Adhesion Behavior in Fish: From Structures to Applications
Abstract
:1. Introduction
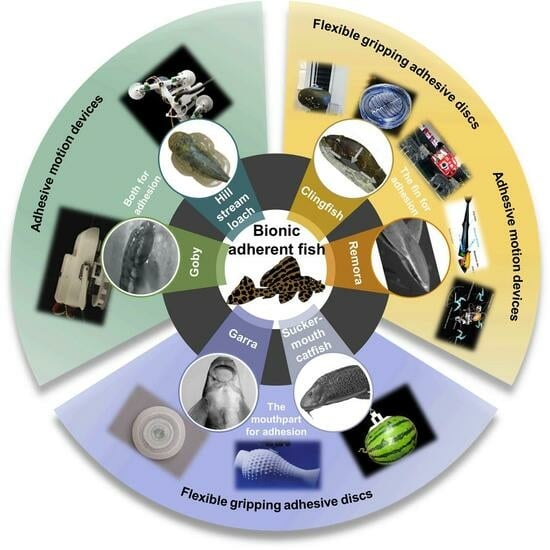
2. Adherent Fish
2.1. The Fin for Adhesion
2.1.1. Clingfish
2.1.2. Remora
2.2. The Mouthpart for Adhesion
2.2.1. Suckermouth Catfish
2.2.2. Garra
2.3. Both for Adhesion
2.3.1. Goby
2.3.2. Hill Stream Loach
3. Underwater Adhesion Systems
3.1. Flexible Gripping Adhesive Discs
3.2. Adhesive Motion Devices
4. Constraints, Limitations, and Future Recommendations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, J.C. Fish swimming efficiency. Curr. Biol. 2022, 32, R666–R671. [Google Scholar] [CrossRef]
- Wainwright, D.K.; Kleinteich, T.; Kleinteich, A.; Gorb, S.N.; Summers, A.P. Stick tight: Suction adhesion on irregular surfaces in the northern clingfish. Biol. Lett. 2013, 9, 20130234. [Google Scholar] [CrossRef]
- Knight, K. Clingfish grip reduced by algae. J. Exp. Biol. 2014, 217, 2431–2432. [Google Scholar] [CrossRef]
- Friedman, M.; Johanson, Z.; Harrington, R.C.; Near, T.J.; Graham, M.R. An early fossil remora (Echeneoidea) reveals the evolutionary assembly of the adhesion disc. Proc. Biol. Sci. 2013, 280, 20131200. [Google Scholar] [CrossRef]
- Nadler, J.H.; Mercer, A.J.; Culler, M.; Ledford, K.A.; Bloomquist, R.; Lin, A. Structures and Function of Remora Adhesion. MRS Online Proc. Libr. 2013, 1498, 159–168. [Google Scholar] [CrossRef]
- Culler, M.; Ledford, K.A.; Nadler, J.H. The Role of Topology and Tissue Mechanics in Remora Attachment. MRS Online Proc. Libr. 2014, 1648, 102. [Google Scholar] [CrossRef]
- Adriaens, D.; Geerinckx, T.; Vlassenbroeck, J.; Van Hoorebeke, L.; Herrel, A. Extensive jaw mobility in suckermouth armored catfishes (Loricariidae): A morphological and kinematic analysis of substrate scraping mode of feeding. Physiol. Biochem. Zool. 2009, 82, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, J.; Liu, X.; Zhang, Q.; Cong, Q.; Chen, T.; Liu, C. Advanced Bionic Attachment Equipment Inspired by the Attachment Performance of Aquatic Organisms: A Review. Biomimetics 2023, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.R. Unculi (Horny Projections Arising from Single Cells), an Adaptive Feature of the Epidermis of Ostariophysan Fishes. Zool. Scr. 1982, 11, 55–76. [Google Scholar] [CrossRef]
- Das, D.; Nag, T.C. Fine structure of the organ of attachment of the teleost, Garra gotyla gotyla (Ham). Zoology 2006, 109, 300–309. [Google Scholar] [CrossRef]
- Pinky, M.S.; Mittal, A.K. Glycoproteins in the epithelium of lips and associated structures of a hill stream fish Garra lamta (Cyprinidae, Cypriniformes): A histochemical investigation. Anat. Histol. Embryol. 2008, 37, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A. Electron Microscopic study of Adhesive organ of Garra lamta (Ham.). Int. J. Biol. Sci. 2012, 1, 43–48. [Google Scholar]
- Sun, C.; Li, X.; Zhou, W.; Li, F. A review of Garra (Teleostei: Cypriniformes) from two rivers in West Yunnan, China with description of a new species. Zootaxa 2018, 4378, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, X. Enhanced Adhesion of Synthetic Discs with Micro-Patterned Margins. Biomimetics 2022, 7, 202. [Google Scholar] [CrossRef]
- Cong, Q.; Xu, J.; Fan, J.; Chen, T.; Ru, S. Insights into the Multilevel Structural Characterization and Adsorption Mechanism of Sinogastromyzon szechuanensis Sucker on the Rough Surface. Life 2021, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wang, J.; Ji, C. The Adhesive System and Anisotropic Shear Force of Guizhou Gastromyzontidae. Sci. Rep. 2016, 6, 37221. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Chang, H.K.; Liu, G.L.; Chen, P.Y. Climbing upstream: Multi-scale structural characterization and underwater adhesion of the Pulin river loach (Sinogastromyzon puliensis). J. Mech. Behav. Biomed. Mater. 2017, 73, 76–85. [Google Scholar] [CrossRef]
- Schoenfuss, H.L.; Blob, R.W. Kinematics of waterfall climbing in Hawaiian freshwater fishes (Gobiidae): Vertical propulsion at the aquatic–terrestrial interface. J. Zool. 2003, 261, 191–205. [Google Scholar] [CrossRef]
- Blob, R.W.; Rai, R.; Julius, M.L.; Schoenfuss, H.L. Functional diversity in extreme environments: Effects of locomotor style and substrate texture on the waterfall-climbing performance of Hawaiian gobiid fishes. J. Zool. 2006, 268, 315–324. [Google Scholar] [CrossRef]
- Maie, T.; Schoenfuss, H.L.; Blob, R.W. Performance and scaling of a novel locomotor structure: Adhesive capacity of climbing gobiid fishes. J. Exp. Biol. 2012, 215, 3925–3936. [Google Scholar] [CrossRef]
- Pennuto, C.M.; Rupprecht, S.M. Upstream range expansion by invasive round gobies: Is functional morphology important? Aquat. Ecol. 2015, 50, 45–57. [Google Scholar] [CrossRef]
- Blob, R.W.; Lagarde, R.; Diamond, K.M.; Keeffe, R.M.; Bertram, R.S.; Ponton, D.; Schoenfuss, H.L. Functional Diversity of Evolutionary Novelties: Insights from Waterfall-Climbing Kinematics and Performance of Juvenile Gobiid Fishes. Integr. Org. Biol. 2019, 1, obz029. [Google Scholar] [CrossRef] [PubMed]
- Cutkosky, M.R. Climbing with adhesion: From bioinspiration to biounderstanding. Interface Focus 2015, 5, 20150015. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liang, Y. Preliminary studies on the basic factors of bionics. Sci. China Technol. Sci. 2014, 57, 520–530. [Google Scholar] [CrossRef]
- Bogatyrev, N.R. A “living” machine. J. Bionic Eng. 2004, 1, 79–87. [Google Scholar] [CrossRef]
- Lu, Y. Significance and Progress of Bionics. J. Bionic Eng. 2004, 1, 1–3. [Google Scholar] [CrossRef]
- Ditsche, P.; Summers, A. Learning from Northern clingfish (Gobiesox maeandricus): Bioinspired suction cups attach to rough surfaces. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190204. [Google Scholar] [CrossRef]
- Sandoval, J.A.; Jadhav, S.; Quan, H.; Deheyn, D.D.; Tolley, M.T. Reversible adhesion to rough surfaces both in and out of water, inspired by the clingfish suction disc. Bioinspir. Biomim. 2019, 14, 066016. [Google Scholar] [CrossRef]
- Lee, S.H.; Song, H.W.; Kang, B.S.; Kwak, M.K. Remora-Inspired Reversible Adhesive for Underwater Applications. ACS Appl. Mater. Interfaces 2019, 11, 47571–47576. [Google Scholar] [CrossRef]
- Su, S.; Wang, S.; Li, L.; Xie, Z.; Hao, F.; Xu, J.; Wang, S.; Guan, J.; Wen, L. Vertical Fibrous Morphology and Structure-Function Relationship in Natural and Biomimetic Suction-Based Adhesion Discs. Matter 2020, 2, 1207–1221. [Google Scholar] [CrossRef]
- Huie, J.M.; Summers, A.P. The effects of soft and rough substrates on suction-based adhesion. J. Exp. Biol. 2022, 225, jeb243773. [Google Scholar] [CrossRef] [PubMed]
- Cockbill, L. Clingfish inspires suction cups for underwater robots. Phys. World. 2019, 32, 6. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Chen, Y.; Wainwright, D.K.; Kenaley, C.P.; Gong, Z.; Liu, Z.; Liu, H.; Guan, J.; Wang, T.; et al. A biorobotic adhesive disc for underwater hitchhiking inspired by the remora suckerfish. Sci. Robot. 2017, 2, eaan8072. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Sun, W.; Wainwright, D.; Wang, H.; Zhao, W.; Chen, B.; Chen, Y.; Wen, L. Detachment of the remora suckerfish disc: Kinematics and a bio-inspired robotic model. Bioinspir. Biomim. 2020, 15, 056018. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Zhao, W.; Zhang, Y.; Wen, L. A biomimetic remora disc with tunable, reversible adhesion for surface sliding and skimming. Bioinspir. Biomim. 2022, 17, 036001. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, S.; Zhang, Y.; Song, S.; Wang, C.; Tan, S.; Zhao, W.; Wang, G.; Sun, W.; Yang, F.; et al. Aerial-aquatic robots capable of crossing the air-water boundary and hitchhiking on surfaces. Sci. Robot. 2022, 7, eabm6695. [Google Scholar] [CrossRef]
- Wang, J.-r.; Xi, Y.-x.; Ji, C.; Zou, J. A biomimetic robot crawling bidirectionally with load inspired by rock-climbing fish. J. Zhejiang Univ. Sci. A 2022, 23, 14–26. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, C.; Wang, R.; Fu, Y.; Chen, Q.; Yang, Y.; Wang, W.; Zhang, M.; Xi, N.; Liu, L. Uncover rock-climbing fish’s secret of balancing tight adhesion and fast sliding for bioinspired robots. Natl. Sci. Rev. 2023, 10, nwad183. [Google Scholar] [CrossRef]
- Honaryar, A.; Ghiasi, M. Design of a Bio-inspired Hull Shape for an AUV from Hydrodynamic Stability Point of View through Experiment and Numerical Analysis. J. Bionic Eng. 2018, 15, 950–959. [Google Scholar] [CrossRef]
- Jacobi, M. Autonomous inspection of underwater structures. Rob. Auton. Syst. 2015, 67, 80–86. [Google Scholar] [CrossRef]
- Ludvigsen, M.; Sørensen, A.J. Towards integrated autonomous underwater operations for ocean mapping and monitoring. Annu. Rev. Control 2016, 42, 145–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Yoder, N.; Kieft, B.; Kukulya, A.; Hobson, B.W.; Ryan, S.; Gawarkiewicz, G.G. Autonomous Tracking of Salinity-Intrusion Fronts by a Long-Range Autonomous Underwater Vehicle. IEEE J. Ocean. Eng. 2022, 47, 950–958. [Google Scholar] [CrossRef]
- Katzschmann, R.K.; DelPreto, J.; MacCurdy, R.; Rus, D. Exploration of underwater life with an acoustically controlled soft robotic fish. Sci. Robot. 2018, 3, eaar3449. [Google Scholar] [CrossRef] [PubMed]
- Gorma, W.; Post, M.A.; White, J.; Gardner, J.; Luo, Y.; Kim, J.; Mitchell, P.D.; Morozs, N.; Wright, M.; Xiao, Q. Development of Modular Bio-Inspired Autonomous Underwater Vehicle for Close Subsea Asset Inspection. Appl. Sci. 2021, 11, 5401. [Google Scholar] [CrossRef]
- Allotta, B.; Costanzi, R.; Ridolfi, A.; Salvetti, O.; Reggiannini, M.; Kruusmaa, M.; Salumae, T.; Lane, D.M.; Frost, G.; Tsiogkas, N.; et al. The ARROWS Project: Robotic technologies for underwater archaeology. IOP Conf. Ser. Mater. Sci. Eng. 2018, 364, 012088. [Google Scholar] [CrossRef]
- Breder, C.M. The locomotion of fishes. Zoologia 1926, 4, 159–297. [Google Scholar] [CrossRef]
- Scaradozzi, D.; Palmieri, G.; Costa, D.; Pinelli, A. BCF swimming locomotion for autonomous underwater robots: A review and a novel solution to improve control and efficiency. Ocean Eng. 2017, 130, 437–453. [Google Scholar] [CrossRef]
- Sfakiotakis, M.; Lane, D.M.; Davies, J.B.C. Review of fish swimming modes for aquatic locomotion. IEEE J. Ocean. Eng. 1999, 24, 237–252. [Google Scholar] [CrossRef]
- Altringham, J.D.; Ellerby, D.J. Fish swimming: Patterns in muscle function. J. Exp. Biol. 1999, 202, 3397–3403. [Google Scholar] [CrossRef]
- Wardle, C.; Videler, J.; Altringham, J. Tuning in to fish swimming waves: Body form, swimming mode and muscle function. J. Exp. Biol. 1995, 198, 1629–1636. [Google Scholar] [CrossRef]
- Di Santo, V.; Goerig, E.; Wainwright, D.K.; Akanyeti, O.; Liao, J.C.; Castro-Santos, T.; Lauder, G.V. Convergence of undulatory swimming kinematics across a diversity of fishes. Proc. Natl. Acad. Sci. USA 2021, 118, e2113206118. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.N.; Lauder, G.V.; Tytell, E.D. Airfoil-like mechanics generate thrust on the anterior body of swimming fishes. Proc. Natl. Acad. Sci. USA 2020, 117, 10585–10592. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.W. Form and Function in Fish Swimming. Sci. Am. 1984, 251, 72–82. [Google Scholar] [CrossRef]
- Heepe, L.; Gorb, S.N. Biologically Inspired Mushroom-Shaped Adhesive Microstructures. Annu. Rev. Mater. Res. 2014, 44, 173–203. [Google Scholar] [CrossRef]
- Federle, W.; Labonte, D. Dynamic biological adhesion: Mechanisms for controlling attachment during locomotion. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190199. [Google Scholar] [CrossRef]
- Pennisi, E. Clingfish Stick Like Geckos. Science 2012, 335, 277. [Google Scholar] [CrossRef]
- Arita, G.S. A Comparative Study of the Structure and Function of the Adhesive Apparatus of the Cyclopteridae and Gobiesocidae. Ph.D. Thesis, University of British Columbia, Kelowna, BC, Canada, 1 August 1967. [Google Scholar]
- Wolff, T.I.D. The Design and Fabrication of a Biomimetic Lifting Aid. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 27 January 2017. [Google Scholar]
- Gorb, S.N.; Varenberg, M. Mushroom-shaped geometry of contact elements in biological adhesive systems. J. Adhes. Sci. Technol. 2007, 21, 1175–1183. [Google Scholar] [CrossRef]
- Ditsche, P.; Wainwright, D.K.; Summers, A.P. Attachment to challenging substrates-fouling, roughness and limits of adhesion in the northern clingfish (Gobiesox maeandricus). J. Exp. Biol. 2014, 217, 2548–2554. [Google Scholar] [CrossRef]
- Britz, R.; Johnson, G.D. Ontogeny and homology of the skeletal elements that form the sucking disc of remoras (Teleostei, Echeneoidei, Echeneidae). J. Morphol. 2012, 273, 1353–1366. [Google Scholar] [CrossRef]
- Cohen, K.E.; Crawford, C.H.; Hernandez, L.P.; Beckert, M.; Nadler, J.H.; Flammang, B.E. Sucker with a fat lip: The soft tissues underlying the viscoelastic grip of remora adhesion. J. Anat. 2020, 237, 643–654. [Google Scholar] [CrossRef]
- Weihs, D.; Fish, F.E.; Nicastro, A.J. Mechanics of Remora Removal by Dolphin Spinning. Mar. Mamm. Sci. 2007, 23, 707–714. [Google Scholar] [CrossRef]
- Fulcher, B.A.; Motta, P.J. Suction disk performance of echeneid fishes. Can. J. Zool. 2006, 84, 42–50. [Google Scholar] [CrossRef]
- Hora, S.L.; Ashworth, J.H.V. Ecology, bionomics and evolution of the torrential fauna, with special reference to the organs of attachment. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1930, 218, 171–282. [Google Scholar] [CrossRef]
- Van Wassenbergh, S.; Lieben, T.; Herrel, A.; Huysentruyt, F.; Geerinckx, T.; Adriaens, D.; Aerts, P. Kinematics of benthic suction feeding in Callichthyidae and Mochokidae, with functional implications for the evolution of food scraping in catfishes. J. Exp. Biol. 2009, 212, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Geerinckx, T.; Herrel, A.; Adriaens, D. Suckermouth armored catfish resolve the paradox of simultaneous respiration and suction attachment: A kinematic study of Pterygoplichthys disjunctivus. J. Exp. Zool. A Ecol. Genet. Physiol. 2011, 315, 121–131. [Google Scholar] [CrossRef]
- Tripathi, P.; Mittal, S.; Ojha, J.; Mittal, A. Scanning Electron Microscopic Study of the Structures Associated with Lips of an Indian Hill Stream Fish Garra lamta (Cyprinidae, Cypriniformes). Eur. J. Morphol. 2002, 40, 161–169. [Google Scholar] [CrossRef]
- Mittal, S.; Yashpal, M.; Ojha, J.; Mittal, A. Occurrence of keratinization in the structures associated with lips of a hill stream fish Garra lamta (Hamilton) (Cyprinidae, Cypriniformes). J. Fish Biol. 2004, 65, 1165–1172. [Google Scholar] [CrossRef]
- Teimori, A.; Esmaeili, H.; Ansari, M. Micro-structure Consideration of the Adhesive Organ in Doctor Fish, Garra rufa (Teleostei; Cyprinidae) from the Persian Gulf Basin. Turk. J. Fish. Aquat. Sci. 2011, 11, 407–411. [Google Scholar] [CrossRef]
- Ojha, J.; Singh, S.K. Functional morphology of the anchorage system and food scrapers of a hillstream fish, Garra lamta (Ham.) (Cyprinidae, Cypriniformes). J. Fish Biol. 1992, 41, 159–161. [Google Scholar] [CrossRef]
- Hussain, J.F.; Bordoloi, S. Adaptive Modifications in Four Fish Species of the Genus Garra (Teleostei; Cyprinidae) in Basistha River, Assam, India. Microsc. Microanal. 2018, 24, 310–317. [Google Scholar] [CrossRef]
- Saxena, S.C.; Chandy, M. Adhesive apparatus in certain Indian hill stream fishes. J. Zool. 1966, 148, 315–340. [Google Scholar] [CrossRef]
- Saxena, S.C.; Chandy, M. The pelvic girdle and fin in certain Indian hill stream fishes. J. Zool. 1966, 148, 167–190. [Google Scholar] [CrossRef]
- Taillebois, L.; Castelin, M.; Lord, C.; Chabarria, R.; Dettai, A.; Keith, P. New Sicydiinae phylogeny (Teleostei: Gobioidei) inferred from mitochondrial and nuclear genes: Insights on systematics and ancestral areas. Mol. Phylogenet. Evol. 2014, 70, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Forker, G.K.; Schoenfuss, H.L.; Blob, R.W.; Diamond, K.M. Bendy to the bone: Links between vertebral morphology and waterfall climbing in amphidromous gobioid fishes. J. Anat. 2021, 239, 747–754. [Google Scholar] [CrossRef]
- McCraney, W.T.; Thacker, C.E.; Alfaro, M.E. Supermatrix phylogeny resolves goby lineages and reveals unstable root of Gobiaria. Mol. Phylogenet. Evol. 2020, 151, 106862. [Google Scholar] [CrossRef]
- Cediel, R.A.; Blob, R.W.; Schrank, G.D.; Plourde, R.C.; Schoenfuss, H.L. Muscle fiber type distribution in climbing Hawaiian gobioid fishes: Ontogeny and correlations with locomotor performance. Zoology 2008, 111, 114–122. [Google Scholar] [CrossRef]
- Blob, R.W.; Wright, K.M.; Becker, M.; Maie, T.; Iverson, T.J.; Julius, M.L.; Schoenfuss, H.L. Ontogenetic change in novel functions: Waterfall climbing in adult Hawaiian gobiid fishes. J. Zool. 2007, 273, 200–209. [Google Scholar] [CrossRef]
- Maie, T.; Schoenfuss, H.L.; Blob, R.W. Ontogenetic Scaling of Body Proportions In Waterfall-climbing Gobiid Fishes from Hawai’i and Dominica: Implications for Locomotor Function. Copeia 2007, 270, 755–764. [Google Scholar] [CrossRef]
- Schoenfuss, H.L.; Maie, T.; Kawano, S.M.; Blob, R.W. Performance across extreme environments: Comparing waterfall climbing among amphidromous gobioid fishes from Caribbean and Pacific Islands. Cybium 2011, 35, 361–369. [Google Scholar]
- Palecek, A.M.; Schoenfuss, H.L.; Blob, R.W. Sticking to it: Testing passive pull-off forces in waterfall-climbing fishes across challenging substrates. J. Exp. Biol. 2021, 224, jeb228718. [Google Scholar] [CrossRef]
- Budney, L.A.; Hall, B.K. Comparative morphology and osteology of pelvic fin-derived midline suckers in lumpfishes, snailfishes and gobies. J. Appl. Ichthyol. 2010, 26, 167–175. [Google Scholar] [CrossRef]
- Maie, T.; Schoenfuss, H.L.; Blob, R.W. Musculoskeletal determinants of pelvic sucker function in Hawaiian stream gobiid fishes: Interspecific comparisons and allometric scaling. J. Morphol. 2013, 274, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Maie, T.; Blob, R.W. Adhesive force and endurance of the pelvic sucker across different modes of waterfall-climbing in gobiid fishes: Contrasting climbing mechanisms share aspects of ontogenetic change. Zoology 2021, 149, 125969. [Google Scholar] [CrossRef] [PubMed]
- Palecek, A.M.; Schoenfuss, H.L.; Blob, R.W. Sucker Shapes, Skeletons, and Bioinspiration: How Hard and Soft Tissue Morphology Generates Adhesive Performance in Waterfall Climbing Goby Fishes. Integr. Comp. Biol. 2022, 62, 934–944. [Google Scholar] [CrossRef]
- De Meyer, J.; Geerinckx, T. Using the whole body as a sucker: Combining respiration and feeding with an attached lifestyle in hill stream loaches (Balitoridae, Cypriniformes). J. Morphol. 2014, 275, 1066–1079. [Google Scholar] [CrossRef]
- Crawford, C.H.; Randall, Z.S.; Hart, P.B.; Page, L.M.; Chakrabarty, P.; Suvarnaraksha, A.; Flammang, B.E. Skeletal and muscular pelvic morphology of hillstream loaches (Cypriniformes: Balitoridae). J. Morphol. 2020, 281, 1280–1295. [Google Scholar] [CrossRef]
- Wang, J.; Ji, C.; Wang, W.; Zou, J.; Yang, H.; Pan, M. An adhesive locomotion model for the rock-climbing fish, Beaufortia kweichowensis. Sci. Rep. 2019, 9, 16571. [Google Scholar] [CrossRef]
- Conway, K.W.; Lujan, N.K.; Lundberg, J.G.; Mayden, R.L.; Siegel, D.S. Microanatomy of the paired-fin pads of ostariophysan fishes (Teleostei: Ostariophysi). J. Morphol. 2012, 273, 1127–1149. [Google Scholar] [CrossRef]
- Frick, K.E.; Corbett, S.C.; Moser, M.L. Climbing success of adult Pacific lamprey on a vertical wetted wall. Fish. Manag. Ecol. 2017, 24, 230–239. [Google Scholar] [CrossRef]
- Pronko, A.J.; Perlman, B.M.; Ashley-Ross, M.A. Launches, squiggles and pounces, oh my! The water-land transition in mangrove rivulus (Kryptolebias marmoratus). J. Exp. Biol. 2013, 216, 3988–3995. [Google Scholar] [CrossRef]
- Flammang, B.E.; Suvarnaraksha, A.; Markiewicz, J.; Soares, D. Tetrapod-like pelvic girdle in a walking cavefish. Sci. Rep. 2016, 6, 23711. [Google Scholar] [CrossRef]
- Coates, M.I. The Evolution of Paired Fins. Theory Biosci. 2003, 122, 266–287. [Google Scholar] [CrossRef]
- King, H.M.; Shubin, N.H.; Coates, M.I.; Hale, M.E. Behavioral evidence for the evolution of walking and bounding before terrestriality in sarcopterygian fishes. Proc. Natl. Acad. Sci. USA 2011, 108, 21146–21151. [Google Scholar] [CrossRef]
- Ekstrom, L.J.; Kajiura, S.M. Pelvic girdle shape predicts locomotion and phylogeny in batoids. J. Morphol. 2014, 275, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Gomez-Skarmeta, J.L.; Rodrigues, P.N. New frontiers in the evolution of fin development. J. Exp. Zool. B Mol. Dev. Evol. 2014, 322, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.V.; Clack, J.A.; Smithson, T.R.; Pierce, S.E. Functional adaptive landscapes predict terrestrial capacity at the origin of limbs. Nature 2021, 589, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Nassiraei, A.A.F.; Sonoda, T.; Ishii, K. Development of Ship Hull Cleaning Underwater Robot. In Proceedings of the 2012 Fifth International Conference on Emerging Trends in Engineering and Technology, Himeji, Japan, 5–7 November 2012; pp. 157–162. [Google Scholar]
- Souto, D.; Faiña, A.; López-Peña, F.; Duro, R.J. Lappa: A new type of robot for underwater non-magnetic and complex hull cleaning. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 3409–3414. [Google Scholar]
- Guo, T.; Liu, X.; Song, D. Innovative sliding negative pressure adsorptive approach applied to an underwater climbing adsorption robot. Phys. Fluids 2021, 33, 117107. [Google Scholar] [CrossRef]
- Guo, T.; Deng, Z.D.; Liu, X.; Song, D.; Yang, H. Development of a new hull adsorptive underwater climbing robot using the Bernoulli negative pressure effect. Ocean Eng. 2022, 243, 110306. [Google Scholar] [CrossRef]
- Guo, T.; Liu, X.; He, T.; Song, D. Synchro-Drive-Based Underwater Climbing Adsorption Robot. IEEE Robot. Autom. Lett. 2022, 7, 6250–6257. [Google Scholar] [CrossRef]
- Yan, H.; Yin, Q.; Peng, J.; Bai, B. Multi-functional Tugboat for Monitoring and Cleaning Bottom Fouling. IOP Conf. Ser. Earth Environ. Sci. 2019, 237, 022045. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Zhang, L.; Zheng, P.; Yang, C. Study on the adsorption performance of underwater propeller-driven Bernoulli adsorption device. Ocean Eng. 2022, 266, 112724. [Google Scholar] [CrossRef]
- Fan, J.; Yang, C.; Chen, Y.; Wang, H.; Huang, Z.; Shou, Z.; Jiang, P.; Wei, Q. An underwater robot with self-adaption mechanism for cleaning steel pipes with variable diameters. Ind. Rob. 2018, 45, 193–205. [Google Scholar] [CrossRef]
- Lu, H.; Yun, G.; Cole, T.; Ouyang, Y.; Ren, H.; Shu, J.; Zhang, Y.; Zhang, S.; Dickey, M.D.; Li, W.; et al. Reversible Underwater Adhesion for Soft Robotic Feet by Leveraging Electrochemically Tunable Liquid Metal Interfaces. ACS Appl. Mater. Interfaces 2021, 13, 37904–37914. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, C.; Chen, Y.; Li, J.; Liu, S.; Ye, G. Study on the Optimal Design for Cavitation Reduction in the Vortex Suction Cup for Underwater Climbing Robot. J. Mar. Sci. Eng. 2022, 10, 70. [Google Scholar] [CrossRef]
- Albitar, H.; Dandan, K.; Ananiev, A.; Kalaykov, I. Underwater Robotics: Surface Cleaning Technics, Adhesion and Locomotion Systems. Int. J. Adv. Robot. Syst. 2016, 13, 7. [Google Scholar] [CrossRef]
- Song, C.; Cui, W. Review of Underwater Ship Hull Cleaning Technologies. J. Mar. Sci. Appl. 2020, 19, 415–429. [Google Scholar] [CrossRef]
- Chen, L.; Cui, R.; Yan, W.; Xu, H.; Zhao, H.; Li, H. Design and climbing control of an underwater robot for ship hull cleaning. Ocean Eng. 2023, 274, 114024. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, J.; Gu, Z.; Wan, X.; Jiang, L.; Wang, S. Bioinspired Multiscale Wet Adhesive Surfaces: Structures and Controlled Adhesion. Adv. Funct. Mater. 2019, 30, 1905287. [Google Scholar] [CrossRef]
- Beckert, M.; Flammang, B.E.; Nadler, J.H. Remora attachment is enhanced by spinule friction. J. Exp. Biol. 2015, 218, 3551–3558. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- van Rees, W.M.; Matsumoto, E.A.; Gladman, A.S.; Lewis, J.A.; Mahadevan, L. Mechanics of biomimetic 4D printed structures. Soft Matter 2018, 14, 8771–8779. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Breakthrough in the printing tactics for stimuli-responsive materials: 4D printing. Chem. Eng. J. 2019, 366, 264–304. [Google Scholar] [CrossRef]
- Cheng, T.; Thielen, M.; Poppinga, S.; Tahouni, Y.; Wood, D.; Steinberg, T.; Menges, A.; Speck, T. Bio-Inspired Motion Mechanisms: Computational Design and Material Programming of Self-Adjusting 4D-Printed Wearable Systems. Adv. Sci. 2021, 8, 2100411. [Google Scholar] [CrossRef] [PubMed]
- Shakibania, S.; Ghazanfari, L.; Raeeszadeh-Sarmazdeh, M.; Khakbiz, M. Medical application of biomimetic 4D printing. Drug Dev. Ind. Pharm. 2021, 47, 521–534. [Google Scholar] [CrossRef]
- Zhou, X.; Ren, L.; Song, Z.; Li, G.; Zhang, J.; Li, B.; Wu, Q.; Li, W.; Ren, L.; Liu, Q. Advances in 3D/4D printing of mechanical metamaterials: From manufacturing to applications. Compos. B Eng. 2023, 254, 110585. [Google Scholar] [CrossRef]
- Lauder, G.V. Fish locomotion: Recent advances and new directions. Annu. Rev. Mar. Sci. 2015, 7, 521–545. [Google Scholar] [CrossRef]
- Lauder, G.V.; Madden, P.G. Advances in comparative physiology from high-speed imaging of animal and fluid motion. Annu. Rev. Physiol. 2008, 70, 143–163. [Google Scholar] [CrossRef]
- Flammang, B.E.; Lauder, G.V.; Troolin, D.R.; Strand, T.E. Volumetric imaging of fish locomotion. Biol. Lett. 2011, 7, 695–698. [Google Scholar] [CrossRef]
- Liao, J.; Beal, D.; Lauder, G.; Triantafyllou, M. Fish Exploiting Vortices Decrease Muscle Activity. Science 2003, 302, 1566–1569. [Google Scholar] [CrossRef]
- Liu, G.; Ren, Y.; Dong, H.; Akanyeti, O.; Liao, J.C.; Lauder, G.V. Computational analysis of vortex dynamics and performance enhancement due to body–fin and fin–fin interactions in fish-like locomotion. J. Fluid Mech. 2017, 829, 65–88. [Google Scholar] [CrossRef]
- Wang, J.; Wainwright, D.K.; Lindengren, R.E.; Lauder, G.V.; Dong, H. Tuna locomotion: A computational hydrodynamic analysis of finlet function. J. R. Soc. Interface 2020, 17, 20190590. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Dong, H.; Bozkurttas, M.; Lauder, G.; Madden, P. Locomotion with flexible propulsors: II. Computational modeling of pectoral fin swimming in sunfish. Bioinspir. Biomim. 2006, 1, S35–S41. [Google Scholar] [CrossRef]
- Arnold, G.P.; Webb, P.W.; Holford, B.H. The Role of the Pectoral Fins in Station-Holding of Atlantic Salmon Parr (Salmo salar L.). J. Exp. Biol. 1991, 156, 625–629. [Google Scholar] [CrossRef]
- Carlson, R.L.; Lauder, G.V. Living on the bottom: Kinematics of benthic station-holding in darter fishes (Percidae: Etheostomatinae). J. Morphol. 2010, 271, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.L.; Lauder, G.V. Escaping the flow: Boundary layer use by the darter Etheostoma tetrazonum (Percidae) during benthic station holding. J. Exp. Biol. 2011, 214, 1181–1193. [Google Scholar] [CrossRef]
- Taft, N.K.; Lauder, G.V.; Madden, P.G.A. Functional regionalization of the pectoral fin of the benthic longhorn sculpin during station holding and swimming. J. Zool. 2008, 276, 159–167. [Google Scholar] [CrossRef]
- Roderick, W.R.; Cutkosky, M.R.; Lentink, D. Touchdown to take-off: At the interface of flight and surface locomotion. Interface Focus 2017, 7, 20160094. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, C.; Liu, L. An Introduction to Biomimetic Underwater Adhesion System. In Proceedings of the 2018 13th World Congress on Intelligent Control and Automation (WCICA), Changsha, China, 4–8 July 2018; pp. 479–483. [Google Scholar]
- Sun, B.; Li, W.; Wang, Z.; Zhu, Y.; He, Q.; Guan, X.; Dai, G.; Yuan, D.; Li, A.; Cui, W.; et al. Recent Progress in Modeling and Control of Bio-Inspired Fish Robots. J. Mar. Sci. Eng. 2022, 10, 773. [Google Scholar] [CrossRef]
- Youssef, S.M.; Soliman, M.; Saleh, M.A.; Mousa, M.A.; Elsamanty, M.; Radwan, A.G. Underwater Soft Robotics: A Review of Bioinspiration in Design, Actuation, Modeling, and Control. Micromachines 2022, 13, 110. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Li, H.; Shen, J.; Zhang, F.; He, J.; Lin, J.; Wang, B.; Niu, S.; Han, Z.; et al. Bioinspired hydrogel actuator for soft robotics: Opportunity and challenges. Nano Today 2023, 49, 101764. [Google Scholar] [CrossRef]
- Zimmerman, S.; Abdelkefi, A. Review of marine animals and bioinspired robotic vehicles: Classifications and characteristics. Prog. Aerosp. Sci. 2017, 93, 95–119. [Google Scholar] [CrossRef]















| Biological Prototype | Characteristics | Pictures |
|---|---|---|
| Clingfish |
|  (Reprinted with permission from Ref. [27]. Copyright 2019, the Royal Society) (Reprinted with permission from Ref. [27]. Copyright 2019, the Royal Society) |
|  (Reprinted with permission from Ref. [28,32]. Copyright 2019, IOP Publishing) (Reprinted with permission from Ref. [28,32]. Copyright 2019, IOP Publishing) | |
|  (Reprinted with permission from Ref. [31]. Copyright 2022, Company of Biologists Ltd.) (Reprinted with permission from Ref. [31]. Copyright 2022, Company of Biologists Ltd.) | |
| Remora |
|  (Reprinted with permission from Ref. [29]. Copyright 2019, American Chemical Society) (Reprinted with permission from Ref. [29]. Copyright 2019, American Chemical Society) |
|  (Reprinted with permission from Ref. [30]. Copyright 2020, Elsevier Ltd.) (Reprinted with permission from Ref. [30]. Copyright 2020, Elsevier Ltd.) |
| Biological Prototype | Characteristics | Pictures |
|---|---|---|
| Remora |
|  (Reprinted with permission from Ref. [33]. Copyright 2017, the American Association for the Advancement of Science) (Reprinted with permission from Ref. [33]. Copyright 2017, the American Association for the Advancement of Science) |
|  (Reprinted with permission from Ref. [34]. Copyright 2020, IOP Publishing) (Reprinted with permission from Ref. [34]. Copyright 2020, IOP Publishing) | |
|  (Reprinted with permission from Ref. [35]. Copyright 2022, IOP Publishing) (Reprinted with permission from Ref. [35]. Copyright 2022, IOP Publishing) | |
|  (Reprinted with permission from Ref. [36]. Copyright 2022, the American Association for the Advancement of Science) (Reprinted with permission from Ref. [36]. Copyright 2022, the American Association for the Advancement of Science) | |
| Hill stream loach |
|  (Reprinted with permission from Ref. [37]. Copyright 2022, Springer Nature) (Reprinted with permission from Ref. [37]. Copyright 2022, Springer Nature) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, S.; Zheng, L.; Ren, L. Adhesion Behavior in Fish: From Structures to Applications. Biomimetics 2023, 8, 534. https://doi.org/10.3390/biomimetics8070534
Wang J, Wang S, Zheng L, Ren L. Adhesion Behavior in Fish: From Structures to Applications. Biomimetics. 2023; 8(7):534. https://doi.org/10.3390/biomimetics8070534
Chicago/Turabian StyleWang, Jinhao, Shukun Wang, Long Zheng, and Luquan Ren. 2023. "Adhesion Behavior in Fish: From Structures to Applications" Biomimetics 8, no. 7: 534. https://doi.org/10.3390/biomimetics8070534