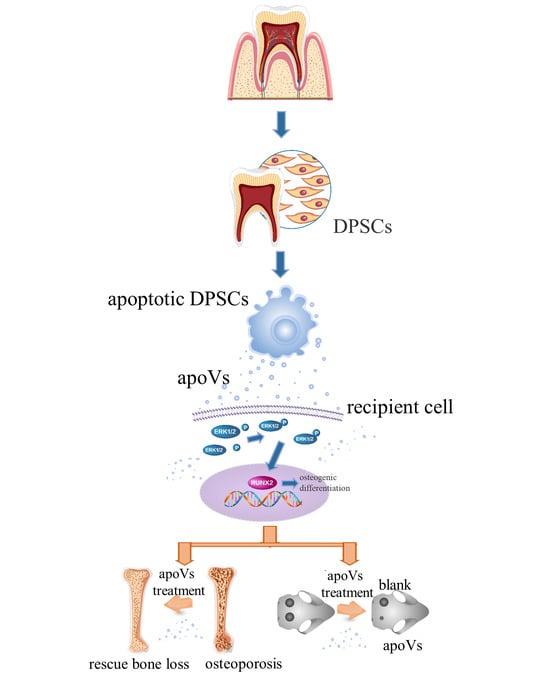
Apoptotic Vesicles Derived from Dental Pulp Stem Cells Promote Bone Formation through the ERK1/2 Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation and Purification of apoVs Derived from DPSCs
2.3. TdT-Mediated dUTP Nick End Labeling (TUNEL) Staining
2.4. DPSC-Derived apoVs Uptake by MSCs In Vitro
2.5. Identification of DPSC-Derived apoVs
2.6. Cell Proliferation Assay
2.7. Alkaline Phosphatase (ALP) Staining and Quantification
2.8. Alizarin Red S (ARS) Staining and Quantification
2.9. RNA Extraction and Quantitative Real-Time Reverse Transcription PCR (qRT-PCR)
2.10. Western Blotting
2.11. Heterotypic Bone Formation Assay In Vivo
2.12. In Vivo Experiment with apoV Injection
2.13. Microcomputed Tomography and Bone Morphometric Analysis
2.14. Osteogenic Efficiency in Bone Defects
2.15. Statistical Analysis
3. Results
3.1. Characterization of DPSC-Derived apoVs and the Uptake by MSCs
3.2. ApoVs’ Internalization by MSCs
3.3. ApoVs Promote the Osteogenic Differentiation of MSCs In Vitro and In Vivo
3.4. ApoVs Reduced the Loss of Microarchitecture and Bone Mass in OVX Mice
3.5. ApoVs Reduced the Loss of Microarchitecture and Bone Mass in Aged Mice
3.6. ApoVs Promoted Critical-Sized Rat Calvarial Defects In Vivo
3.7. ApoVs Promote the Osteogenic Development of MSCs via the ERK1/2 Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A








References
- Delaisse, J.M.; Andersen, T.L.; Kristensen, H.B.; Jensen, P.R.; Andreasen, C.M.; Søe, K. Re-thinking the bone remodeling cycle mechanism and the origin of bone loss. Bone 2020, 141, 115628. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xiao, L.; Peng, J.; Peng, J.; Qian, Y.Q.; Huang, C.C. Exosomes derived from bone marrow mesenchymal stem cells improve osteoporosis through promoting osteoblast proliferation via MAPK pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3962–3970. [Google Scholar] [PubMed]
- Jiang, H.T.; Ran, C.C.; Liao, Y.P.; Zhu, J.H.; Wang, H.; Deng, R.; Nie, M.; He, B.C.; Deng, Z.L. IGF-1 reverses the osteogenic inhibitory effect of dexamethasone on BMP9-induced osteogenic differentiation in mouse embryonic fibroblasts via PI3K/AKT/COX-2 pathway. J. Steroid Biochem. Mol. Biol. 2019, 191, 105363. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, Y.; Hu, X.; Shang, X.; Ma, S.; Guo, H.; Ma, X.; Cai, D.; Hu, Z.; Zhao, Y.; et al. Titanium dioxide nanotubes increase purinergic receptor P2Y6 expression and activate its downstream PKCα-ERK1/2 pathway in bone marrow mesenchymal stem cells under osteogenic induction. Acta Biomater. 2023, 157, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shibata, Y.; Zhu, T.; Zhou, J.; Zhang, J. Osteocytes in bone aging: Advances, challenges, and future perspectives. Ageing Res. Rev. 2022, 77, 101608. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Tang, Y.; Zhou, T.; Yang, L.; Zhang, G.; Meng, Y.; Zhang, H.; Gao, J.; Wang, C.; Su, Y.X.; et al. Exosomes derived from mesenchymal stromal cells promote bone regeneration by delivering miR-182-5p-inhibitor. Pharmacol. Res. 2023, 192, 106798. [Google Scholar] [CrossRef]
- Liao, F.; Liao, Z.; Zhang, T.; Jiang, W.; Zhu, P.; Zhao, Z.; Shi, H.; Zhao, D.; Zhou, N.; Huang, X. ECFC-derived exosomal THBS1 mediates angiogenesis and osteogenesis in distraction osteogenesis via the PI3K/AKT/ERK pathway. J. Orthop. Transl. 2022, 37, 12–22. [Google Scholar] [CrossRef]
- Keremu, A.; Aila, P.; Tusun, A.; Abulikemu, M.; Zou, X. Extracellular vesicles from bone mesenchymal stem cells transport microRNA-206 into osteosarcoma cells and target NRSN2 to block the ERK1/2-Bcl-xL signaling pathway. Eur. J. Histochem. 2022, 66, 3394. [Google Scholar] [CrossRef]
- Chu, D.T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, D.K.; Thanh, V.V.; Quang, T.L.; Truong, D.T.; Pham, V.H.; Ngoc, V.T.N.; Chu-Dinh, T.; et al. An Update on the Progress of Isolation, Culture, Storage, and Clinical Application of Human Bone Marrow Mesenchymal Stem/Stromal Cells. Int. J. Mol. Sci. 2020, 21, 708. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tang, J.; Kou, X.; Huang, W.; Zhu, Y.; Jiang, Y.; Yang, K.; Li, C.; Hao, M.; Qu, Y.; et al. Proteomic analysis of MSC-derived apoptotic vesicles identifies Fas inheritance to ameliorate haemophilia a via activating platelet functions. J. Extracell. Vesicles 2022, 11, e12240. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, S.; Qiu, X.; Yang, X.; Bao, L.; Pu, F.; Liu, X.; Li, C.; Xuan, K.; Zhou, J.; et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy 2020, 16, 2140–2155. [Google Scholar] [CrossRef] [PubMed]
- Dou, G.; Tian, R.; Liu, X.; Yuan, P.; Ye, Q.; Liu, J.; Liu, S.; Zhou, J.; Deng, Z.; Chen, X.; et al. Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci. Adv. 2020, 6, eaba2987. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Kou, X.; Chen, C.; Liu, S.; Liu, Y.; Yu, W.; Yu, T.; Yang, R.; Wang, R.; Zhou, Y.; et al. Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res. 2018, 28, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254. [Google Scholar] [CrossRef]
- Zhang, W.; Yelick, P.C. Tooth Repair and Regeneration: Potential of Dental Stem Cells. Trends Mol. Med. 2021, 27, 501–511. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 2019, 67, 432–442. [Google Scholar] [CrossRef]
- Imanishi, Y.; Hata, M.; Matsukawa, R.; Aoyagi, A.; Omi, M.; Mizutani, M.; Naruse, K.; Ozawa, S.; Honda, M.; Matsubara, T.; et al. Efficacy of extracellular vesicles from dental pulp stem cells for bone regeneration in rat calvarial bone defects. Inflamm. Regen. 2021, 41, 12. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X.; Chen, Y.; Liu, B.; Chen, G. Dental stem cell-derived extracellular vesicles as promising therapeutic agents in the treatment of diseases. Int. J. Oral. Sci. 2022, 14, 2. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Yin, Y.; He, X.T.; An, Y.; Tian, B.M.; Hong, Y.L.; Wu, L.A.; Chen, F.M. The proangiogenic effects of extracellular vesicles secreted by dental pulp stem cells derived from periodontally compromised teeth. Stem Cell Res. Ther. 2020, 11, 110. [Google Scholar] [CrossRef]
- Liu, C.; Hu, F.; Jiao, G.; Guo, Y.; Zhou, P.; Zhang, Y.; Zhang, Z.; Yi, J.; You, Y.; Li, Z.; et al. Dental pulp stem cell-derived exosomes suppress M1 macrophage polarization through the ROS-MAPK-NFκB P65 signaling pathway after spinal cord injury. J. Nanobiotechnol. 2022, 20, 65. [Google Scholar] [CrossRef]
- Tian, J.; Chen, W.; Xiong, Y.; Li, Q.; Kong, S.; Li, M.; Pang, C.; Qiu, Y.; Xu, Z.; Gong, Q.; et al. Small extracellular vesicles derived from hypoxic preconditioned dental pulp stem cells ameliorate inflammatory osteolysis by modulating macrophage polarization and osteoclastogenesis. Bioact. Mater. 2023, 22, 326–342. [Google Scholar] [CrossRef]
- Qiao, X.; Tang, J.; Dou, L.; Yang, S.; Sun, Y.; Mao, H.; Yang, D. Dental Pulp Stem Cell-Derived Exosomes Regulate Anti-Inflammatory and Osteogenesis in Periodontal Ligament Stem Cells and Promote the Repair of Experimental Periodontitis in Rats. Int. J. Nanomed. 2023, 18, 4683–4703. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, K.; Cheng, Y.; Liu, Y.; Gu, R.; Liu, X.; Liu, H.; Zhang, X.; Liu, Y. Apoptotic Vesicles Regulate Bone Metabolism via the miR1324/SNX14/SMAD1/5 Signaling Axis. Small 2023, 19, e2205813. [Google Scholar] [CrossRef]
- Li, Z.; Wu, M.; Liu, S.; Liu, X.; Huan, Y.; Ye, Q.; Yang, X.; Guo, H.; Liu, A.; Huang, X.; et al. Apoptotic vesicles activate autophagy in recipient cells to induce angiogenesis and dental pulp regeneration. Mol. Ther. 2022, 30, 3193–3208. [Google Scholar] [CrossRef]
- Lu, Y.; Mai, Z.; Cui, L.; Zhao, X. Engineering exosomes and biomaterial-assisted exosomes as therapeutic carriers for bone regeneration. Stem Cell Res. Ther. 2023, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Feltrin, F.D.S.; Agner, T.; Sayer, C.; Lona, L.M.F. Curcumin encapsulation in functional PLGA nanoparticles: A promising strategy for cancer therapies. Adv. Colloid. Interface Sci. 2022, 300, 102582. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Du, Y.; Hu, M.; Tian, Y.; Li, Z.; Lv, L.; Zhang, X.; Liu, Y.; Zhou, Y.; et al. DUSP5 promotes osteogenic differentiation through SCP1/2-dependent phosphorylation of SMAD1. Stem Cells 2021, 39, 1395–1409. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Yu, S.; Jin, R.; Xiao, Y.; Pan, M.; Pei, F.; Zhu, X.; Huang, H.; Zhang, Z.; Chen, S.; et al. Circulating miR-338 Cluster activities on osteoblast differentiation: Potential Diagnostic and Therapeutic Targets for Postmenopausal Osteoporosis. Theranostics 2019, 9, 3780–3797. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Li, W.; Zhang, W.; Yang, C.; Zhang, C.; Liang, X.; Yin, J.; Bai, J.; Ge, G.; Zhang, H.; et al. Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-κB activated pyroptosis pathways. Pharmacol. Res. 2021, 174, 105967. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Hong, C.-G.; Hu, W.-B.; Chen, M.-L.; Duan, R.; Li, H.-M.; Yue, T.; Cao, J.; Wang, Z.-X.; Chen, C.-Y.; et al. Autophagy receptor OPTN (optineurin) regulates mesenchymal stem cell fate and bone-fat balance during aging by clearing FABP3. Autophagy 2020, 17, 2766–2782. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Tao, Y.; Yang, C.; Li, W.; Zhang, W.; Li, X.; Gu, Y.; Hong, Y.; Yang, H.; Liu, Y.; et al. Gut Metabolite Urolithin A Inhibits Osteoclastogenesis and Senile Osteoporosis by Enhancing the Autophagy Capacity of Bone Marrow Macrophages. Front. Pharmacol. 2022, 13, 875611. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Yang, Y.-S.; Hong, J.; Chaugule, S.; Chun, H.; van der Meulen, M.C.H.; Xu, R.; Greenblatt, M.B.; Shim, J.-H. Biphasic regulation of osteoblast development via the ERK MAPK–mTOR pathway. eLife 2022, 11, e78069. [Google Scholar] [CrossRef]
- Li, J.; Du, H.; Ji, X.; Chen, Y.; Li, Y.; Heng, B.C.; Xu, J. ETV2 promotes osteogenic differentiation of human dental pulp stem cells through the ERK/MAPK and PI3K-Akt signaling pathways. Stem Cell Res. Ther. 2022, 13, 495. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Targeting ERK1/2 protein-serine/threonine kinases in human cancers. Pharmacol. Res. 2019, 142, 151–168. [Google Scholar] [CrossRef]
- Shin, M.J.; Park, J.Y.; Lee, D.H.; Khang, D. Stem Cell Mimicking Nanoencapsulation for Targeting Arthritis. Int. J. Nanomed. 2021, 16, 8485–8507. [Google Scholar] [CrossRef]
- Ma, L.; Chen, C.; Liu, D.; Huang, Z.; Li, J.; Liu, H.; Kin, K.R.T.; Tang, B.; Sui, B.; Zhang, X.; et al. Apoptotic extracellular vesicles are metabolized regulators nurturing the skin and hair. Bioact. Mater. 2023, 19, 626–641. [Google Scholar] [CrossRef]
- Sui, B.; Wang, R.; Chen, C.; Kou, X.; Wu, D.; Fu, Y.; Lei, F.; Wang, Y.; Liu, Y.; Chen, X.; et al. Apoptotic Vesicular Metabolism Contributes to Organelle Assembly and Safeguards Liver Homeostasis and Regeneration. Gastroenterology, 2024; ahead of print. [Google Scholar]
- Piszczatowska, K.; Czerwaty, K.; Cyran, A.M.; Fiedler, M.; Ludwig, N.; Brzost, J.; Szczepański, M.J. The Emerging Role of Small Extracellular Vesicles in Inflammatory Airway Diseases. Diagnostics 2021, 11, 222. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Lai, E.C. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol. Cell 2011, 43, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Hayder, H.; O’Brien, J.; Nadeem, U.; Peng, C. MicroRNAs: Crucial regulators of placental development. Reproduction 2018, 155, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liang, M.; Wu, Y.; Ding, N.; Duan, L.; Yu, T.; Bai, Y.; Kang, F.; Dong, S.; Xu, J.; et al. Mature osteoclast-derived apoptotic bodies promote osteogenic differentiation via RANKL-mediated reverse signaling. J. Biol. Chem. 2019, 294, 11240–11247. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.L.; Wu, Q.Y.; Miao, Z.N.; Xu, M.H.; Xu, R.S.; Jiang, D.L.; Ye, J.X.; Chen, F.H.; Zhao, M.D.; Wang, H.J.; et al. Osteoclast-Derived Extracellular Vesicles: Novel Regulators of Osteoclastogenesis and Osteoclast-Osteoblasts Communication in Bone Remodeling. Front. Physiol. 2018, 9, 628. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Partridge, N.C. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology 2016, 31, 233–245. [Google Scholar] [CrossRef]
- Yahara, Y.; Barrientos, T.; Tang, Y.J.; Puviindran, V.; Nadesan, P.; Zhang, H.; Gibson, J.R.; Gregory, S.G.; Diao, Y.; Xiang, Y.; et al. Erythromyeloid progenitors give rise to a population of osteoclasts that contribute to bone homeostasis and repair. Nat. Cell Biol. 2020, 22, 49–59. [Google Scholar] [CrossRef]
- Veis, D.J.; O’Brien, C.A. Osteoclasts, Master Sculptors of Bone. Annu. Rev. Pathol. 2023, 18, 257–281. [Google Scholar] [CrossRef]
- Song, S.; Guo, Y.; Yang, Y.; Fu, D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther. 2022, 237, 108168. [Google Scholar] [CrossRef]
- Dzau, V.J.; Balatbat, C.A. Health and societal implications of medical and technological advances. Sci. Transl. Med. 2018, 10, eaau4778. [Google Scholar] [CrossRef]
- Foessl, I.; Dimai, H.P.; Obermayer-Pietsch, B. Long-term and sequential treatment for osteoporosis. Nat. Rev. Endocrinol. 2023, 19, 520–533. [Google Scholar] [CrossRef]
- Li, H.; Xiao, Z.; Quarles, L.D.; Li, W. Osteoporosis: Mechanism, Molecular Target and Current Status on Drug Development. Curr. Med. Chem. 2021, 28, 1489–1507. [Google Scholar] [CrossRef]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Chen, L.R.; Chen, K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022, 23, 1376. [Google Scholar] [CrossRef]
- Khan, M.; Cheung, A.M.; Khan, A.A. Drug-Related Adverse Events of Osteoporosis Therapy. Endocrinol. Metab. Clin. N. Am. 2017, 46, 181–192. [Google Scholar] [CrossRef]
- Misch, C.M. Autogenous bone: Is it still the gold standard? Implant Dent. 2010, 19, 361. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, P.; Xue, S.; Xu, Y.; Tan, J.; Liu, G. Adipose-derived stem cells alleviate osteoporosis by enhancing osteogenesis and inhibiting adipogenesis in a rabbit model. Cytotherapy 2014, 16, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhang, J.; Yu, Y.; Ni, Y.; Wei, Y.; Cheng, Y.; Han, L.; Xiao, L.; Ma, X.; Wei, H.; et al. Mitochondrial Transfer Regulates Cell Fate Through Metabolic Remodeling in Osteoporosis. Adv. Sci. 2023, 10, e2204871. [Google Scholar] [CrossRef] [PubMed]
- Macías, I.; Alcorta-Sevillano, N.; Rodríguez, C.I.; Infante, A. Osteoporosis and the Potential of Cell-Based Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 1653. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.H.; Ho, K.N.; Lee, Y.C.; Chou, M.J.; Lew, W.Z.; Huang, H.M.; Lai, P.C.; Feng, S.W. Melatonin enhances osteogenic differentiation of dental pulp mesenchymal stem cells by regulating MAPK pathways and promotes the efficiency of bone regeneration in calvarial bone defects. Stem Cell Res. Ther. 2022, 13, 73. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, L.; Song, L.; Li, X.; Chen, D.; Bai, X. miR-155 Inhibits Mouse Osteoblast Differentiation by Suppressing SMAD5 Expression. Biomed. Res. Int. 2017, 2017, 1893520. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Zhou, M.M. Structure and regulation of MAPK phosphatases. Cell Signal 2004, 16, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Hao, M.; Jiang, F.; Li, J.; Yang, K.; Li, C.; Ma, L.; Liu, S.; Kou, X.; Shi, S.; et al. Lyophilized apoptotic vesicle-encapsulated adhesive hydrogel sponge as a rapid hemostat for traumatic hemorrhage in coagulopathy. J. Nanobiotechnol. 2023, 21, 407. [Google Scholar] [CrossRef] [PubMed]








| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| OCN | AGCCACCGAGACACCATGAGA | GGCTGCACCTTTGCTGGACT |
| RUNX2 | ACTACCAGCCACCGAGACCA | ACTGCTTGCAGCCTTAAATGACTCT |
| COL1A1 | TGGTCCCAAGGGTAACAGCG | AACACCAACAGGGCCAGGCT |
| ALP | ATGGGATGGGTGTCTCCACA | CCACGAAGGGGAACTTGTC |
| GAPDH | AAGGTCGGAGTCAACGGATTTG | TCCTGGAAGATGGTGATGGGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Zhu, Y.; Shao, Y.; Jiang, Y.; Zhu, L.; Liu, Y.; Zhang, P.; Liu, Y.; Zhang, X.; Zhou, Y. Apoptotic Vesicles Derived from Dental Pulp Stem Cells Promote Bone Formation through the ERK1/2 Signaling Pathway. Biomedicines 2024, 12, 730. https://doi.org/10.3390/biomedicines12040730
Yang K, Zhu Y, Shao Y, Jiang Y, Zhu L, Liu Y, Zhang P, Liu Y, Zhang X, Zhou Y. Apoptotic Vesicles Derived from Dental Pulp Stem Cells Promote Bone Formation through the ERK1/2 Signaling Pathway. Biomedicines. 2024; 12(4):730. https://doi.org/10.3390/biomedicines12040730
Chicago/Turabian StyleYang, Kunkun, Yuan Zhu, Yuzi Shao, Yuhe Jiang, Lei Zhu, Yaoshan Liu, Ping Zhang, Yunsong Liu, Xiao Zhang, and Yongsheng Zhou. 2024. "Apoptotic Vesicles Derived from Dental Pulp Stem Cells Promote Bone Formation through the ERK1/2 Signaling Pathway" Biomedicines 12, no. 4: 730. https://doi.org/10.3390/biomedicines12040730