Prospect of Mesenchymal Stem-Cell-Conditioned Medium in the Treatment of Acute Pancreatitis: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Study subjects: in vivo studies using animal models with AP;
- Interventions: any application of MSC-CM, EVs/MVs, or exosomes to the study groups;
- Outcomes: any functional, histological, physiological, and biomechanical outcomes;
- Study design: comparative studies.
2.2. Literature Search and Study Selection
2.3. Methodological Quality Assessment and Risk of Bias
2.4. Data Extraction
3. Results
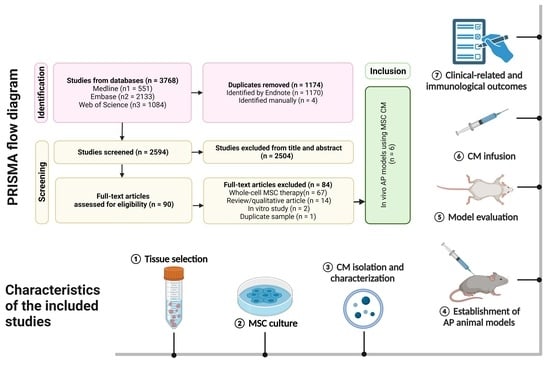
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Donor Pretreatment and Identification of MSCs
3.2.2. Isolation and Identification of MSC-CM/Exosome
3.2.3. Model Establishment and Grouping
3.2.4. Timing of Therapy and Observation
3.3. Methodological Quality
Assessment of Risk of Bias
3.4. Clinical Related Outcomes
3.4.1. MSC Cultured Medium Alleviates Pancreatic Injury and Reduces Serum Pancreatic Enzyme
3.4.2. MSC Cultured Medium Lessens Myocardial Injury and Restores Cardiac Function
3.5. Immunological Outcomes
MSC Culture Medium Reduces Inflammatory Responses and Apoptotic Processes
4. Discussion
4.1. The Role of MSC Cultured Medium in Alleviating Pancreatic Injury and Suppressing Inflammation in Murine Models
4.2. Challenges in MSC-CM Preparation
4.3. Limitations in AP Models and Study Designs
4.4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Authors/Year/Ref | MSC Identification | Identification of CM or Exo | Intervention Group | Controls | Group Size | Timing of Therapy Start/: Observation Period |
|---|---|---|---|---|---|---|
| Zhao et al. (2022) [32] |
−: CD34, CD45 |
| AP/Exo |
| 8 | Directly following induction/12 h |
| Li et al. (2022) [33] |
−: CD31− CD45−
|
| AP/Exo(intraperitoneal) AP/Exo (tail vein) |
| 6 | Directly following induction/24 h |
| Chen et al. (2022) [34] |
−: CD45, CD34 |
| SAP/Exo |
| 6 | One dose directly following induction, the other after 12 h/24 h |
| Roch et al. (2020) [35] |
−: CD31, CD45, CD106, CD144 |
| AP/AD-MSC-CM |
| 6 | 24 h after induction/48 h |
| Abdolmohammadi et al. (2020) [36] |
−: CD34, CD45 |
| AP/CM |
| 7 | 1 h after induction/12 h |
| Abdolmohammadi et al. (2020) [36] |
−: CD45, CD34 |
| AP/CM |
| 7 | 1 h after induction/12 h |
| Yin et al. (2016) [37] |
−: CD34, CD45 | - | SAP/MV |
| 18 | 2 h after induction/12 h, 24 h, 48 h separately |
| Yin et al. (2016) [37] |
−: CD34, CD45 | - | MAP/MV |
| 18 | 2 h after induction/6 h, 12 h, 24 h separately |
Appendix B

References
- Márta, K.; Lazarescu, A.M.; Farkas, N.; Mátrai, P.; Cazacu, I.; Ottóffy, M.; Habon, T.; Erőss, B.; Vincze, À.; Veres, G.; et al. Aging and Comorbidities in Acute Pancreatitis I: A Meta-Analysis and Systematic Review Based on 194,702 Patients. Front. Physiol. 2019, 10, 328. [Google Scholar] [CrossRef]
- Tenner, S.; Baillie, J.; DeWitt, J.; Vege, S.S. American College of Gastroenterology guideline: Management of acute pancreatitis. Am. J. Gastroenterol. 2013, 108, 1400–1415. [Google Scholar] [CrossRef]
- Mayerle, J.; Sendler, M.; Hegyi, E.; Beyer, G.; Lerch, M.M.; Sahin-Tóth, M. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology 2019, 156, 1951–1968e1951. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Papachristou, G.I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Saluja, A.; Dudeja, V.; Dawra, R.; Sah, R.P. Early Intra-Acinar Events in Pathogenesis of Pancreatitis. Gastroenterology 2019, 156, 1979–1993. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.P.; Saluja, A. Molecular mechanisms of pancreatic injury. Curr. Opin. Gastroenterol. 2011, 27, 444–451. [Google Scholar] [CrossRef]
- Gukovskaya, A.S.; Gukovsky, I.; Algül, H.; Habtezion, A. Autophagy, Inflammation, and Immune Dysfunction in the Pathogenesis of Pancreatitis. Gastroenterology 2017, 153, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, D.W.; Zeng, C.Y.; Chen, Y.X. Effects of the allogeneic bone marrow mesenchymal stem cells on inflammatory factors of rats with severe acute pancreatitis. J. Gastroenterol. Hepatol. 2011, 26, 231. [Google Scholar] [CrossRef]
- Jung, K.H.; Song, S.U.; Yi, T.; Jeon, M.-S.; Hong, S.-W.; Zheng, H.-M.; Lee, H.-S.; Choi, M.-J.; Lee, D.-H.; Hong, S.-S. Human Bone Marrow-Derived Clonal Mesenchymal Stem Cells Inhibit Inflammation and Reduce Acute Pancreatitis in Rats. Gastroenterology 2011, 140, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhou, J.; Yang, T.; Xie, W.; Song, G.; Song, Z.; Chen, J. Mesenchymal stromal cell therapy for pancreatitis: Progress and challenges. Med. Res. Rev. 2021, 41, 2474–2488. [Google Scholar] [CrossRef]
- Shekari, F.; Nazari, A.; Kashani, S.A.; Hajizadeh-Saffar, E.; Lim, R.; Baharvand, H. Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: A systematic review. Cytotherapy 2021, 23, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.J.; Wang, X.P. Role of bone marrow mesenchymal stem cells and derived microvesicles in acute pancreatitis: Effects and mechanisms. J. Dig. Dis. 2015, 16, 94–95. [Google Scholar]
- Goodman, R.R.; Jong, M.K.; Davies, J.E. Concise review: The challenges and opportunities of employing mesenchymal stromal cells in the treatment of acute pancreatitis. Biotechnol. Adv. 2020, 42, 107338. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhao, Z.; Yang, K.; Xin, M.; Zhou, L.; Chen, S.; Zhou, S.; Tang, Z.; Ji, H.; Dai, R. Application of exosomes in the diagnosis and treatment of pancreatic diseases. Stem Cell Res. Ther. 2022, 13, 153. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, M.J.; Bae, E.H.; Ryu, J.S.; Kaur, G.; Kim, H.J.; Kim, J.Y.; Barreda, H.; Jung, S.Y.; Choi, J.M.; et al. Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Mol. Ther. 2020, 28, 1628–1644. [Google Scholar] [CrossRef]
- Harrell, C.R.; Miloradovic, D.; Sadikot, R.; Fellabaum, C.; Markovic, B.S.; Miloradovic, D.; Acovic, A.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular and Cellular Mechanisms Responsible for Beneficial Effects of Mesenchymal Stem Cell-Derived Product “Exo-d-MAPPS” in Attenuation of Chronic Airway Inflammation. Anal. Cell. Pathol. 2020, 2020, 3153891. [Google Scholar] [CrossRef]
- Fujii, S.; Miura, Y. Immunomodulatory and Regenerative Effects of MSC-Derived Extracellular Vesicles to Treat Acute GVHD. Stem Cells 2022, 40, 977–990. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Prat-Vidal, C.; Martínez-Falguera, D.; Teis, A.; Soler-Botija, C.; Courageux, Y.; Munizaga-Larroudé, M.; Moron-Font, M.; Bayes-Genis, A.; Borràs, F.E.; et al. Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model. Theranostics 2022, 12, 4656–4670. [Google Scholar] [CrossRef]
- Pak, H.; Hadizadeh, A.; Heirani-Tabasi, A.; Soleimani, M.; Asbagh, R.A.; Fazeli, M.S.; Kazemeini, A.; Keshvari, A.; Keramati, M.R.; Salahshour, F.; et al. Safety and efficacy of injection of human placenta mesenchymal stem cells derived exosomes for treatment of complex perianal fistula in non-Crohn’s cases: Clinical trial phase I. J. Gastroenterol. Hepatol. 2023, 38, 539–547. [Google Scholar] [CrossRef]
- Zarrabi, M.; Shahrbaf, M.A.; Nouri, M.; Shekari, F.; Hosseini, S.E.; Hashemian, S.R.; Aliannejad, R.; Jamaati, H.; Khavandgar, N.; Alemi, H.; et al. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: A randomized controlled trial. Stem Cell Res. Ther. 2023, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Ma, J.; Ren, Y.; Xiang, S.; Jia, R. Secreted klotho from exosomes alleviates inflammation and apoptosis in acute pancreatitis. Am. J. Transl. Res. 2019, 11, 3375–3383. [Google Scholar] [PubMed]
- Huang, J.H.; Xu, Y.; Yin, X.M.; Lin, F.Y. Exosomes Derived from miR-126-modified MSCs Promote Angiogenesis and Neurogenesis and Attenuate Apoptosis after Spinal Cord Injury in Rats. Neuroscience 2020, 424, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, J.H.; Umezu, T.; Ohyashiki, K. Exosomes promote bone marrow angiogenesis in hematologic neoplasia: The role of hypoxia. Curr. Opin. Hematol. 2016, 23, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; He, X.; Zheng, J. Exosomes and regenerative medicine: State of the art and perspectives. Transl. Res. 2018, 196, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yi, Y.; Zhu, Y.; Wang, Z.; Wu, S.; Zhang, J.; Hu, X.; Nie, J. Effects of adipose-derived stem cell released exosomes on wound healing in diabetic mice. Chin. J. Reparative Reconstr. Surg. 2020, 34, 124–131. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151, W-65. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments--the ARRIVE guidelines. J. Cereb. Blood Flow. Metab. 2011, 31, 991–993. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 explanation and elaboration: Updated guidelines for reporting parallel group randomised trials. Int. J. Surg. 2012, 10, 28–55. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhirong, Z.; Li, H.; Yiqun, H.; Chunyang, H.; Lichen, Z.; Zhen, T.; Tao, W.; Ruiwu, D. Enhancing or inhibiting apoptosis? The effects of ucMSC-Ex in the treatment of different degrees of traumatic pancreatitis. Apoptosis 2022, 27, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, H.; Zhangdi, H.; Xu, R.; Zhang, X.; Liu, J.; Hu, Y.; Ning, D.; Jin, S. Hair follicle-MSC-derived small extracellular vesicles as a novel remedy for acute pancreatitis. J. Control. Release 2022, 352, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, J.; Huang, W.; Li, C.; Luo, H.; Xue, Z.; Xiao, Y.; Wu, Q.; Chen, C. Exosomes from human induced pluripotent stem cells derived mesenchymal stem cells improved myocardial injury caused by severe acute pancreatitis through activating Akt/Nrf2/HO-1 axis. Cell Cycle 2022, 21, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Roch, A.M.; Maatman, T.K.; Cook, T.G.; Wu, H.H.; Merfeld-Clauss, S.; Traktuev, D.O.; March, K.L.; Zyromski, N.J. Therapeutic Use of Adipose-Derived Stromal Cells in a Murine Model of Acute Pancreatitis. J. Gastrointest. Surg. 2020, 24, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi, K.; Mahmoudi, T.; Nojehdehi, S.; Tayebi, L.; Hashemi, S.M.; Noorbakhsh, F.; Abdollahi, A.; Soleimani, M.; Nikbin, B.; Nicknam, M.H. Effect of Hypoxia Preconditioned Adipose-Derived Mesenchymal Stem Cell Conditioned Medium on Cerulein-Induced Acute Pancreatitis in Mice. Adv. Pharm. Bull. 2020, 10, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Hu, G.; Wan, R.; Yu, G.; Cang, X.; Xiong, J.; Ni, J.; Hu, Y.; Xing, M.; Fan, Y.; et al. Role of Microvesicles from Bone Marrow Mesenchymal Stem Cells in Acute Pancreatitis. Pancreas 2016, 45, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Ali, A.A.A.; Shahror, R.A.; Chen, K.Y. Efficient Labeling of Mesenchymal Stem Cells For High Sensitivity Long-Term MRI Monitoring In Live Mice Brains. Int. J. Nanomed. 2020, 15, 97–114. [Google Scholar] [CrossRef]
- Ziegler, K.M.; Wade, T.E.; Wang, S.; Swartz-Basile, D.A.; Pitt, H.A.; Zyromski, N.J. Validation of a novel, physiologic model of experimental acute pancreatitis in the mouse. Am. J. Transl. Res. 2011, 3, 159–165. [Google Scholar]
- Schmidt, J.; Rattner, D.W.; Lewandrowski, K.; Compton, C.C.; Mandavilli, U.; Knoefel, W.T.; Warshaw, A.L. A better model of acute pancreatitis for evaluating therapy. Ann. Surg. 1992, 215, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi, K.; Mahmoudi, T.; Alimohammadi, M.; Tahmasebi, S.; Zavvar, M.; Hashemi, S.M. Mesenchymal stem cell-based therapy as a new therapeutic approach for acute inflammation. Life Sci. 2023, 312, 121206. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lyon, C.J.; Fletcher, J.K.; Tang, W.; Wan, M.; Hu, T.Y. Extracellular vesicle activities regulating macrophage- and tissue-mediated injury and repair responses. Acta Pharm. Sin. B 2021, 11, 1493–1512. [Google Scholar] [CrossRef]
- Munir, F.; Jamshed, M.B.; Shahid, N.; Hussain, H.M.; Muhammad, S.A.; Al Mamun, A.; Zhang, Q. Advances in immunomodulatory therapy for severe acute pancreatitis. Immunol. Lett. 2020, 217, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Uder, C.; Brückner, S.; Winkler, S.; Tautenhahn, H.M.; Christ, B. Mammalian MSC from selected species: Features and applications. Cytom. A 2018, 93, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T.; et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yuen, P.S.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.H.; Huang, W.; Latawiec, D.; Jiang, K.; Booth, D.M.; Elliott, V.; Mukherjee, R.; Xia, Q. Review of experimental animal models of biliary acute pancreatitis and recent advances in basic research. HPB 2012, 14, 73–81. [Google Scholar] [CrossRef]
- Yang, X.; Yao, L.; Fu, X.; Mukherjee, R.; Xia, Q.; Jakubowska, M.A.; Ferdek, P.E.; Huang, W. Experimental Acute Pancreatitis Models: History, Current Status, and Role in Translational Research. Front. Physiol. 2020, 11, 614591. [Google Scholar] [CrossRef]
- Ceranowicz, P.; Cieszkowski, J.; Warzecha, Z.; Dembiński, A. Experimental models of acute pancreatitis. Postępy Hig. I Med. Doświadczalnej 2015, 69, 264–269. [Google Scholar] [CrossRef]
- Taha, H.S.; Moustafa, E.M.; Moawed, F.S.M.; Hegazy, M.G.A. Curative role of mesenchymal stromal cells in chronic pancreatitis: Modulation of MAPK and TGF-β1/SMAD factors. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211054036. [Google Scholar] [CrossRef]
- Kong, L.; Xu, X.; Zhang, H.; Zhou, Y.; Huang, H.; Chen, B.; Zhou, Z. Human umbilical cord-derived mesenchymal stem cells improve chronic pancreatitis in rats via the AKT-mTOR-S6K1 signaling pathway. Bioengineered 2021, 12, 1986–1996. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]


| Authors/Year/Ref | Donor/Type/Pre-Treatment | Level of CM | Model/Induction Method/Severity of AP | Routs/Dose/Number of Doses Mouse: 20 g Rat: 250 g | Immunological Outcome | Clinical Outcome | Level of Evidence |
|---|---|---|---|---|---|---|---|
| Zhao et al. (2022) [32] | Human/UC-MSC/- | Exo | Rat/extrusion stress/traumatic AP | Tail vein/20 μg/single | ↓ Apoptosis | ↓ Histopathological scores ↓ Serum amylase & lipase | Moderate |
| Li et al. (2022) [33] | Mice/HF-MSC/- | sEV | Mice/cerulein/AP | Intraperitoneal and tail vein/100 μg/double | ↓ TNF-α ↓ IL-6 ↑ IL-4 ↑ IL-10 ↓ MPO | ↓ Histopathological scores ↓ Serum amylase & lipase | Moderate |
| Chen et al. (2022) [34] | Human/iMSCs/- | Exo | Rat/NaT/AP | Tail vein/100 μg/single | ↑ Akt/Nrf2/HO-1 ↑ vWF and VEGF | ↓ Myocardial injury ↓ Oxidative stress ↑ Cardiac function | Moderate |
| Roch et al. (2020) [35] | Human/AD-MSC | CM | Mice/cerulein/AP | Tail vein/100 μL/single | - | ↓ Histopathological scores | Low |
| Abdolmohammadi et al. (2020) [36] | Mice/AD-MSC/- | CM | Mice/cerulein/AP | Tail vein/500 μL/triple | ↓ IL-6 ↓ MPO | ↓ Histopathological scores ↓ Serum amylase & lipase | Moderate |
| Abdolmohammadi et al. (2020) [36] | Mice/AD-MSC/hypoxia-preconditioned | CM | Mice/cerulein/AP | Intraperitoneal/500 μL/triple | ↓ IL-6 ↓ MPO No significant difference with CM | ↓ Histopathological scores ↓ Serum amylase & lipase No significant difference with CM | Moderate |
| Yin et al. (2016) [37] | Rat/BM-MSC/- | MV | Rat/NaT/SAP | Tail vein/1000 μg/single | ↓ NF-κB, p65 expression ↓ MPO ↑ Acinar cells survival | ↓ Histopathological scores ↓ Serum amylase & lipase | Moderate |
| Yin et al. (2016) [37] | Rat/BM-MSC/- | MV | Mice/Cerulein/MAP | Tail vein/100 μg/single | ↓ NF-κB, p65 expression ↓ MPO ↑ Acinar cells survival | ↓ Histopathological scores ↓ Serum amylase & lipase | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, K.; Kong, F.; Wu, D. Prospect of Mesenchymal Stem-Cell-Conditioned Medium in the Treatment of Acute Pancreatitis: A Systematic Review. Biomedicines 2023, 11, 2343. https://doi.org/10.3390/biomedicines11092343
Pang K, Kong F, Wu D. Prospect of Mesenchymal Stem-Cell-Conditioned Medium in the Treatment of Acute Pancreatitis: A Systematic Review. Biomedicines. 2023; 11(9):2343. https://doi.org/10.3390/biomedicines11092343
Chicago/Turabian StylePang, Ke, Fanyi Kong, and Dong Wu. 2023. "Prospect of Mesenchymal Stem-Cell-Conditioned Medium in the Treatment of Acute Pancreatitis: A Systematic Review" Biomedicines 11, no. 9: 2343. https://doi.org/10.3390/biomedicines11092343