Mesencephalic Locomotor Region and Presynaptic Inhibition during Anticipatory Postural Adjustments in People with Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants
2.3. Study Procedures
2.4. Outcome Assessments
2.4.1. Clinical Assessments
2.4.2. Behavioral Assessments
2.4.3. Test and Conditioned H-Reflexes
2.4.4. Assessment of PSI of the Soleus Muscle during APA for Step Initiation
2.4.5. Beta of the BOLD Signal Change of Locomotor Regions during Step Initiation
2.5. Statistical Analyses
3. Results
3.1. Participants
3.2. MLR Activity and APA Amplitude during Step Initiation Explain the Loss of PSI of the Soleus Muscle for Step Initiation
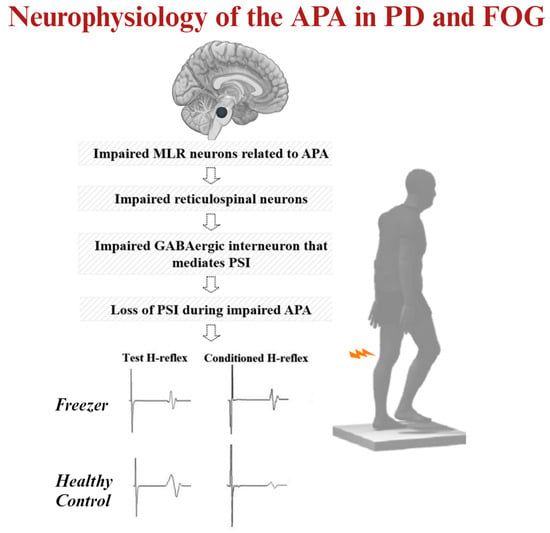
4. Discussion
4.1. Why Do MLR Activity and APA Amplitudes Explain the Loss of PSI of the Soleus Muscle during Step Initiation in Freezers?
4.2. Future Directions for Treatment Strategies
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amboni, M.; Stocchi, F.; Abbruzzese, G.; Morgante, L.; Onofrj, M.; Ruggieri, S.; Tinazzi, M.; Zappia, M.; Attar, M.; Colombo, D.; et al. Prevalence and associated features of self-reported freezing of gait in Parkinson disease: The DEEP FOG study. Park. Relat. Disord. 2015, 21, 644–649. [Google Scholar] [CrossRef]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and freezing of gait in Parkinson’s disease: A review of two interconnected, episodic phenomena. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Bloem, B.R.; Giladi, N.; Hallett, M.; Horak, F.B.; Nieuwboer, A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011, 10, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Yiou, E.; Caderby, T.; Delafontaine, A.; Fourcade, P.; Honeine, J.L. Balance control during gait initiation: State-of-the-art and research perspectives. World J. Orthop. 2017, 8, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Lira, J.L.O.; Ugrinowitsch, C.; Coelho, D.B.; Teixeira, L.A.; de Lima-Pardini, A.C.; Magalhaes, F.H.; Barbosa, E.R.; Horak, F.B.; Silva-Batista, C. Loss of presynaptic inhibition for step initiation in parkinsonian individuals with freezing of gait. J. Physiol. 2020, 598, 1611–1624. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.G.; Nutt, J.G.; Horak, F.B. Recovery from Multiple APAs Delays Gait Initiation in Parkinson’s Disease. Front. Hum. Neurosci. 2017, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Fling, B.W.; Cohen, R.G.; Mancini, M.; Nutt, J.G.; Fair, D.A.; Horak, F.B. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 2013, 136 Pt 8, 2405–2418. [Google Scholar] [CrossRef]
- Filho, S.S.; Coelho, D.B.; Ugrinowitsch, C.; de Souza, C.R.; Magalhaes, F.H.; de Lima-Pardini, A.C.; de Oliveira, E.M.B.; Mattos, E.; Teixeira, L.A.; Silva-Batista, C. Age-Related Changes in Presynaptic Inhibition During Gait Initiation. J. Gerontol. Ser. A 2021, 76, 568–575. [Google Scholar] [CrossRef]
- Knikou, M. The H-reflex as a probe: Pathways and pitfalls. J. Neurosci. Methods 2008, 171, 1–12. [Google Scholar] [CrossRef]
- Fink, A.J.; Croce, K.R.; Huang, Z.J.; Abbott, L.F.; Jessell, T.M.; Azim, E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature 2014, 509, 43–48. [Google Scholar] [CrossRef]
- Capaday, C.; Lavoie, B.A.; Comeau, F. Differential effects of a flexor nerve input on the human soleus H-reflex during standing versus walking. Can. J. Physiol. Pharmacol. 1995, 73, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Hultborn, H.; Meunier, S.; Morin, C.; Pierrot-Deseilligny, E. Assessing changes in presynaptic inhibition of I a fibres: A study in man and the cat. J. Physiol. 1987, 389, 729–756. [Google Scholar] [CrossRef]
- Katz, R.; Meunier, S.; Pierrot-Deseilligny, E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain 1988, 111 Pt 2, 417–437. [Google Scholar] [CrossRef] [PubMed]
- Rudomin, P.; Schmidt, R.F. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp. Brain Res. 1999, 129, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Sirois, J.; Frigon, A.; Gossard, J.P. Independent control of presynaptic inhibition by reticulospinal and sensory inputs at rest and during rhythmic activities in the cat. J. Neurosci. 2013, 33, 8055–8067. [Google Scholar] [CrossRef]
- Baudry, S.; Duchateau, J. Age-related influence of vision and proprioception on Ia presynaptic inhibition in soleus muscle during upright stance. J. Physiol. 2012, 590, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Fling, B.W.; Cohen, R.G.; Mancini, M.; Carpenter, S.D.; Fair, D.A.; Nutt, J.G.; Horak, F.B. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS ONE 2014, 9, e100291. [Google Scholar] [CrossRef]
- Sinnamon, H.M.; Jassen, A.K.; Vita, L.A. Brainstem regions with neuronal activity patterns correlated with priming of locomotor stepping in the anesthetized rat. Neuroscience 2000, 99, 77–91. [Google Scholar] [CrossRef]
- Gurfinkel, V.S.; Lipshits, M.I.; Lestienne, F.G. Anticipatory neck muscle activity associated with rapid arm movements. Neurosci. Lett. 1988, 94, 104–108. [Google Scholar] [CrossRef]
- Viallet, F.; Massion, J.; Massarino, R.; Khalil, R. Coordination between posture and movement in a bimanual load lifting task: Putative role of a medial frontal region including the supplementary motor area. Exp. Brain Res. 1992, 88, 674–684. [Google Scholar] [CrossRef]
- Garcia-Rill, E.; Skinner, R.D. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res. 1987, 411, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, R.; Kojima, S.; Edama, M.; Onishi, H. Activation of the Supplementary Motor Areas Enhances Spinal Reciprocal Inhibition in Healthy Individuals. Brain Sci. 2020, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Schepens, B.; Drew, T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J. Neurophysiol. 2004, 92, 2217–2238. [Google Scholar] [CrossRef]
- de Lima-Pardini, A.C.; de Azevedo Neto, R.M.; Coelho, D.B.; Boffino, C.C.; Shergill, S.S.; de Oliveira Souza, C.; Brant, R.; Barbosa, E.R.; Cardoso, E.F.; Teixeira, L.A.; et al. An fMRI-compatible force measurement system for the evaluation of the neural correlates of step initiation. Sci. Rep. 2017, 7, 43088. [Google Scholar] [CrossRef]
- Silva-Batista, C.; de Lima-Pardini, A.C.; Nucci, M.P.; Coelho, D.B.; Batista, A.; Piemonte, M.E.P.; Barbosa, E.R.; Teixeira, L.A.; Corcos, D.M.; Amaro, E.; et al. A Randomized, Controlled Trial of Exercise for Parkinsonian Individuals with Freezing of Gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2020, 35, 1607–1617. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Matar, E.; Ward, P.B.; Bolitho, S.J.; Gilat, M.; Pearson, M.; Naismith, S.L.; Lewis, S.J. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain 2013, 136 Pt 4, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Nieuwboer, A.; Rochester, L.; Herman, T.; Vandenberghe, W.; Emil, G.E.; Thomaes, T.; Giladi, N. Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson’s disease and their carers. Gait Posture 2009, 30, 459–463. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Seto, E.; Sela, G.; McIlroy, W.E.; Black, S.E.; Staines, W.R.; Bronskill, M.J.; McIntosh, A.R.; Graham, S.J. Quantifying head motion associated with motor tasks used in fMRI. Neuroimage 2001, 14, 284–297. [Google Scholar] [CrossRef]
- Fahn, S.; Elton, R.; UPDRS Program Members. Unified Parkinson’s disease rating scale. In Recent Developments in Parkinson’s Disease; Fahn, S., Marsden, C.D., Goldstein, M., Calne, D.B., Eds.; Macmillan Healthcare Information: Princeton, NJ, USA, 1987; Volume 2, pp. 153–163, 293–304. [Google Scholar]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Troyer, A.K.; Leach, L.; Strauss, E. Aging and response inhibition: Normative data for the Victoria Stroop Test. Aging Neuropsychol. Cogn. 2006, 13, 20–35. [Google Scholar] [CrossRef]
- Mancini, M.; Smulders, K.; Cohen, R.G.; Horak, F.B.; Giladi, N.; Nutt, J.G. The clinical significance of freezing while turning in Parkinson’s disease. Neuroscience 2017, 343, 222–228. [Google Scholar] [CrossRef]
- Crone, C.; Hultborn, H.; Mazieres, L.; Morin, C.; Nielsen, J.; Pierrot-Deseilligny, E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: A study in man and the cat. Exp. Brain Res. 1990, 81, 35–45. [Google Scholar] [CrossRef]
- Crone, C.; Hultborn, H.; Jespersen, B.; Nielsen, J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J. Physiol. 1987, 389, 163–185. [Google Scholar] [CrossRef]
- Patikas, D.A.; Kotzamanidis, C.; Robertson, C.T.; Koceja, D.M. The effect of the ankle joint angle in the level of soleus Ia afferent presynaptic inhibition. Electromyogr. Clin. Neurophysiol. 2004, 44, 503–511. [Google Scholar] [PubMed]
- Silva-Batista, C.; Mattos, E.C.; Corcos, D.M.; Wilson, J.M.; Heckman, C.J.; Kanegusuku, H.; Piemonte, M.E.; Tulio de Mello, M.; Forjaz, C.; Roschel, H.; et al. Resistance training with instability is more effective than resistance training in improving spinal inhibitory mechanisms in Parkinson’s disease. J. Appl. Physiol. 2017, 122, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.B. Presynaptic inhibition in humans. Prog. Neurobiol. 1995, 47, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Knikou, M.; Taglianetti, C. On the methods employed to record and measure the human soleus H-reflex. Somatosens. Mot. Res. 2006, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Iles, J.F. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J. Physiol. 1996, 491 Pt 1, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Earles, D.; Vardaxis, V.; Koceja, D. Regulation of motor output between young and elderly subjects. Clin. Neurophysiol. 2001, 112, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Geertsen, S.S.; Lundbye-Jensen, J.; Nielsen, J.B. Increased central facilitation of antagonist reciprocal inhibition at the onset of dorsiflexion following explosive strength training. J. Appl. Physiol 2008, 105, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Baudry, S.; Lecoeuvre, G.; Duchateau, J. Age-related changes in the behavior of the muscle-tendon unit of the gastrocnemius medialis during upright stance. J. Appl. Physiol. 2012, 112, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Yuan, Y.; Zhang, L.; Ding, J.; Wang, J.; Zhang, J.; Zhang, K.; Wang, J. Alterations of functional and structural connectivity of freezing of gait in Parkinson’s disease. J. Neurol. 2016, 263, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Amboni, M.; Esposito, F.; Russo, A.; Picillo, M.; Marcuccio, L.; Pellecchia, M.T.; Vitale, C.; Cirillo, M.; Tedeschi, G.; et al. Resting-state brain connectivity in patients with Parkinson’s disease and freezing of gait. Park. Relat. Disord. 2012, 18, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.L.; Leenders, K.L. Brain imaging in patients with freezing of gait. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23 (Suppl. 2), S461–S467. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Jenkinson, M.; Woolrich, M.W.; Beckmann, C.F.; Behrens, T.E.; Johansen-Berg, H.; Bannister, P.R.; De Luca, M.; Drobnjak, I.; Flitney, D.E.; et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004, 23 (Suppl. S1), S208–S219. [Google Scholar] [CrossRef] [PubMed]
- Poldrack, R.A.; Fletcher, P.C.; Henson, R.N.; Worsley, K.J.; Brett, M.; Nichols, T.E. Guidelines for reporting an fMRI study. Neuroimage 2008, 40, 409–414. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Buschbacher, R.M. Normal range for H-reflex recording from the calf muscles. Am J. Phys. Med. Rehabil. 1999, 78 (Suppl. 6), S75–S79. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Shindo, M.; Yanagawa, S.; Yoshida, T.; Momoi, H.; Yanagisawa, N. Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp. Brain Res. 1995, 104, 167–170. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Carré, G.C.G.; García Márquez, J.R.; Gruber, B.; Lafourcade, B.; Leitao, P.J.; Münkemüller, T.; McClean, C.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2012, 36, 27–46. [Google Scholar] [CrossRef]
- Lewis, S.J.; Barker, R.A. A pathophysiological model of freezing of gait in Parkinson’s disease. Park. Relat. Disord. 2009, 15, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Sherman, D.; Fuller, P.M.; Marcus, J.; Yu, J.; Zhang, P.; Chamberlin, N.L.; Saper, C.B.; Lu, J. Anatomical Location of the Mesencephalic Locomotor Region and Its Possible Role in Locomotion, Posture, Cataplexy, and Parkinsonism. Front. Neurol. 2015, 6, 140. [Google Scholar] [CrossRef]
- Snijders, A.H.; Leunissen, I.; Bakker, M.; Overeem, S.; Helmich, R.C.; Bloem, B.R.; Toni, I. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain 2011, 134 Pt 1, 59–72. [Google Scholar] [CrossRef]
- Shik, M.L.; Orlovsky, G.N. Neurophysiology of locomotor automatism. Physiol. Rev. 1976, 56, 465–501. [Google Scholar] [CrossRef] [PubMed]
- Shik, M.L.; Severin, F.V.; Orlovsky, G.N. Control of walking and running by means of electrical stimulation of the mesencephalon. Electroencephalogr. Clin. Neurophysiol. 1969, 26, 549. [Google Scholar]
- Takakusaki, K.; Chiba, R.; Nozu, T.; Okumura, T. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J. Neural Transm. 2016, 123, 695–729. [Google Scholar] [CrossRef]
- Takakusaki, K.; Habaguchi, T.; Ohtinata-Sugimoto, J.; Saitoh, K.; Sakamoto, T. Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: A new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 2003, 119, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, S.; Dubuc, R.; Gossard, J.P. Dynamic sensorimotor interactions in locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef] [PubMed]
- Jordan, L.M.; Liu, J.; Hedlund, P.B.; Akay, T.; Pearson, K.G. Descending command systems for the initiation of locomotion in mammals. Brain Res. Rev. 2008, 57, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K.; Kohyama, J.; Matsuyama, K.; Mori, S. Medullary reticulospinal tract mediating the generalized motor inhibition in cats: Parallel inhibitory mechanisms acting on motoneurons and on interneuronal transmission in reflex pathways. Neuroscience 2001, 103, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.; Engberg, I.; Lundberg, A. Primary afferent depolarization evoked from the brain stem and the cerebellum. Arch. Ital. Biol. 1966, 104, 73–85. [Google Scholar] [PubMed]
- Jankowska, E.; Lund, S.; Lundberg, A.; Pompeiano, O. Inhibitory effects evoked through ventral reticulospinal pathways. Arch. Ital. Biol. 1968, 106, 124–140. [Google Scholar]
- Takakusaki, K.; Ohta, Y.; Mori, S. Single medullary reticulospinal neurons exert postsynaptic inhibitory effects via inhibitory interneurons upon alpha-motoneurons innervating cat hindlimb muscles. Exp. Brain Res. 1989, 74, 11–23. [Google Scholar] [CrossRef]
- Sakai, S.T.; Davidson, A.G.; Buford, J.A. Reticulospinal neurons in the pontomedullary reticular formation of the monkey (Macaca fascicularis). Neuroscience 2009, 163, 1158–1170. [Google Scholar] [CrossRef]
- Riddle, C.N.; Edgley, S.A.; Baker, S.N. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J. Neurosci. 2009, 29, 4993–4999. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Nutt, J.G.; Carlson-Kuhta, P.; Stephens, M.; Horak, F.B. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp. Neurol. 2009, 215, 334–341. [Google Scholar] [CrossRef]
- Takakusaki, K. Functional Neuroanatomy for Posture and Gait Control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Takakusaki, K.; Kohyama, J.; Matsuyama, K. Medullary reticulospinal tract mediating a generalized motor inhibition in cats: III. Functional organization of spinal interneurons in the lower lumbar segments. Neuroscience 2003, 121, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Nachev, P.; Kennard, C.; Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef]
- Takakusaki, K. Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, A.; Voorhoeve, P. Effects from the pyramidal tract on spinal reflex arcs. Acta Physiol. Scand. 1962, 56, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Meunier, S.; Pierrot-Deseilligny, E. Cortical control of presynaptic inhibition of Ia afferents in humans. Exp. Brain Res. 1998, 119, 415–426. [Google Scholar] [CrossRef]
- Vercruysse, S.; Spildooren, J.; Heremans, E.; Wenderoth, N.; Swinnen, S.P.; Vandenberghe, W.; Nieuwboer, A. The neural correlates of upper limb motor blocks in Parkinson’s disease and their relation to freezing of gait. Cereb. Cortex 2014, 24, 3154–3166. [Google Scholar] [CrossRef]
- Takakusaki, K.; Takahashi, M.; Noguchi, T.; Chiba, R.J.N.; Neuroscience, C. Neurophysiological mechanisms of gait disturbance in advanced Parkinson’s disease patients. Neurol. Clin. Neurosci. 2022, 11, 201–217. [Google Scholar] [CrossRef]
- Lewis, S.J.; Shine, J.M. The Next Step: A Common Neural Mechanism for Freezing of Gait. Neuroscientist 2016, 22, 72–82. [Google Scholar] [CrossRef]
- Peterka, R.J. Sensorimotor integration in human postural control. J. Neurophysiol. 2002, 88, 1097–1118. [Google Scholar] [CrossRef]
- Smith, P.F. Vestibular Functions and Parkinson’s Disease. Front. Neurol. 2018, 9, 1085. [Google Scholar] [CrossRef]
- Manchester, D.; Woollacott, M.; Zederbauer-Hylton, N.; Marin, O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J. Gerontol. 1989, 44, M118–M127. [Google Scholar] [CrossRef]
- Pahapill, P.A.; Lozano, A.M. The pedunculopontine nucleus and Parkinson’s disease. Brain 2000, 123 Pt 9, 1767–1783. [Google Scholar] [CrossRef]
- Zweig, R.M.; Jankel, W.R.; Hedreen, J.C.; Mayeux, R.; Price, D.L. The pedunculopontine nucleus in Parkinson’s disease. Ann. Neurol. 1989, 26, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Curtze, C.; Nutt, J.G.; Carlson-Kuhta, P.; Mancini, M.; Horak, F.B. Levodopa Is a Double-Edged Sword for Balance and Gait in People with Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wu, D.; Lin, C.; Cai, H.; Chen, L.; Cai, G.; Ye, Q.; Cai, G. Pedunculopontine Nucleus Deep Brain Stimulation Improves Gait Disorder in Parkinson’s Disease: A Systematic Review and Meta-analysis. Neurochem. Res. 2020, 45, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Pierantozzi, M.; Palmieri, M.G.; Galati, S.; Stanzione, P.; Peppe, A.; Tropepi, D.; Brusa, L.; Pisani, A.; Moschella, V.; Marciani, M.G.; et al. Pedunculopontine nucleus deep brain stimulation changes spinal cord excitability in Parkinson’s disease patients. J. Neural. Transm. 2008, 115, 731–735. [Google Scholar] [CrossRef]
- Streumer, J.; Selvaraj, A.K.; Kurt, E.; Bloem, B.R.; Esselink, R.A.J.; Bartels, R.; Georgiev, D.; Vinke, R.S. Does spinal cord stimulation improve gait in Parkinson’s disease: A comprehensive review. Park. Relat. Disord. 2023, 109, 105331. [Google Scholar] [CrossRef] [PubMed]
- Fonoff, E.T.; de Lima-Pardini, A.C.; Coelho, D.B.; Monaco, B.A.; Machado, B.; Pinto de Souza, C.; Dos Santos Ghilardi, M.G.; Hamani, C. Spinal Cord Stimulation for Freezing of Gait: From Bench to Bedside. Front. Neurol. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- de Lima-Pardini, A.C.; Coelho, D.B.; Souza, C.P.; Souza, C.O.; Ghilardi, M.; Garcia, T.; Voos, M.; Milosevic, M.; Hamani, C.; Teixeira, L.A.; et al. Effects of spinal cord stimulation on postural control in Parkinson’s disease patients with freezing of gait. eLife. 2018, 7, e37727. [Google Scholar] [CrossRef] [PubMed]
- Samotus, O.; Parrent, A.; Jog, M. Spinal Cord Stimulation Therapy for Gait Dysfunction in Advanced Parkinson’s Disease Patients. Mov. Disord. Off. J. Mov. Disord. Soc. 2018, 33, 783–792. [Google Scholar] [CrossRef]
- Fuentes, R.; Petersson, P.; Siesser, W.B.; Caron, M.G.; Nicolelis, M.A. Spinal cord stimulation restores locomotion in animal models of Parkinson’s disease. Science 2009, 323, 1578–1582. [Google Scholar] [CrossRef]
- Yadav, A.P.; Nicolelis, M.A.L. Electrical stimulation of the dorsal columns of the spinal cord for Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2017, 32, 820–832. [Google Scholar] [CrossRef]
- Fanselow, E.E.; Reid, A.P.; Nicolelis, M.A. Reduction of pentylenetetrazole-induced seizure activity in awake rats by seizure-triggered trigeminal nerve stimulation. J. Neurosci. 2000, 20, 8160–8168. [Google Scholar] [CrossRef]
- Jasmin, L.; Wu, M.V.; Ohara, P.T. GABA puts a stop to pain. Curr. Drug Targets-CNS Neurol. Disord. 2004, 3, 487–505. [Google Scholar] [CrossRef]
- Magoul, R.; Onteniente, B.; Geffard, M.; Calas, A. Anatomical distribution and ultrastructural organization of the GABAergic system in the rat spinal cord. An immunocytochemical study using anti-GABA antibodies. Neuroscience 1987, 20, 1001–1009. [Google Scholar] [CrossRef]
- Emborg, M.E.; Carbon, M.; Holden, J.E.; During, M.J.; Ma, Y.; Tang, C.; Moirano, J.; Fitzsimons, H.; Roitberg, B.Z.; Tuccar, E.; et al. Subthalamic glutamic acid decarboxylase gene therapy: Changes in motor function and cortical metabolism. J. Cereb. Blood Flow Metab. 2007, 27, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Kaplitt, M.G.; Feigin, A.; Tang, C.; Fitzsimons, H.L.; Mattis, P.; Lawlor, P.A.; Bland, R.J.; Young, D.; Strybing, K.; Eidelberg, D.; et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: An open label, phase I trial. Lancet 2007, 369, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Mackie, M.; Hughes, D.I.; Maxwell, D.J.; Tillakaratne, N.J.; Todd, A.J. Distribution and colocalisation of glutamate decarboxylase isoforms in the rat spinal cord. Neuroscience 2003, 119, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Kaplitt, M.G.; Fitzsimons, H.L.; Zuzga, D.S.; Liu, Y.; Oshinsky, M.L.; During, M.J. Subthalamic GAD gene therapy in a Parkinson’s disease rat model. Science 2002, 298, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Novak, P.; Novak, V. Effect of step-synchronized vibration stimulation of soles on gait in Parkinson’s disease: A pilot study. J. Neuroeng. Rehabil. 2006, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Mancini, M.; Smulders, K.; Harker, G.; Stuart, S.; Nutt, J.G. Assessment of the ability of open- and closed-loop cueing to improve turning and freezing in people with Parkinson’s disease. Sci. Rep. 2018, 8, 12773. [Google Scholar] [CrossRef]
- Klaver, E.C.; van Vugt, J.P.P.; Bloem, B.R.; van Wezel, R.J.A.; Nonnekes, J.; Tjepkema-Cloostermans, M.C. Good vibrations: Tactile cueing for freezing of gait in Parkinson’s disease. J. Neurol. 2023, 270, 3424–3432. [Google Scholar] [CrossRef]
- Nolano, M.; Provitera, V.; Estraneo, A.; Selim, M.M.; Caporaso, G.; Stancanelli, A.; Saltalamacchia, A.M.; Lanzillo, B.; Santoro, L. Sensory deficit in Parkinson’s disease: Evidence of a cutaneous denervation. Brain 2008, 131 Pt 7, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Gillies, J.D.; Lance, J.W.; Neilson, P.D.; Tassinari, C.A. Presynaptic inhibition of the monosynaptic reflex by vibration. J. Physiol. 1969, 205, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Lapole, T.; Deroussen, F.; Perot, C.; Petitjean, M. Acute effects of Achilles tendon vibration on soleus and tibialis anterior spinal and cortical excitability. Appl. Physiol. Nutr. Metab. 2012, 37, 657–663. [Google Scholar] [CrossRef]
- Souron, R.; Baudry, S.; Millet, G.Y.; Lapole, T. Vibration-induced depression in spinal loop excitability revisited. J. Physiol. 2019, 597, 5179–5193. [Google Scholar] [CrossRef]
- Schaafsma, J.D.; Balash, Y.; Gurevich, T.; Bartels, A.L.; Hausdorff, J.M.; Giladi, N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur. J. Neurol. 2003, 10, 391–398. [Google Scholar] [CrossRef]
- McNeely, M.E.; Earhart, G.M. The effects of medication on turning in people with Parkinson disease with and without freezing of gait. J. Park. Dis. 2011, 1, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Bray, S.; Bryant, D.M.; Glover, G.H.; Reiss, A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 2011, 54, 2808–2821. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, G.E.; Zito, G.; Patria, F.; Sanes, J.N. Improved detection of event-related functional MRI signals using probability functions. Neuroimage 2001, 14, 1193–1205. [Google Scholar] [CrossRef] [PubMed]




| Freezers (n = 34) | Range | |
|---|---|---|
| Demographics | ||
| Men/women (number) | 26/8 | - |
| Age (years) | 63.7 (9.0) | 49 to 80 |
| Educational level (years) | 10.1 (5.2) | 4 to 24 |
| Body mass (kg) | 69.6 (11.2) | 49 to 94 |
| Height (cm) | 1.5 (0.2) | 1 to 2 |
| Body mass index (kg/m2) | 25.7 (3.1) | 20 to 32 |
| MMSE (score) | 26.0 (1.6) | 24 to 29 |
| Clinical variables | ||
| Years since diagnosis (years) | 8.8 (5.1) | 2 to 25 |
| Hoehn and Yahr staging scale (a.u) | 3.2 (0.4) | 3 to 4 |
| Number of participants in stage 3 | 28 | - |
| Number of participants in stage 4 | 6 | - |
| Symptom-dominant side (R/L/B) | 0/6/28 | |
| Right-legged (number) | 20 | |
| Left-legged (number) | 14 | |
| UPDRS-III (score) | 49.9 (11.2) | 23 to 67 |
| PIGD (score) | 8.6 (2.3) | 4 to 13 |
| NFOGQ (score) | 22.2 (5.4) | 12 to 28 |
| Stroop Color-Word Test (a.u) | 69.5 (42.9) | 20.8 to 160.2 |
| L-Dopa equivalent units (mg·day−1) | 802.6 (270.7) | 300 to 1300 |
| Behavioral variables | ||
| The ratio of the conditioned H-reflex relative to the test H-reflex (%) | 115.1 (8.6) | 103.0 to 135.6 |
| FOG-ratio (a.u) | 12.3 (10.8) | 2.1 to 54.6 |
| APA amplitude (N/cm) | 1.9 (0.4) | 1.1 to 3.0 |
| APA duration (ms) | 483.1 (59.9) | 402.6 to 676.7 |
| raEMG of the tibial anterior muscle (mV) | 0.06 (0.03) | 0.1 to 0.10 |
| raEMG of the soleus muscle (mV) | 0.08 (0.04) | 0.02 to 0.19 |
| Co-contraction ratio (%) | 89.8 (58.9) | 0 to 219.9 |
| Beta of the BOLD signal change | ||
| Beta of BOLD signal change of the right SMA (a.u.) | 0.3 (0.4) | −0.5 to 1.1 |
| Beta of BOLD signal change of the right STN (a.u.) | 0.3 (0.5) | −1.0 to 0.9 |
| Beta of BOLD signal change of the right MLR (a.u.) | 0.4 (0.3) | −0.4 to 0.8 |
| Beta of BOLD signal change of the right CLR (a.u.) | 0.3 (0.5) | −1.1 to 1.2 |
| Independent Factors | Partial R2 | Model R2 Change | F Value | p Value | Adjusted Model R2 Change (Variance Explained) |
|---|---|---|---|---|---|
| Beta of BOLD signal change of the MLR during step initiation (a.u) | 0.3152 | 0.3152 | 14.73 | 0.0006 | 0.49 |
| APA amplitude (N/cm) | 0.1347 | 0.4500 | 7.59 | 0.0097 | |
| FOG-ratio (a.u.) | 0.0541 | 0.5041 | 3.28 | 0.0804 | |
| Disease duration (years) | 0.0549 | 0.5590 | 3.61 | 0.0674 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva-Batista, C.; Lira, J.; Coelho, D.B.; de Lima-Pardini, A.C.; Nucci, M.P.; Mattos, E.C.T.; Magalhaes, F.H.; Barbosa, E.R.; Teixeira, L.A.; Amaro Junior, E.; et al. Mesencephalic Locomotor Region and Presynaptic Inhibition during Anticipatory Postural Adjustments in People with Parkinson’s Disease. Brain Sci. 2024, 14, 178. https://doi.org/10.3390/brainsci14020178
Silva-Batista C, Lira J, Coelho DB, de Lima-Pardini AC, Nucci MP, Mattos ECT, Magalhaes FH, Barbosa ER, Teixeira LA, Amaro Junior E, et al. Mesencephalic Locomotor Region and Presynaptic Inhibition during Anticipatory Postural Adjustments in People with Parkinson’s Disease. Brain Sciences. 2024; 14(2):178. https://doi.org/10.3390/brainsci14020178
Chicago/Turabian StyleSilva-Batista, Carla, Jumes Lira, Daniel Boari Coelho, Andrea Cristina de Lima-Pardini, Mariana Penteado Nucci, Eugenia Casella Tavares Mattos, Fernando Henrique Magalhaes, Egberto Reis Barbosa, Luis Augusto Teixeira, Edson Amaro Junior, and et al. 2024. "Mesencephalic Locomotor Region and Presynaptic Inhibition during Anticipatory Postural Adjustments in People with Parkinson’s Disease" Brain Sciences 14, no. 2: 178. https://doi.org/10.3390/brainsci14020178