The Effects of Age on Prostatic Responses to Oxytocin and the Effects of Antagonists
Abstract
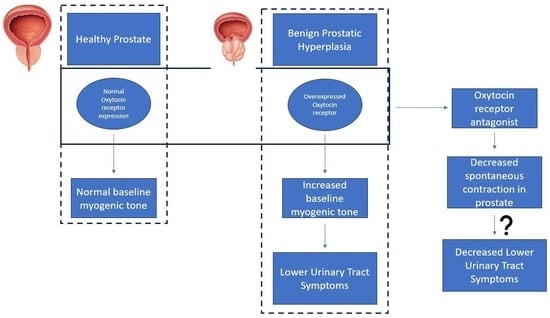
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Tissue Collection
2.3. Reagent Preparation
2.4. Organ Bath Studies
2.5. Spontaneous Contractions
2.6. Statistical Analysis of Tension Recordings
2.7. Immunohistochemistry and Tissue Stains
2.8. Analysis of IHC Experiments
3. Results
3.1. Organ Bath Findings
3.1.1. Atosiban Significantly Downregulated the Frequency of Spontaneous Contractions in the Prostate Tissue
3.1.2. Cligosiban Achieved Significant Inhibition of Spontaneous Activity in Older Rats
3.1.3. β-Mercapto-β,β-cyclopentamethylenepropionyl1, O-Me-Tyr2, Orn8]-Oxytocin (ßMßßC) Significantly Reduced the Spontaneous Contractions in Older Rats
3.2. Immunohistochemistry (IHC) Findings
Oxytocin Receptor (OXTR) Is Widely Expressed within the Epithelial Compartment of Rat Prostate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roehrborn, C. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, E. Prevalence of benign prostatic hyperplasia and prostate cancer in Africans and Africans in the diaspora. J. West Afr. Coll. Surg. 2016, 6, 1–30. [Google Scholar] [PubMed]
- Briganti, A.; Capitanio, U.; Suardi, N.; Gallina, A.; Salonia, A.; Bianchi, M.; Tutolo, M.; Di Girolamo, V.; Guazzoni, G.; Rigatti, P. Benign prostatic hyperplasia and its aetiologies. Eur. Urol. Suppl. 2009, 8, 865–871. [Google Scholar] [CrossRef]
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur. Urol. 2006, 50, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.M.; Ventura, S. Current models of human prostate contractility. Clin. Exp. Pharmacol. Physiol. 2005, 32, 797–804. [Google Scholar] [CrossRef]
- Hyman, M.J.; Groutz, A.; Blaivas, J.G. Detrusor instability in men: Correlation of lower urinary tract symptoms with urodynamic findings. J. Urol. 2001, 166, 550–553. [Google Scholar] [CrossRef]
- Berry, S.J.; Coffey, D.S.; Walsh, P.C.; Ewing, L.L. The development of human benign prostatic hyperplasia with age. J. Urol. 1984, 132, 474–479. [Google Scholar] [CrossRef]
- McConnell, J.D. The epidemiology and pathophysiology of benign prostatic hyperplasia. In Atlas of the Prostate; Current Medicine Group: London, UK, 2003; pp. 1–10. [Google Scholar]
- Shibata, Y.; Ito, K.; Suzuki, K.; Nakano, K.; Fukabori, Y.; Suzuki, R.; Kawabe, Y.; Honma, S.; Yamanaka, H. Changes in the endocrine environment of the human prostate transition zone with aging: Simultaneous quantitative analysis of prostatic sex steroids and comparison with human prostatic histological composition. Prostate 2000, 42, 45–55. [Google Scholar] [CrossRef]
- Carson, C., III; Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003, 61, 2–7. [Google Scholar] [CrossRef]
- Corti, M.; Lorenzetti, S.; Ubaldi, A.; Zilli, R.; Marcoccia, D. Endocrine disruptors and prostate cancer. Int. J. Mol. Sci. 2022, 23, 1216. [Google Scholar] [CrossRef]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F. Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef]
- Salisbury, B.H.; Tadi, P. 5 Alpha reductase inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Lepor, H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev. Urol. 2007, 9, 181. [Google Scholar] [CrossRef]
- Dhaliwal, A.; Gupta, M. PDE5 Inhibitor. [Updated 2020 Jun 23]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gacci, M.; Ficarra, V.; Sebastianelli, A.; Corona, G.; Serni, S.; Shariat, S.F.; Maggi, M.; Zattoni, F.; Carini, M.; Novara, G. Impact of medical treatments for male lower urinary tract symptoms due to benign prostatic hyperplasia on ejaculatory function: A systematic review and meta-analysis. J. Sex. Med. 2014, 11, 1554–1566. [Google Scholar] [CrossRef]
- Gacci, M.; Corona, G.; Salvi, M.; Vignozzi, L.; McVary, K.T.; Kaplan, S.A.; Roehrborn, C.G.; Serni, S.; Mirone, V.; Carini, M. A systematic review and meta-analysis on the use of phosphodiesterase 5 inhibitors alone or in combination with α-blockers for lower urinary tract symptoms due to benign prostatic hyperplasia. Eur. Urol. 2012, 61, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.S.; Nied, R.J.; Dhanani, N. Health-related quality of life in men with erectile dysfunction. J. Gen. Intern. Med. 1998, 13, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ow, D.; Papa, N.; Perera, M.; Liodakis, P.; Sengupta, S.; Clarke, S.; Bolton, D.M.; Lawrentschuk, N. Trends in the surgical treatment of benign prostatic hyperplasia in a tertiary hospital. ANZ J. Surg. 2018, 88, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Bennett, P.R.; Terzidou, V. Advances in the role of oxytocin receptors in human parturition. Mol. Cell. Endocrinol. 2017, 449, 56–63. [Google Scholar] [CrossRef]
- Moberg, K.U.; Prime, D.K. Oxytocin effects in mothers and infants during breastfeeding. Infant 2013, 9, 201–206. [Google Scholar]
- Ludwig, M.; Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 2006, 7, 126–136. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Vasopressin and oxytocin in the mammalian brain and spinal cord. Trends Neurosci. 1983, 6, 467–472. [Google Scholar] [CrossRef]
- Baribeau, D.A.; Anagnostou, E. Oxytocin and vasopressin: Linking pituitary neuropeptides and their receptors to social neurocircuits. Front. Neurosci. 2015, 9, 335. [Google Scholar] [CrossRef]
- Ivell, R.; Balvers, M.; Rust, W.; Bathgate, R.; Einspanier, A. Oxytocin and male reproductive function. Fate Male Germ Cell 1997, 424, 253–264. [Google Scholar]
- Thackare, H.; Nicholson, H.D.; Whittington, K. Oxytocin—Its role in male reproduction and new potential therapeutic uses. Hum. Reprod. Update 2006, 12, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Kiss, A.; Mikkelsen, J.D. Oxytocin—Anatomy and functional assignments: A minireview. Endocr. Regul. 2005, 39, 97–105. [Google Scholar] [PubMed]
- Stadler, B. Role of Oxytocin in the Contractility of the Male Reproductive Tract: Implications for the Treatment of Benign Prostatic Hyperplasia; VVB Laufersweiler Verlag: Gießen, Germany, 2021. [Google Scholar]
- Kunit, T.; Gratzke, C.; Schreiber, A.; Strittmatter, F.; Waidelich, R.; Rutz, B.; Loidl, W.; Andersson, K.-E.; Stief, C.G.; Hennenberg, M. Inhibition of smooth muscle force generation by focal adhesion kinase inhibitors in the hyperplastic human prostate. Am. J. Physiol. Ren. Physiol. 2014, 307, F823–F832. [Google Scholar] [CrossRef]
- Dey, A.; Lang, R.J.; Exintaris, B. Nitric oxide signaling pathways involved in the inhibition of spontaneous activity in the guinea pig prostate. J. Urol. 2012, 187, 2254–2260. [Google Scholar] [CrossRef]
- Exintaris, B.; Klemm, M.F.; Lang, R.J. Spontaneous slow wave and contractile activity of the guinea pig prostate. J. Urol. 2002, 168, 315–322. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Dey, A.; Lam, M.; Ventura, S.; Exintaris, B. Tamsulosin modulates, but does not abolish the spontaneous activity in the guinea pig prostate gland. Neurourol. Urodyn. 2015, 34, 482–488. [Google Scholar] [CrossRef]
- Bodanszky, M.; Sharaf, H.; Roy, J.B.; Said, S.I. Contractile activity of vasotocin, oxytocin, and vasopressin on mammalian prostate. Eur. J. Pharmacol. 1992, 216, 311–313. [Google Scholar] [CrossRef]
- Barberis, C.; Mouillac, B.; Durroux, T. Structural bases of vasopressin/oxytocin receptor function. J. Endocrinol. 1998, 156, 223–229. [Google Scholar] [CrossRef]
- Song, Z.; Albers, H.E. Cross-talk among oxytocin and arginine-vasopressin receptors: Relevance for basic and clinical studies of the brain and periphery. Front. Neuroendocrinol. 2018, 51, 14–24. [Google Scholar] [CrossRef]
- Kroeger, M. Oxytocin: Key hormone in sexual intercourse, parturition, and lactation. Birth Gaz. 1996, 13, 28–30. [Google Scholar] [PubMed]
- Stadler, B.; Whittaker, M.R.; Exintaris, B.; Middendorff, R. Oxytocin in the male reproductive tract; the therapeutic potential of oxytocin-agonists and-antagonists. Front. Endocrinol. 2020, 753, 565731. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, G.M.; Black, M.; Shetty, A.; Bhattacharya, S. Atosiban in the management of preterm labour. Clin. Med. Insights Women’s Health 2011, 4, 9–16. [Google Scholar] [CrossRef]
- Wayman, C.; Russell, R.; Tang, K.; Weibly, L.; Gaboardi, S.; Fisher, L.; Allers, K.; Jackson, M.; Hawcock, T.; Robinson, N. Cligosiban, a novel brain-penetrant, selective oxytocin receptor antagonist, inhibits ejaculatory physiology in rodents. J. Sex. Med. 2018, 15, 1698–1706. [Google Scholar] [CrossRef]
- Althof, S.; Osterloh, I.H.; Muirhead, G.J.; George, K.; Girard, N.; Jennings, W.; Adams, M.; Althof, S.; Shockey, G.; Kaminetsky, J. The oxytocin antagonist cligosiban fails to prolong intravaginal ejaculatory latency in men with lifelong premature ejaculation: Results of a randomized, double-blind, placebo-controlled phase IIb trial (PEDRIX). J. Sex. Med. 2019, 16, 1188–1198. [Google Scholar] [CrossRef]
- Cafarchio, E.M.; Da Silva, L.A.; Auresco, L.C.; Rodart, I.F.; De Souza, J.S.; Antonio, B.B.; Venancio, D.P.; Maifrino, L.B.; Maciel, R.M.; Giannocco, G. Oxytocin reduces intravesical pressure in anesthetized female rats: Action on oxytocin receptors of the urinary bladder. Front. Physiol. 2020, 11, 382. [Google Scholar] [CrossRef]
- Gupta, J.; Russell, R.; Wayman, C.; Hurley, D.; Jackson, V. Oxytocin-induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors. Br. J. Pharmacol. 2008, 155, 118–126. [Google Scholar] [CrossRef]
- Lim, M.M.; Young, L.J. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm. Behav. 2006, 50, 506–517. [Google Scholar] [CrossRef]
- Boivin, B.; Vaniotis, G.; Allen, B.G.; Hebert, T.E. G protein-coupled receptors in and on the cell nucleus: A new signaling paradigm? J. Recept. Signal Transduct. 2008, 28, 15–28. [Google Scholar] [CrossRef]
- Gobeil, F.; Fortier, A.; Zhu, T.; Bossolasco, M.; Leduc, M.; Grandbois, M.; Heveker, N.; Bkaily, G.; Chemtob, S.; Barbaz, D. G-protein-coupled receptors signalling at the cell nucleus: An emerging paradigm. Can. J. Physiol. Pharmacol. 2006, 84, 287–297. [Google Scholar] [CrossRef]
- Verzijl, D.; Peters, S.L.; Alewijnse, A.E. Sphingosine-1-phosphate receptors: Zooming in on ligand-induced intracellular trafficking and its functional implications. Mol. Cells 2010, 29, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Preininger, A.M.; Meiler, J.; Hamm, H.E. Conformational flexibility and structural dynamics in GPCR-mediated G protein activation: A perspective. J. Mol. Biol. 2013, 425, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Herbert, Z.; Bötticher, G.; Aschoff, A.; Sendemir, E.; Zermann, D.H.; Arnold, R.; Mall, G.; Jirikowski, G. Changing Caveolin-1 and oxytocin receptor distribution in the ageing human prostate. Anat. Histol. Embryol. 2007, 36, 361–365. [Google Scholar] [CrossRef]
- Kinsey, C.G.; Bussolati, G.; Bosco, M.; Kimura, T.; Pizzorno, M.C.; Chernin, M.I.; Cassoni, P.; Novak, J.F. Constitutive and ligand-induced nuclear localization of oxytocin receptor. J. Cell. Mol. Med. 2007, 11, 96–110. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Sun, L.; Zambonin, C.G.; Tamma, R.; Nico, B.; Calvano, C.D.; Colaianni, G.; Ji, Y.; Mori, G.; Grano, M. Osteoblast regulation via ligand-activated nuclear trafficking of the oxytocin receptor. Proc. Natl. Acad. Sci. USA 2014, 111, 16502–16507. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chambliss, K.; Umetani, M.; Mineo, C.; Shaul, P.W. Non-nuclear estrogen receptor signaling in the endothelium. J. Biol. Chem. 2011, 286, 14737–14743. [Google Scholar] [CrossRef]
- Stow, J.L.; de Almeida, J.B.; Narula, N.; Holtzman, E.J.; Ercolani, L.; Ausiello, D.A. A heterotrimeric G protein, G alpha i-3, on Golgi membranes regulates the secretion of a heparan sulfate proteoglycan in LLC-PK1 epithelial cells. J. Cell Biol. 1991, 114, 1113–1124. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, H.; Wang, K.; Zheng, Y.; Chen, P.; Wang, X.; DiSanto, M.E.; Zhang, X. Upregulation of oxytocin receptor in the hyperplastic prostate. Front. Endocrinol. 2018, 9, 403. [Google Scholar] [CrossRef]
- Lee, S.N.; Kraska, J.; Papargiris, M.; Teng, L.; Niranjan, B.; Hammar, J.; Ryan, A.; Frydenberg, M.; Lawrentschuk, N.; Middendorff, R. Oxytocin receptor antagonists as a novel pharmacological agent for reducing smooth muscle tone in the human prostate. Sci. Rep. 2021, 11, 6352. [Google Scholar] [CrossRef]
- Einspanier, A.; Ivell, R. Oxytocin and oxytocin receptor expression in reproductive tissues of the male marmoset monkey. Biol. Reprod. 1997, 56, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Whittington, K.; Assinder, S.; Gould, M.; Nicholson, H. Oxytocin, oxytocin-associated neurophysin and the oxytocin receptor in the human prostate. Cell Tissue Res. 2004, 318, 375–382. [Google Scholar] [CrossRef]
- Frayne, J.; Nicholson, H. Localization of oxytocin receptors in the human and macaque monkey male reproductive tracts: Evidence for a physiological role of oxytocin in the male. Mol. Hum. Reprod. 1998, 4, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Ellem, S.J.; Risbridger, G.P. The dual, opposing roles of estrogen in the prostate. Ann. N. Y. Acad. Sci. 2009, 1155, 174–186. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badshah, M.; Ibrahim, J.; Su, N.; Whiley, P.; Whittaker, M.; Exintaris, B. The Effects of Age on Prostatic Responses to Oxytocin and the Effects of Antagonists. Biomedicines 2023, 11, 2956. https://doi.org/10.3390/biomedicines11112956
Badshah M, Ibrahim J, Su N, Whiley P, Whittaker M, Exintaris B. The Effects of Age on Prostatic Responses to Oxytocin and the Effects of Antagonists. Biomedicines. 2023; 11(11):2956. https://doi.org/10.3390/biomedicines11112956
Chicago/Turabian StyleBadshah, Masroor, Jibriil Ibrahim, Nguok Su, Penny Whiley, Michael Whittaker, and Betty Exintaris. 2023. "The Effects of Age on Prostatic Responses to Oxytocin and the Effects of Antagonists" Biomedicines 11, no. 11: 2956. https://doi.org/10.3390/biomedicines11112956