Eziokwu, A.S.; Jia, X.; Kruse, M.; Moore, H.C.F.; Budd, G.T.; Abraham, J.; Montero, A.J. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res. Treat. 2019, 176, 429–434. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C.; Talalay, P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chengedza, S.; Benbrook, D.M. NF-kappaB is involved in SHetA2 circumvention of TNF-alpha resistance, but not induction of intrinsic apoptosis. Anticancer Drugs 2010, 21, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.H.; Pfahl, M.; Lotan, R. Induction of apoptosis by the synthetic retinoid MX3350-1 through extrinsic and intrinsic pathways in head and neck squamous carcinoma cells. Oncogene 2005, 24, 3669–3677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lans, T.E.; Bartlett, D.L.; Libutti, S.K.; Gnant, M.F.; Liewehr, D.J.; Venzon, D.J.; Turner, E.M.; Alexander, H.R. Role of tumor necrosis factor on toxicity and cytokine production after isolated hepatic perfusion. Clin. Cancer Res. 2001, 7, 784–790. [Google Scholar] [PubMed]
- Lin, Y.; Liu, X.; Yue, P.; Benbrook, D.M.; Berlin, K.D.; Khuri, F.R.; Sun, S.Y. Involvement of c-FLIP and survivin down-regulation in flexible heteroarotinoid-induced apoptosis and enhancement of TRAIL-initiated apoptosis in lung cancer cells. Mol. Cancer Ther. 2008, 7, 3556–3565. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.D.; Chen, S.; Yue, P.; Zou, W.; Benbrook, D.M.; Liu, S.; Le, T.C.; Berlin, K.D.; Khuri, F.R.; Sun, S.Y. CAAT/enhancer binding protein homologous protein-dependent death receptor 5 induction is a major component of SHetA2-induced apoptosis in lung cancer cells. Cancer Res. 2008, 68, 5335–5344. [Google Scholar] [CrossRef] [Green Version]
- Nomanbhoy, T.K.; Sharma, G.; Brown, H.; Wu, J.; Aban, A.; Vogeti, S.; Alemayehu, S.; Sykes, M.; Rosenblum, J.S.; Kozarich, J.W. Chemoproteomic Evaluation of Target Engagement by the Cyclin-Dependent Kinase 4 and 6 Inhibitor Palbociclib Correlates with Cancer Cell Response. Biochemistry 2016, 55, 5434–5441. [Google Scholar] [CrossRef]
- Sumi, N.J.; Kuenzi, B.M.; Knezevic, C.E.; Remsing Rix, L.L.; Rix, U. Chemoproteomics Reveals Novel Protein and Lipid Kinase Targets of Clinical CDK4/6 Inhibitors in Lung Cancer. ACS Chem. Biol. 2015, 10, 2680–2686. [Google Scholar] [CrossRef] [Green Version]
- Privratsky, J.R.; Newman, P.J. PECAM-1: Regulator of endothelial junctional integrity. Cell Tissue Res. 2014, 355, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Kollmann, K.; Heller, G.; Schneckenleithner, C.; Warsch, W.; Scheicher, R.; Ott, R.G.; Schafer, M.; Fajmann, S.; Schlederer, M.; Schiefer, A.I.; et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell. 2013, 24, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Benbrook, D.M.; Guruswamy, S.; Wang, Y.; Sun, Z.; Mohammed, A.; Zhang, Y.; Li, Q.; Rao, C.V. Chemoprevention of colon and small intestinal tumorigenesis in APC(min/+) mice by SHetA2 (NSC721689) without toxicity. Cancer Prev. Res. 2013, 6, 908–916. [Google Scholar] [CrossRef] [Green Version]
- Doppalapudi, R.S.; Riccio, E.S.; Davis, Z.; Menda, S.; Wang, A.; Du, N.; Green, C.; Kopelovich, L.; Rao, C.V.; Benbrook, D.M.; et al. Genotoxicity of the cancer chemopreventive drug candidates CP-31398, SHetA2, and phospho-ibuprofen. Mutat. Res. 2012, 746, 78–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Cho, M.; Yu, K.W.; Waisman, J.; Yuan, Y.; Mortimer, J. A single institution experience with palbociclib toxicity requiring dose modifications. Breast Cancer Res. Treat. 2018, 168, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Brown, C.W.; Berlin, K.D.; Dhar, A.; Guruswamy, S.; Brown, D.; Gardner, G.J.; Birrer, M.J.; Benbrook, D.M. Synthesis of flexible sulfur-containing heteroarotinoids that induce apoptosis and reactive oxygen species with discrimination between malignant and benign cells. J. Med. Chem. 2004, 47, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madka, V.; Kumar, G.; Pathuri, G.; Zhang, Y.; Lightfoot, S.; Asch, A.S.; Mohammed, A.; Steele, V.E.; Rao, C.V. Bisphosphonates Zometa and Fosamax Synergize with Metformin to Prevent AOM-induced Colon Cancer in F344 Rat Model. Cancer Prev. Res. 2019. [Google Scholar] [CrossRef] [Green Version]






| Cell Line | IC50 | DRI | CI | Interpretation | ||
|---|---|---|---|---|---|---|
| IC50 | IC95 | IC50 | IC95 | |||
| SiHa | 5.0 µM SHetA2 11.5 µM palbociclib | 11.03 µM 8.73 µM | 11.47 µM 580.40 µM | 0.205 | 0.089 | Very Strong Synergism |
| CaSki | 5.0 µM SHetA2 13.0 µM palbociclib | 8.75 µM 9.60 µM | 3.07 µM 28.57 µM | 0.218 | 0.361 | Synergism |
| C33A | 1.6 µM SHetA2 5.2 µM palbociclib | 2.29 µM 6.27 µM | 8.31 µM 145.90 µM | 0.205 | 0.116 | Strong Synergism |
| End Point | Normal | Control | 60 mg/kg SHetA2 | 100 mg/kg Palbociclib | SHetA2 + Palbociclib | ANOVA |
|---|---|---|---|---|---|---|
| BUN (mg/dL) | 18–29 | 15.67 ± 1.53 | 19.33 ± 3.215 | 12 ± 6.055 | 10.50 ± 1.291 | F = 3.767, p = 0.048 |
| CREA(mg/dL) | 0.2–0.8 | 0.045 ± 0 | 0.197 ± 0.263 | 0.273 ± 0.263 | 0.12 ± 0.05 | F = 0.0580, p = 0.984 |
| ALKP(U/L) | 62–209 | 14.8 ± 17.89 | 25.17 ± 17.89 | 27.75 ± 15.5 | 20 ± 17.89 | F = 0.0784, p = 0.970 |
| AST(U/L) | 59–247 | 131 ± 59.65 | 264 ± 138.7 | 74.75 ± 21.82 | 41.75 ± 18.77 | F = 6.499, p = 0.0074 |
| Organs | Control | 60 mg/kg SHetA2 | 100 mg/kg Palbociclib | Combination | Kruskal-Wallis Test |
|---|---|---|---|---|---|
| Kidney | 0.017 ± 0.001 | 0.018 ± 0.002 | 0.016 ± 0.002 | 0.010 ± 0.002 | p = 0.850 |
| Liver | 0.053 ± 0.002 | 0.056 ± 0.007 | 0.050 ± 0.002 | 0.050 ± 0.008 | p = 0.246 |
| Spleen | 0.012 ± 0.001 | 0.0125 ± 0.001 | 0.010 ± 0 | 0.010 ± 0 | p = 0.281 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy, A.L.; Rai, R.; Isingizwe, Z.R.; Zhao, Y.D.; Lightfoot, S.A.; Benbrook, D.M. Complementary Targeting of Rb Phosphorylation and Growth in Cervical Cancer Cell Cultures and a Xenograft Mouse Model by SHetA2 and Palbociclib. Cancers 2020, 12, 1269. https://doi.org/10.3390/cancers12051269
Kennedy AL, Rai R, Isingizwe ZR, Zhao YD, Lightfoot SA, Benbrook DM. Complementary Targeting of Rb Phosphorylation and Growth in Cervical Cancer Cell Cultures and a Xenograft Mouse Model by SHetA2 and Palbociclib. Cancers. 2020; 12(5):1269. https://doi.org/10.3390/cancers12051269
Chicago/Turabian StyleKennedy, Amy L., Rajani Rai, Zitha Redempta Isingizwe, Yan Daniel Zhao, Stanley A. Lightfoot, and Doris M. Benbrook. 2020. "Complementary Targeting of Rb Phosphorylation and Growth in Cervical Cancer Cell Cultures and a Xenograft Mouse Model by SHetA2 and Palbociclib" Cancers 12, no. 5: 1269. https://doi.org/10.3390/cancers12051269