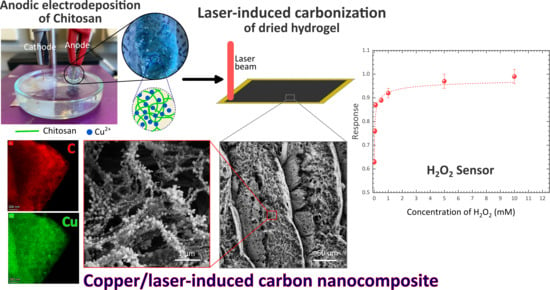
Laser-Induced Copper/Carbon Nanocomposite from Anodically Electrodeposited Chitosan for H2O2 Sensing
Abstract
:1. Introduction
2. Experimental
2.1. Copper/Carbon Nanocomposite Electrode Fabrication
2.2. Characterization
2.3. H2O2 Sensing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirvanimoghaddam, K.; Hamim, S.U.; Akbari, M.K.; Fakhrhoseini, S.M.; Khayyam, H.; Pakseresht, A.H.; Ghasali, E.; Zabet, M.; Munir, K.S.; Jia, S.; et al. Carbon fiber reinforced metal matrix composites: Fabrication processes and properties. Compos. Part A Appl. Sci. Manuf. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (Bio) sensors with biomedical applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Schultes, G.; Schmid-Engel, H.; Schwebke, S.; Werner, U. Granular metal–carbon nanocomposites as piezoresistive sensor films–Part 1: Experimental results and morphology. J. Sens. Sens. Syst. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Zhou, S.; Zhou, J.; Pan, Y.; Wu, Q.; Ping, J. Wearable electrochemical sensors for plant small-molecule detection. Trends Plant Sci. 2024, 29, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Ta, Q.T.H.; Tran, N.M.; Tri, N.N.; Sreedhar, A.; Noh, J.S. Highly surface-active Si-doped TiO2/Ti3C2Tx heterostructure for gas sensing and photodegradation of toxic matters. Chem. Eng. J. 2021, 425, 131437. [Google Scholar] [CrossRef]
- Yu, R.; Chen, L.; Liu, Q.; Lin, J.; Tan, K.L.; Ng, S.C.; Chan, H.S.; Xu, G.Q.; Hor, T.A. Platinum deposition on carbon nanotubes via chemical modification. Chem. Mater. 1998, 10, 718–722. [Google Scholar] [CrossRef]
- Tessonnier, J.P.; Ersen, O.; Weinberg, G.; Pham-Huu, C.; Su, D.S.; Schlogl, R. Selective deposition of metal nanoparticles inside or outside multiwalled carbon nanotubes. ACS Nano 2009, 3, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Mamleyev, E.R.; Falk, F.; Weidler, P.G.; Heissler, S.; Wadhwa, S.; Nassar, O.; Shyam Kumar, C.; Kübel, C.; Wöll, C.; Islam, M.; et al. Polyaramid-based flexible antibacterial coatings fabricated using laser-induced carbonization and copper electroplating. ACS Appl. Mater. Interfaces 2020, 12, 53193–53205. [Google Scholar] [CrossRef]
- Chauhan, G.; Ángeles, A.L.; Gonzalez-González, E.; Kulkarni, M.M.; Cardenas-Benitez, B.; Jiménez, M.F.; Trujillo-de Santiago, G.; Alvarez, M.M.; Madou, M.; Martinez-Chapa, S.O. Nano-spaced Gold on Glassy Carbon Substrate for Controlling Cell Behavior. Adv. Mater. Interfaces 2020, 7, 2000238. [Google Scholar] [CrossRef]
- Islam, M.; Dolle, C.; Sadaf, A.; Weidler, P.G.; Sharma, B.; Eggeler, Y.M.; Mager, D.; Korvink, J.G. Electrospun carbon nanofibre-assisted patterning of metal oxide nanostructures. Microsyst. Nanoeng. 2022, 8, 71. [Google Scholar] [CrossRef]
- Mondal, K.; Ali, M.A.; Singh, C.; Sumana, G.; Malhotra, B.D.; Sharma, A. Highly sensitive porous carbon and metal/carbon conducting nanofiber based enzymatic biosensors for triglyceride detection. Sens. Actuators B Chem. 2017, 246, 202–214. [Google Scholar] [CrossRef]
- Islam, M.; Keck, D.; Martinez-Duarte, R. Architected tungsten carbide electrodes using origami techniques. Adv. Eng. Mater. 2019, 21, 1900290. [Google Scholar] [CrossRef]
- Islam, M.; Arya, N.; Weidler, P.; Korvink, J.; Badilita, V. Electrodeposition of chitosan enables synthesis of copper/carbon composites for H2O2 sensing. Mater. Today Chem. 2020, 17, 100338. [Google Scholar] [CrossRef]
- Rai, P.K.; Gupta, A. Development of durable anticorrosion superhydrophobic electroformed copper tubular structures. J. Manuf. Process. 2023, 85, 236–245. [Google Scholar] [CrossRef]
- Helú, M.A.B.; Liu, L. Rational shaping of hydrogel by electrodeposition under fluid mechanics for electrochemical writing on complex shaped surfaces at microscale. Chem. Eng. J. 2021, 416, 129029. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wang, S.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
- Avcu, E.; Baştan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Fusco, S.; Chatzipirpiridis, G.; Sivaraman, K.M.; Ergeneman, O.; Nelson, B.J.; Pané, S. Chitosan electrodeposition for microrobotic drug delivery. Adv. Healthc. Mater. 2013, 2, 1037–1044. [Google Scholar] [CrossRef]
- Li, B.; Cheng, Y.; Dong, L.; Wang, Y.; Chen, J.; Huang, C.; Wei, D.; Feng, Y.; Jia, D.; Zhou, Y. Nitrogen doped and hierarchically porous carbons derived from chitosan hydrogel via rapid microwave carbonization for high-performance supercapacitors. Carbon 2017, 122, 592–603. [Google Scholar] [CrossRef]
- Ababneh, H.; Hameed, B. Chitosan-derived hydrothermally carbonized materials and its applications: A review of recent literature. Int. J. Biol. Macromol. 2021, 186, 314–327. [Google Scholar] [CrossRef]
- Tian, Y.; Estevez, D.; Wei, H.; Peng, M.; Zhou, L.; Xu, P.; Wu, C.; Yan, M.; Wang, H.; Peng, H.X.; et al. Chitosan-derived carbon aerogels with multiscale features for efficient microwave absorption. Chem. Eng. J. 2021, 421, 129781. [Google Scholar] [CrossRef]
- Shi, N.; Liu, Q.; He, X.; Wang, G.; Chen, N.; Peng, J.; Ma, L. Molecular structure and formation mechanism of hydrochar from hydrothermal carbonization of carbohydrates. Energy Fuels 2019, 33, 9904–9915. [Google Scholar] [CrossRef]
- Shen, Y. A review on hydrothermal carbonization of biomass and plastic wastes to energy products. Biomass Bioenergy 2020, 134, 105479. [Google Scholar] [CrossRef]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene. Accounts Chem. Res. 2018, 51, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, Z.; Liu, P.; Guo, X. Laser-induced graphene based flexible electronic devices. Biosensors 2022, 12, 55. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; James, D.K.; Tour, J.M. Laser-induced graphene: From discovery to translation. Adv. Mater. 2019, 31, 1803621. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Mager, D.; Korvink, J.G.; Islam, M. Unraveling the dependency on multiple passes in laser-induced graphene electrodes for supercapacitor and H2O2 sensing. Mater. Sci. Energy Technol. 2021, 4, 407–412. [Google Scholar] [CrossRef]
- Chyan, Y.; Ye, R.; Li, Y.; Singh, S.P.; Arnusch, C.J.; Tour, J.M. Laser-induced graphene by multiple lasing: Toward electronics on cloth, paper, and food. ACS Nano 2018, 12, 2176–2183. [Google Scholar] [CrossRef]
- Mamleyev, E.R.; Weidler, P.G.; Nefedov, A.; Szabó, D.V.; Islam, M.; Mager, D.; Korvink, J.G. Nano-and microstructured copper/copper oxide composites on laser-induced carbon for enzyme-free glucose sensors. ACS Appl. Nano Mater. 2021, 4, 13747–13760. [Google Scholar] [CrossRef]
- Bachmann, A.L.; Ferris, A.L.; Im, S.; Dickey, M.D.; Lazarus, N. Laser-Induced Graphene from SU-8 Photoresist: Toward Functional Micromolding. ACS Appl. Eng. Mater. 2022, 1, 222–228. [Google Scholar] [CrossRef]
- Wan, Z.; Nguyen, N.T.; Gao, Y.; Li, Q. Laser induced graphene for biosensors. Sustain. Mater. Technol. 2020, 25, e00205. [Google Scholar] [CrossRef]
- Liu, J.; Ji, H.; Lv, X.; Zeng, C.; Li, H.; Li, F.; Qu, B.; Cui, F.; Zhou, Q. Laser-induced graphene (LIG)-driven medical sensors for health monitoring and diseases diagnosis. Microchim. Acta 2022, 189, 54. [Google Scholar] [CrossRef] [PubMed]
- Wanjari, V.P.; Reddy, A.S.; Duttagupta, S.P.; Singh, S.P. Laser-induced graphene-based electrochemical biosensors for environmental applications: A perspective. Environ. Sci. Pollut. Res. 2023, 30, 42643–42657. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X.; Song, W. Physical and chemical sensors on the basis of laser-induced graphene: Mechanisms, applications, and perspectives. ACS Nano 2021, 15, 18708–18741. [Google Scholar] [CrossRef] [PubMed]
- Mamleyev, E.R.; Heissler, S.; Nefedov, A.; Weidler, P.G.; Nordin, N.; Kudryashov, V.V.; Länge, K.; MacKinnon, N.; Sharma, S. Laser-induced hierarchical carbon patterns on polyimide substrates for flexible urea sensors. NPJ Flex. Electron. 2019, 3, 2. [Google Scholar] [CrossRef]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, P.A.; Travas-Sejdic, J. Sensitive, selective, disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, H.; Sun, P.; Sun, C.K.; Huang, H.; Guan, S.; Liu, H.; Zhang, H.; Zhang, C.; Qin, K.R. Laser-induced graphene-based non-enzymatic sensor for detection of hydrogen peroxide. Electroanalysis 2019, 31, 1334–1341. [Google Scholar] [CrossRef]
- Matias, T.A.; de Faria, L.V.; Rocha, R.G.; Silva, M.N.; Nossol, E.; Richter, E.M.; Muñoz, R.A. Prussian blue-modified laser-induced graphene platforms for detection of hydrogen peroxide. Microchim. Acta 2022, 189, 188. [Google Scholar] [CrossRef]
- Fenzl, C.; Nayak, P.; Hirsch, T.; Wolfbeis, O.S.; Alshareef, H.N.; Baeumner, A.J. Laser-scribed graphene electrodes for aptamer-based biosensing. ACS Sens. 2017, 2, 616–620. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, S.; Hu, Z.; Zhang, X.; Yi, N.; Tang, K.; Dexheimer, M.G.; Lian, X.; Wang, Q.; Yang, J.; et al. Laser-induced graphene non-enzymatic glucose sensors for on-body measurements. Biosens. Bioelectron. 2021, 193, 113606. [Google Scholar] [CrossRef]
- Yu, S.; Cui, J.; Wang, J.; Zhong, C.; Wang, X.; Wang, N. Facile fabrication of Cu (II) coordinated chitosan-based magnetic material for effective adsorption of reactive brilliant red from aqueous solution. Int. J. Biol. Macromol. 2020, 149, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Shen, G.; Zheng, W.; Fu, J.; Fu, F.; Hu, X.; Jin, Z.; Liu, X. Remarkable durability of the antibacterial function achieved via a coordination effect of Cu (II) ion and chitosan grafted on cotton fibers. Cellulose 2022, 29, 1003–1015. [Google Scholar] [CrossRef]
- Deng, Z.; Li, L.; Ren, Y.; Ma, C.; Liang, J.; Dong, K.; Liu, Q.; Luo, Y.; Li, T.; Tang, B.; et al. Highly efficient two-electron electroreduction of oxygen into hydrogen peroxide over Cu-doped TiO2. Nano Res. 2022, 15, 3880–3885. [Google Scholar] [CrossRef]
- Alencar, L.M.; Silva, A.W.; Trindade, M.A.; Salvatierra, R.V.; Martins, C.A.; Souza, V.H. One-step synthesis of crumpled graphene fully decorated by copper-based nanoparticles: Application in H2O2 sensing. Sens. Actuators B Chem. 2022, 360, 131649. [Google Scholar] [CrossRef]
- Yuan, K.; Zhang, Y.; Huang, S.; Yang, S.; Zhao, S.; Liu, F.; Peng, Q.; Zhao, Y.; Zhang, G.; Fan, J.; et al. Copper nanoflowers on carbon cloth as a flexible electrode toward both enzymeless electrocatalytic glucose and H2O2. Electroanalysis 2021, 33, 1800–1809. [Google Scholar] [CrossRef]
- Shaner, S.W.; Islam, M.; Kristoffersen, M.B.; Azmi, R.; Heissler, S.; Ortiz-Catalan, M.; Korvink, J.G.; Asplund, M. Skin stimulation and recording: Moving towards metal-free electrodes. Biosens. Bioelectron. X 2022, 11, 100143. [Google Scholar] [CrossRef]
- Cançado, L.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.; Mizusaki, H.; Jorio, A.; Coelho, L.; Magalhães-Paniago, R.; Pimenta, M. General equation for the determination of the crystallite size L a of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Kulyk, B.; Silva, B.F.; Carvalho, A.F.; Silvestre, S.; Fernandes, A.J.; Martins, R.; Fortunato, E.; Costa, F.M. Laser-induced graphene from paper for mechanical sensing. ACS Appl. Mater. Interfaces 2021, 13, 10210–10221. [Google Scholar] [CrossRef]
- Huang, L.; Su, J.; Song, Y.; Ye, R. Laser-induced graphene: En route to smart sensing. Nano-Micro Lett. 2020, 12, 157. [Google Scholar] [CrossRef]
- Barbhuiya, N.H.; Kumar, A.; Singh, S.P. A journey of laser-induced graphene in water treatment. Trans. Indian Natl. Acad. Eng. 2021, 6, 159–171. [Google Scholar] [CrossRef]
- Abdou, S.M.; Moharam, H. Characterization of table salt samples from different origins and ESR detection of the induced effects due to gamma irradiation. Proc. J. Phys. Conf. Ser. 2019, 1253, 012036. [Google Scholar] [CrossRef]
- Dabera, G.D.M.; Walker, M.; Sanchez, A.M.; Pereira, H.J.; Beanland, R.; Hatton, R.A. Retarding oxidation of copper nanoparticles without electrical isolation and the size dependence of work function. Nat. Commun. 2017, 8, 1894. [Google Scholar] [CrossRef] [PubMed]
- Popok, V.N.; Novikov, S.M.; Lebedinskij, Y.Y.; Markeev, A.M.; Andreev, A.A.; Trunkin, I.N.; Arsenin, A.V.; Volkov, V.S. Gas-aggregated copper nanoparticles with long-term plasmon resonance stability. Plasmonics 2021, 16, 333–340. [Google Scholar] [CrossRef]
- Dennison, J.R.; Holtz, M.; Swain, G. Raman spectroscopy of carbon materials. Spectroscopy 1996, 11, 38–45. [Google Scholar]
- Dresselhaus, M.; Jorio, A.; Saito, R. Characterizing graphene, graphite, and carbon nanotubes by Raman spectroscopy. Annu. Rev. Condens. Matter Phys. 2010, 1, 89–108. [Google Scholar] [CrossRef]
- Ribeiro-Soares, J.; Oliveros, M.; Garin, C.; David, M.; Martins, L.; Almeida, C.; Martins-Ferreira, E.; Takai, K.; Enoki, T.; Magalhães-Paniago, R.; et al. Structural analysis of polycrystalline graphene systems by Raman spectroscopy. Carbon 2015, 95, 646–652. [Google Scholar] [CrossRef]
- Pillet, G.; Sapelkin, A.; Bacsa, W.; Monthioux, M.; Puech, P. Size-controlled graphene-based materials prepared by annealing of pitch-based cokes: G band phonon line broadening effects due to high pressure, crystallite size, and merging with D’ band. J. Raman Spectrosc. 2019, 50, 1861–1866. [Google Scholar] [CrossRef]
- Beyssac, O.; Lazzeri, M. Application of Raman spectroscopy to the study of graphitic carbons in the Earth Sciences. Eur. Mineral. Union Notes Mineral. 2012, 12, 415–454. [Google Scholar]
- Sole, C.; Drewett, N.E.; Hardwick, L.J. In situ Raman study of lithium-ion intercalation into microcrystalline graphite. Faraday Discuss. 2014, 172, 223–237. [Google Scholar] [CrossRef]
- Tian, W.; Ding, X.; Jiang, F.; Du, X.; Shi, J.; Zhang, J. Green Preparation of Cu Nanoparticles via Gallic Acid Applied to H2O2 Detection. J. Electron. Mater. 2022, 51, 1752–1758. [Google Scholar] [CrossRef]
- Ammara, S.; Shamaila, S.; Bokhari, A.; Sabah, A. Nonenzymatic glucose sensor with high performance electrodeposited nickel/copper/carbon nanotubes nanocomposite electrode. J. Phys. Chem. Solids 2018, 120, 12–19. [Google Scholar] [CrossRef]
- Xie, L.; Asiri, A.M.; Sun, X. Monolithically integrated copper phosphide nanowire: An efficient electrocatalyst for sensitive and selective nonenzymatic glucose detection. Sens. Actuators B Chem. 2017, 244, 11–16. [Google Scholar] [CrossRef]
- Lee, W.C.; Kim, K.B.; Gurudatt, N.; Hussain, K.K.; Choi, C.S.; Park, D.S.; Shim, Y.B. Comparison of enzymatic and non-enzymatic glucose sensors based on hierarchical Au-Ni alloy with conductive polymer. Biosens. Bioelectron. 2019, 130, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Tan, X.; Tang, D.; Li, J.; Ma, J. A tale of two metal ions: Contrasting behaviors of high oxidation states of Cu and Mn in a bicarbonate–H2O2 system. Environ. Sci. Water Res. Technol. 2021, 7, 479–486. [Google Scholar] [CrossRef]
- Alegret, S.; Merkoçi, A. Electrochemical Sensor Analysis; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Molnár, Á. The use of chitosan-based metal catalysts in organic transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Varma, A.; Deshpande, S.; Kennedy, J. Metal complexation by chitosan and its derivatives: A review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Mei, L.; Zhang, P.; Chen, J.; Chen, D.; Quan, Y.; Gu, N.; Zhang, G.; Cui, R. Non-enzymatic sensing of glucose and hydrogen peroxide using a glassy carbon electrode modified with a nanocomposite consisting of nanoporous copper, carbon black and nafion. Microchim. Acta 2016, 183, 1359–1365. [Google Scholar] [CrossRef]
- Rezaei, B.; Askarpour, N.; Ghiaci, M.; Niyazian, F.; Ensafi, A. Synthesis of functionalized MWCNTs decorated with copper nanoparticles and its application as a sensitive sensor for amperometric detection of H2O2. Electroanalysis 2015, 27, 1457–1465. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Y.; Li, X.; Zhao, J.; Ren, Z.; Pang, H. Nitrogen-Doped Carbon–Copper Nanohybrids as Electrocatalysts in H2O2 and Glucose Sensing. ChemElectroChem 2014, 1, 799–807. [Google Scholar] [CrossRef]
- Nia, P.M.; Woi, P.M.; Alias, Y. Facile one-step electrochemical deposition of copper nanoparticles and reduced graphene oxide as nonenzymatic hydrogen peroxide sensor. Appl. Surf. Sci. 2017, 413, 56–65. [Google Scholar]
- Temur, E.; Eryiğit, M.; Urhan, B.K.; Demir, Ü.; Özer, T.Ö. Cu/Electrochemically reduced graphene oxide layered nanocomposite for non-enzymatic H2O2 sensor. Mater. Today Proc. 2021, 46, 6971–6975. [Google Scholar] [CrossRef]






| Materials | Synthesis Method | Linear Range | Sensitivity | Reference |
|---|---|---|---|---|
| Cu/carbon black | Physical mixing | 0.003–2.338 mM | 3.91 μA·cm−2·mM−1 | [68] |
| Cu/MWCNT 1 | Chemical reduction | 0.5–10,000 μM/L | 0.37 μA·L·μM−1 | [69] |
| N-doped Carbon/Cu | Calcination | 0.1–0.9 mM | 1.27 mA·mM−1 | [70] |
| Cu-rGO 2 | Electrodeposition | 0.1–18 mM | 119.75 μA·mM−1 | [71] |
| Cu-rGO | Electrochemical reduction | 0.01–1 mM | 20 μA·cm−2·mM−1 | [72] |
| Cu-Graphene | Spray pyrolysis | 32–803 μM/L | 370 μA·L·cm−2·mM−1 | [44] |
| Cu/carbon | Anodic electrodeposition and pyrolysis | 0.1–3 mM | 58.9 μA·cm−2·mM−1 | [13] |
| Cu/LIC | Anodic electrodeposition and laser carbonization | 0.01–1 mM | 2.65 mM−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, U.; Rai, P.K.; Gupta, A.; Korvink, J.G.; Badilita, V.; Islam, M. Laser-Induced Copper/Carbon Nanocomposite from Anodically Electrodeposited Chitosan for H2O2 Sensing. C 2024, 10, 28. https://doi.org/10.3390/c10020028
Zafar U, Rai PK, Gupta A, Korvink JG, Badilita V, Islam M. Laser-Induced Copper/Carbon Nanocomposite from Anodically Electrodeposited Chitosan for H2O2 Sensing. C. 2024; 10(2):28. https://doi.org/10.3390/c10020028
Chicago/Turabian StyleZafar, Usama, Prince Kumar Rai, Ankur Gupta, Jan G. Korvink, Vlad Badilita, and Monsur Islam. 2024. "Laser-Induced Copper/Carbon Nanocomposite from Anodically Electrodeposited Chitosan for H2O2 Sensing" C 10, no. 2: 28. https://doi.org/10.3390/c10020028