Cerebral Blood Flow in Healthy Subjects with Different Hypnotizability Scores
Abstract
:1. Introduction
1.1. Changes in the Cerebral Artery Diameter Induced by Hypercapnia/Hypocapnia and Arterial Pressure
1.2. Changes in the Cerebral Artery Diameter Induced by Brain Activity
1.3. Aims of the Study
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedure
2.3. Tests
2.3.1. Trail Making Task (TMT)
2.3.2. Mental Arithmetic Task (MAT)
2.3.3. Hyperventilation (HVT)
2.3.4. Rebreathing (RBT)
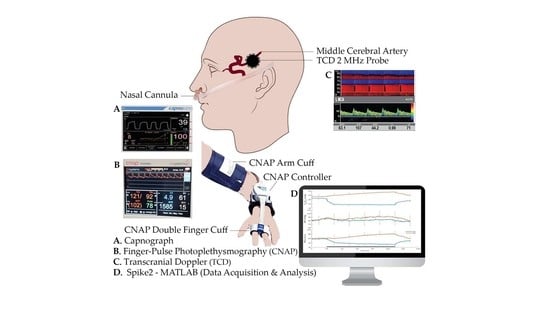
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. Differences between Med-Highs and Med-Lows
3.1.1. Systemic Measures
3.1.2. Doppler Measures
3.2. Associations between Systemic and Doppler Variables
3.2.1. Hyperventilation (HVT)
3.2.2. Rebreathing (RBT)
4. Discussion
4.1. Comparisons between Groups
4.2. Association between Systemic and Doppler Variables
5. Limitations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elkins, G.R.; Barabasz, A.F.; Council, J.R.; Spiegel, D. Advancing Research and Practice: The Revised APA Division 30 Definition of Hypnosis. Int. J. Clin. Exp. Hypn. 2015, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Santarcangelo, E.L.; Scattina, E. Complementing the Latest APA Definition of Hypnosis: Sensory-Motor and Vascular Peculiarities Involved in Hypnotizability. Int. J. Clin. Exp. Hypn. 2016, 64, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Piccione, C.; Hilgard, E.R.; Zimbardo, P.G. On the Degree of Stability of Measured Hypnotizability Over a 25-Year Period. J. Pers. Soc. Psychol. 1989, 56, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.; Lifshitz, M.; Raz, A. Brain Correlates of Hypnosis: A Systematic Review and Meta-Analytic Exploration. Neurosci. Biobehav. Rev. 2017, 81, 75–98. [Google Scholar] [CrossRef]
- Picerni, E.; Santarcangelo, E.L.; Laricchiuta, D.; Cutuli, D.; Petrosini, L.; Spalletta, G.; Piras, F. Cerebellar Structural Variations in Subjects with Different Hypnotizability. Cerebellum 2019, 18, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Raz, A. Attention and Hypnosis: Neural Substrates and Genetic Associations of Two Converging Processes. Int. J. Clin. Exp. Hypn. 2005, 53, 237–258. [Google Scholar] [CrossRef]
- Cojan, Y.; Waber, L.; Schwartz, S.; Rossier, L.; Forster, A.; Vuilleumier, P. The Brain under Self-Control: Modulation of Inhibitory and Monitoring Cortical Networks during Hypnotic Paralysis. Neuron 2009, 62, 862–875. [Google Scholar] [CrossRef] [Green Version]
- McGeown, W.J.; Mazzoni, G.; Vannucci, M.; Venneri, A. Structural and Functional Correlates of Hypnotic Depth and Suggestibility. Psychiatry Res. Neuroimaging 2015, 231, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Ibáñez-Marcelo, E.; Campioni, L.; Phinyomark, A.; Petri, G.; Santarcangelo, E.L. Topology Highlights Mesoscopic Functional Equivalence between Imagery and Perception: The Case of Hypnotizability. Neuroimage 2019, 200, 437–449. [Google Scholar] [CrossRef]
- Spina, V.; Chisari, C.; Santarcangelo, E.L. High Motor Cortex Excitability in Highly Hypnotizable Individuals: A Favourable Factor for Neuroplasticity? Neuroscience 2020, 430, 125–130. [Google Scholar] [CrossRef]
- Rosati, A.; Belcari, I.; Santarcangelo, E.L.; Sebastiani, L. Interoceptive Accuracy as a Function of Hypnotizability. Int. J. Clin. Exp. Hypn. 2021, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Diolaiuti, F.; Huber, A.; Ciaramella, A.; Santarcangelo, E.L.; Sebastiani, L. Hypnotisability-related Interoceptive Awareness and Inhibitory/Activating Emotional Traits. Arch. Ital. Biol. 2019, 157, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Jambrik, Z.; Santarcangelo, E.L.; Rudisch, T.; Varga, A.; Forster, T.; Carli, G. Modulation of pain-induced endothelial dysfunction by hypnotizability. Pain 2005, 116, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Jambrik, Z.; Santarcangelo, E.L.; Ghelarducci, B.; Picano, E.; Sebastiani, L. Does hypnotizability modulate the stress-related endothelial dysfunction? Brain Res. Bull. 2004, 63, 213–216. [Google Scholar] [CrossRef]
- Jambrik, Z.; Chunzeng, L.; Santarcangelo, E.L.; Sebastiani, L.; Ghelarducci, B.; Picano, E. Traditional acupuncture does not modulate the endothelial dysfunction induced by mental stress. Int. J. Cardiovasc. Imaging 2004, 20, 357–362. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, M.; Wang, M.; Li, X. Flow-Mediated Vasodilation through Mechanosensitive G Protein-Coupled Receptors in Endothelial Cells. Trends Cardiovasc. Med. 2021, 32, 61–70. [Google Scholar] [CrossRef]
- Contestabile, A. Role of Nitric Oxide in Cerebellar Development and Function: Focus on Granule Neurons. Cerebellum 2012, 11, 50–61. [Google Scholar] [CrossRef]
- Fantini, S.; Sassaroli, A.; Tgavalekos, K.T.; Kornbluth, J. Cerebral Blood Flow and Autoregulation: Current Measurement Techniques and Prospects for Noninvasive Optical Methods. Neurophotonics 2016, 3, 031411. [Google Scholar] [CrossRef]
- Severinghaus, J.W.; Lassen, N. Step Hypocapnia to Separate Arterial from Tissue PCO2 in the Regulation of Cerebral Blood Flow. Circ. Res. 1967, 20, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Fathi, A.R.; Yang, C.; Bakhtian, K.D.; Qi, M.; Lonser, R.R.; Pluta, R.M. Carbon dioxide influence on nitric oxide production in endothelial cells and astrocytes: Cellular mechanisms. Brain Res. 2011, 1386, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Hoiland, R.L.; Fisher, J.A.; Ainslie, P.N. Regulation of the Cerebral Circulation by Arterial Carbon Dioxide. Compr. Physiol. 2019, 9, 1101–1154. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, H.G.; Howe, C.A.; Hoiland, R.L.; Carr, J.M.J.R.; Chalifoux, C.J.; Brown, C.V.; Patrician, A.; Tremblay, J.C.; Panerai, R.B.; Robinson, T.G.; et al. Alterations in Arterial CO2 rather than pH affect the Kinetics of Neurovascular Coupling in Humans. J. Physiol. 2021, 599, 3663–3676. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M.; Brian, J.E. Nitric oxide and the cerebral circulation. Stroke 1994, 25, 692–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leffler, C.W. Nitric oxide in control of the cerebral circulation. In Nitric Oxide and the Regulation of the Peripheral Circulation; Kadowitz, P.J., McNamara, D.B., Eds.; Birkhäuser Boston: Boston, MA, USA, 2000; pp. 113–127. ISBN 978-1-4612-1326-0. [Google Scholar]
- Shalit, M.N.; Shimojyo, S.; Reinmuth, O.M.; Shalit, M.N. Carbon dioxide and cerebral circulatory control. I. The extravascular effect. Arch. Neurol. 1967, 17, 298–303. [Google Scholar] [CrossRef]
- Skinhoj, E.; Paulson, O.B. Carbon Dioxide and Cerebral Circulatory Control: Evidence of a Nonfocal Site of Action of Carbon Dioxide on Cerebral Circulation. Arch. Neurol. 1969, 20, 249–252. [Google Scholar] [CrossRef]
- Seylaz, J. Contribution to the study of the mechanism of cerebral blood flow regulation. Helv. Physiol. Pharmacol. Acta 1968, 26, 33–61. [Google Scholar]
- Seylaz, J.; Pinard, E.; Dittmar, A.; Birer, A. Measurement of Blood Flow, Tissue PO2 and Tissue PCO2 Continuously and Simultaneously in the Same Structure of the Brain. Med. Biol. Eng. Comput. 1979, 17, 19–24. [Google Scholar] [CrossRef]
- Scremin, O.U.; Scremin, A.M.; Somani, S.M.; Giacobini, E. Brain Regional Distribution of Physostigmine and Its Relation to Cerebral Blood Flow following Intravenous Administration in Rats. J. Neurosci. Res. 1990, 26, 188–195. [Google Scholar] [CrossRef]
- Rovere, A.A.; Scremin, O.U.; Beresi, M.R.; Raynald, A.C.; Giardini, A. Cholinergic Mechanism in the Cerebrovascular Action of Carbon Dioxide. Stroke 1973, 4, 969–972. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.; Meyer, J.S.; Hiromoto, H.; Aoyagi, M.; Tagashira, Y.; Ott, E.O. Neurogenic Control of Cerebral Blood Flow in the Baboon. J. Neurosurg. 1975, 43, 676–688. [Google Scholar] [CrossRef]
- Busija, D.W.; Heistad, D.D. Effects of Cholinergic Nerves on Cerebral Blood Flow in Cats. Circ. Res. 1981, 48, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scremin, O.U.; Sonnenschein, R.R.; Rubinstein, E.H. Cholinergic Cerebral Vasodilatation in the Rabbit: Absence of Concomitant Metabolic Activation. J. Cereb. Blood Flow Metab. 1982, 2, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Shohami, E.; Sidi, A. Accumulation of Prostacyclin in Rat Brain During Haemorrhagic Hypotension-Possible Role of PGI2 in Autoregulation. J. Cereb. Blood Flow Metab. 1984, 4, 107–109. [Google Scholar] [CrossRef] [Green Version]
- Pickard, J.D. Role of Prostaglandins and Arachidonic Acid Derivatives in the Coupling of Cerebral Blood Flow to Cerebral Metabolism. J. Cereb. Blood Flow Metab. 2016, 1, 361–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadecola, C. Does Nitric Oxide Mediate the Increases in Cerebral Blood Flow Elicited by Hypercapnia? Proc. Natl. Acad. Sci. USA 1992, 89, 3913–3916. [Google Scholar] [CrossRef] [Green Version]
- Panerai, R.B.; Evans, D.H.; Naylor, A.R. Influence of arterial blood pressure on cerebrovascular reactivity. Stroke 1999, 30, 1293–1295. [Google Scholar] [CrossRef] [Green Version]
- Hoiland, R.L.; Caldwell, H.G.; Carr, J.M.J.R.; Howe, C.A.; Stacey, B.S.; Dawkins, T.; Wakeham, D.J.; Tremblay, J.C.; Tymko, M.M.; Patrician, A.; et al. Nitric oxide contributes to cerebrovascular shear-mediated dilatation but not steady-state cerebrovascular reactivity to carbon dioxide. J. Physiol. 2021, 600, 1385–1403. [Google Scholar] [CrossRef]
- Lucas, S.J.E.; Tzeng, Y.C.; Galvin, S.D.; Thomas, K.N.; Ogoh, S.; Ainslie, P.N. Influence of changes in blood pressure on cerebral perfusion and oxygenation. Hypertension 2010, 55, 698–705. [Google Scholar] [CrossRef] [Green Version]
- Smirl, J.D.; Hoffman, K.; Tzeng, Y.C.; Hansen, A.; Ainslie, A.P.N. Methodological comparison of active- and passive-driven oscillations in blood pressure; implications for the assessment of cerebral pressure-flow relationships. J. Appl. Physiol. 2015, 119, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Nippert, A.R.; Biesecker, K.R.; Newman, E.A. Mechanisms Mediating Functional Hyperemia in the Brain. Neuroscientist 2018, 24, 73–83. [Google Scholar] [CrossRef]
- Poplawsky, A.J.; Iordanova, B.; Vazquez, A.L.; Kim, S.-G.; Fukuda, M. Postsynaptic Activity of Inhibitory Neurons evokes Hemodynamic fMRI Responses. Neuroimage 2021, 225, 117457. [Google Scholar] [CrossRef] [PubMed]
- Tellegen, A.; Atkinson, G. Openness to Absorbing and Self-Altering Experiences (“Absorption”), A Trait related to Hypnotic Susceptibility. J. Abnorm. Psychol. 1974, 83, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Presciuttini, S.; Gialluisi, A.; Barbuti, S.; Curcio, M.; Scatena, F.; Carli, G.; Santarcangelo, E.L. Hypnotizability and Catechol-O-Methyltransferase (COMT) polymorphysms in Italians. Front. Hum. Neurosci. 2014, 7, 929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rominger, C.; Weiss, E.M.; Nagl, S.; Niederstätter, H.; Parson, W.; Papousek, I. Carriers of the COMT Met/Met allele have higher degrees of hypnotizability, provided that they have good attentional control: A case of gene-trait interaction. Int. J. Clin. Exp. Hypn. 2014, 62, 455–482. [Google Scholar] [CrossRef]
- Guillot, A.; Collet, C.; Nguyen, V.A.; Malouin, F.; Richards, C.; Doyon, J. Functional Neuroanatomical Networks Associated with Expertise in Motor Imagery. Neuroimage 2008, 41, 1471–1483. [Google Scholar] [CrossRef]
- Cavallaro, F.I.; Cacace, I.; Del Testa, M.; Andre, P.; Carli, G.; De Pascalis, V.; Rocchi, R.; Santarcangelo, E.L. Hypnotizability-related EEG Alpha and Theta Activities during Visual and Somesthetic Imageries. Neurosci. Lett. 2010, 470, 13–18. [Google Scholar] [CrossRef]
- Ibáñez-Marcelo, E.; Campioni, L.; Manzoni, D.; Santarcangelo, E.L.; Petri, G. Spectral and Topological Analyses of the Cortical Representation of the Head Position: Does Hypnotizability Matter? Brain Behav. 2019, 9, e01277. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Weitzenhoffer, A.M.; Hilgard, E.R. Stanford Hypnotic Susceptibility Scale, Forms A and B; Consulting Psychologists Press: Palo Alto, CA, USA, 1959. [Google Scholar]
- Csipo, T.; Lipecz, A.; Mukli, P.; Bahadli, D.; Abdulhussein, O.; Owens, C.D.; Tarantini, S.; Hand, R.A.; Yabluchanska, V.; Mikhail Kellawan, J.; et al. Increased cognitive workload evokes greater neurovascular coupling responses in healthy young adults. PLoS ONE 2021, 16, e0250043. [Google Scholar] [CrossRef]
- De Pascalis, V.; Bellusci, A.; Russo, P.M. Italian Norms for the Stanford Hypnotic Susceptibility Scale, Form C. Int. J. Clin. Exp. Hypn. 2000, 48, 315–323. [Google Scholar] [CrossRef]
- Rastogi, R.; Morgan, B.J.; Badr, M.S.; Chowdhuri, S. Hypercapnia-induced vasodilation in the cerebral circulation is reduced in older adults with sleep-disordered breathing. J. Appl. Physiol. 2022, 132, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Zambach, S.A.; Cai, C.; Helms, H.C.C.; Hald, B.O.; Dong, Y.; Fordsmann, J.C.; Nielsen, R.M.; Hu, J.; Lønstrup, M.; Brodin, B.; et al. Precapillary sphincters and pericytes at first-order capillaries as key regulators for brain capillary perfusion. Proc. Natl. Acad. Sci. USA 2021, 118, e2023749118. [Google Scholar] [CrossRef] [PubMed]
- Porta, A.; Gelpi, F.; Bari, V.; Cairo, B.; De Maria, B.; Panzetti, C.M.; Cornara, N.; Bertoldo, E.G.; Fiolo, V.; Callus, E.; et al. Monitoring the Evolution of Asynchrony between Mean Arterial Pressure and Mean Cerebral Blood Flow via Cross-Entropy Methods. Entropy 2022, 24, 80. [Google Scholar] [CrossRef]
- Younger, J.W.; Rossetti, G.C.; Borckardt, J.J.; Smith, A.R.; Tasso, A.F.; Nash, M.R. Hypnotizability and somatic complaints: A gender-specific phenomenon. Int. J. Clin. Exp. Hypn. 2007, 55, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facco, E.; Testoni, I.; Ronconi, L.; Casiglia, E.; Zanette, G.; Spiegel, D. Psychological Features of Hypnotizability: A First Step Towards Its Empirical Definition. Int. J. Clin. Exp. Hypn. 2017, 65, 98–119. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.R.J.; Howarth, C.; MacVicar, B.A. Bidirectional Control of Blood Flow by Astrocytes: A Role for Tissue Oxygen and Other Metabolic Factors. Adv. Exp. Med. Biol. 2016, 903, 209–219. [Google Scholar] [CrossRef]
- Acunzo, D.J.; Oakley, D.A.; Terhune, D.B. The neurochemistry of hypnotic suggestion. Am. J. Clin. Hypn. 2021, 63, 355–371. [Google Scholar] [CrossRef]
- Santarcangelo, E.L.; Scattina, E. Responding to Sensorimotor Suggestions: From Endothelial Nitric Oxide to the Functional Equivalence Between Imagery and Perception. Int. J. Clin. Exp. Hypn. 2019, 67, 394–407. [Google Scholar] [CrossRef]




| Condition | Variable | Med-Lows | Med-Highs | ||
|---|---|---|---|---|---|
| TMT | Mean | SD | Mean | SD | |
| Basal | HR (bpm) | 72.02 | 12.08 | 78.50 | 14.66 |
| ABP (mmHg) | 82.29 | 17.23 | 80.71 | 14.66 | |
| PETCO2 (mmHg) | 37.31 | 1.91 | 34.63 | 3.45 | |
| MCAv (cm/s) | 59.52 | 9.61 | 52.13 | 10.43 | |
| Task | HR * (bpm) | 79.09 | 13.66 | 81.62 | 14.46 |
| ABP * (mmHg) | 87.95 | 14.72 | 86.56 | 13.98 | |
| PETCO2 (mmHg) | 37.13 | 1.40 | 34.46 | 3.43 | |
| MCAv (cm/s) | 59.02 | 10.10 | 50.18 | 9.87 | |
| MAT | |||||
| Basal | HR (bpm) | 75.98 | 11.61 | 76.50 | 14.10 |
| ABP (mmHg) | 78.19 | 14.59 | 83.71 | 15.15 | |
| PETCO2 (mmHg) | 36.51 | 1.92 | 34.53 | 3.29 | |
| MCAv (cm/s) | 60.25 | 8.70 | 52.56 | 10.61 | |
| Task | HR * (bpm) | 84.20 | 12.41 | 84.51 | 15.00 |
| ABP * (mmHg) | 88.64 | 12.91 | 96.89 | 9.80 | |
| PETCO2 (mmHg) | 36.96 | 2.61 | 34.91 | 3.68 | |
| MCAv (cm/s) | 61.60 | 10.27 | 55.66 | 11.87 | |
| HVT | |||||
| Basal | HR (bpm) | 72.93 | 10.09 | 77.88 | 11.36 |
| ABP (mmHg) | 85.65 | 12.47 | 83.03 | 14.77 | |
| PETCO2 (mmHg) | 33.61 | 2.49 | 32.21 | 3.73 | |
| MCAv (cm/s) | 61.25 | 7.24 | 59.68 | 6.67 | |
| Task | HR * (bpm) | 99.34 | 19.71 | 84.12 | 13.01 |
| ABP (mmHg) | 83.58 | 12.93 | 82.53 | 10.65 | |
| PETCO2 * (mmHg) | 18.34 | 1.46 | 17.92 | 1.59 | |
| MCAv * (cm/s) | 34.19 | 7.07 | 33.35 | 5.17 | |
| RBT | |||||
| Basal | HR (bpm) | 74.26 | 9.36 | 76.77 | 11.24 |
| ABP (mmHg) | 78.35 | 12.82 | 85.23 | 11.25 | |
| PETCO2 (mmHg) | 38.15 | 4.47 | 35.96 | 4.05 | |
| MCAv (cm/s) | 58.38 | 6.51 | 56.53 | 9.58 | |
| Task | HR * (bpm) | 87.52 | 11.91 | 87.57 | 13.70 |
| ABP * (mmHg) | 88.47 | 11.57 | 95.33 | 17.84 | |
| PETCO2 * (mmHg) | 48.12 | 3.13 | 46.08 | 3.22 | |
| MCAv * (cm/s) | 82.71 | 13.50 | 75.59 | 11.53 | |
| Task | Variable | Med-Lows | Med-Highs | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| HVT | ∆CVCi (cm/s/mmHg) | −0.32 | 0.12 | −0.33 | 0.07 |
| CVR (cm/s/mmHg) | 1.81 | 0.46 | 1.91 | 0.49 | |
| RBT | ∆CVCi (cm/s/mmHg) | 0.18 | 0.12 | 0.16 | 0.13 |
| CVR (cm/s/mmHg) | 2.49 | 0.76 | 2.17 | 1.66 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, A.; Santarcangelo, E.L.; Roatta, S. Cerebral Blood Flow in Healthy Subjects with Different Hypnotizability Scores. Brain Sci. 2022, 12, 558. https://doi.org/10.3390/brainsci12050558
Rashid A, Santarcangelo EL, Roatta S. Cerebral Blood Flow in Healthy Subjects with Different Hypnotizability Scores. Brain Sciences. 2022; 12(5):558. https://doi.org/10.3390/brainsci12050558
Chicago/Turabian StyleRashid, Anas, Enrica Laura Santarcangelo, and Silvestro Roatta. 2022. "Cerebral Blood Flow in Healthy Subjects with Different Hypnotizability Scores" Brain Sciences 12, no. 5: 558. https://doi.org/10.3390/brainsci12050558