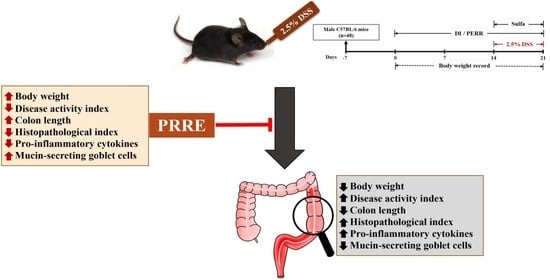
Protective Effect of Red Rice Extract Rich in Proanthocyanidins in a Murine Colitis Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Proanthocyanidins-Rich Red Rice Extract (PRRE)
2.2. Measurement of Total Phenolic Content
2.3. Measurement of Total Proanthocyanidins Content
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.5. Animals and Experimental Design
2.6. Assessment of Disease Activity Index
2.7. Histopathological Analysis of Colon Tissues
2.8. Measurement of Pro-Inflammatory CytokinePproductions
2.9. Periodic Acid—Schiff Taining
2.10. Statistical Analysis
3. Results
3.1. Total Phenolic Content and Total Proanthocyanidins Content of Fractionated Extracts FromRed Rice
3.2. Identification of Proanthocyanidin Monomeric Units in PRRE
3.3. PRRE Alleviates Clinical Manifestations in DSS-Induced Colitis
3.4. PRRE Prevents DSS-Induced Histopathological Damage
3.5. PRRE Attenuates the Production of Proinflammatory Cytokines in DSS-Induced Colitis
3.6. PRRE Prevents Mucin-Secreting Goblet Cells in DSS-Induced Colitis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruemmele, F.M. Role of Diet in Inflammatory Bowel Disease. Ann. Nutr. Metab. 2016, 68, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Olfatifar, M.; Zali, M.R.; Pourhoseingholi, M.A.; Balaii, H.; Ghavami, S.B.; Ivanchuk, M.; Ivanchuk, P.; Nazari, S.H.; Shahrokh, S.; Sabour, S.; et al. The emerging epidemic of inflammatory bowel disease in Asia and Iran by 2035: A modeling study. BMC Gastroenterol. 2021, 21, 204. [Google Scholar] [CrossRef]
- Ramos, G.P.; Papadakis, K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019, 94, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxid Med. Cell Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [Green Version]
- Greuter, T.; Vavricka, S.; Konig, A.O.; Beaugerie, L.; Scharl, M.; Swiss Ibdnet, a.o.w.g.o.t.S.S.o.G. Malignancies in Inflammatory Bowel Disease. Digestion 2020, 101, 136–145. [Google Scholar] [CrossRef]
- Das, K.M.; Farag, S.A. Current medical therapy of inflammatory bowel disease. World J. Gastroenterol. 2000, 6, 483–489. [Google Scholar] [CrossRef]
- Linares, V.; Alonso, V.; Domingo, J.L. Oxidative stress as a mechanism underlying sulfasalazine-induced toxicity. Expert Opin. Drug Saf. 2011, 10, 253–263. [Google Scholar] [CrossRef]
- Li, Y.C.; Shen, J.D.; Lu, S.F.; Zhu, L.L.; Wang, B.Y.; Bai, M.; Xu, E.P. Transcriptomic analysis reveals the mechanism of sulfasalazine-induced liver injury in mice. Toxicol. Lett. 2020, 321, 12–20. [Google Scholar] [CrossRef]
- Fukushima, T.; Kato, M.; Adachi, T.; Hamada, Y.; Horimoto, M.; Komiyama, M.; Mori, C.; Horii, I. Effects of sulfasalazine on sperm acrosome reaction and gene expression in the male reproductive organs of rats. Toxicol. Sci. 2005, 85, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.J.; Park, J.M.; Lee, J.S.; Kim, Y.S.; Kangwan, N.; Han, Y.M.; Kang, E.A.; An, J.M.; Park, Y.K.; Hahm, K.B. Oligonol prevented the relapse of dextran sulfate sodium-ulcerative colitis through enhancing NRF2-mediated antioxidative defense mechanism. J. Physiol Pharm. 2018, 69, 359–371. [Google Scholar] [CrossRef]
- Li, X.L.; Cai, Y.Q.; Qin, H.; Wu, Y.J. Therapeutic effect and mechanism of proanthocyanidins from grape seeds in rats with TNBS-induced ulcerative colitis. Can. J. Physiol Pharm. 2008, 86, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Oz, H.S.; Chen, T.; de Villiers, W.J. Green Tea Polyphenols and Sulfasalazine have Parallel Anti-Inflammatory Properties in Colitis Models. Front. Immunol. 2013, 4, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashidian, A.; Mehrzadi, S.; Ghannadi, A.R.; Mahzooni, P.; Sadr, S.; Minaiyan, M. Protective effect of ginger volatile oil against acetic acid-induced colitis in rats: A light microscopic evaluation. J. Integr. Med. 2014, 12, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Paudel, K.R.; Sharma, B.; Karki, R. Phytochemical profile and pharmacological activity of Aegle marmelos Linn. J. Integr. Med. 2018, 16, 153–163. [Google Scholar] [CrossRef]
- Hosoda, K.; Sasahara, H.; Matsushita, K.; Tamura, Y.; Miyaji, M.; Matsuyama, H. Anthocyanin and proanthocyanidin contents, antioxidant activity, and in situ degradability of black and red rice grains. Asian-Australas. J. Anim. Sci. 2018, 31, 1213–1220. [Google Scholar] [CrossRef]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-kappaB pathways in Raw 264.7 macrophages. Nutr. Res. Pr. 2016, 10, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Pintha, K.; Yodkeeree, S.; Pitchakarn, P.; Limtrakul, P. Anti-invasive activity against cancer cells of phytochemicals in red jasmine rice (Oryza sativa L.). Asian Pac. J. Cancer Prev. 2014, 15, 4601–4607. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, T.; Maekawa, M.; Oki, T.; Suda, I.; Iida, S.; Shimada, H.; Takamure, I.; Kadowaki, K. The Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant. J. 2007, 49, 91–102. [Google Scholar] [CrossRef]
- Tantipaiboonwonga, P.; Pintha, K.; Chaiwangyen, W.; Chewonarin, T.; Pangjit, K.; Chumphukam, O.; Kangwan, K.; Suttajit, M. Anti-hyperglycaemic and anti-hyperlipidaemic effects of black and red rice in streptozotocin-induced diabetic rats. ScienceAsia 2017, 43, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.P.; Li, P.; Du, T.; Du, X.J.; Wang, S. Protective effect and mechanism of Monascus-fermented red yeast rice against colitis caused by Salmonella enterica serotype Typhimurium ATCC 14028. Food Funct. 2020, 11, 6363–6375. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharm. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Gunaratne, A.; Wu, K.; Li, D.; Bentota, A.; Corke, H.; Cai, Y.Z. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013, 138, 1153–1161. [Google Scholar] [CrossRef]
- Holkem, A.T.; Favaro-Trindade, C.S.; Lacroix, M. Study of anticancer properties of proanthocyanidin-rich cinnamon extract in combination with Bifidobacterium animalis subsp. lactis BLC1 and resistance of these free and co-encapsulated materials under in vitro simulated gastrointestinal conditions. Food Res. Int. 2020, 134, 109274. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Chinigo, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution, and Effect on Human Health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Cros, G.; Yokota, T.; Crozier, A. Phytochemical profiles of black, red, brown, and white rice from the Camargue region of France. J. Agric. Food Chem. 2013, 61, 7976–7986. [Google Scholar] [CrossRef]
- Lee, Y.A.; Cho, E.J.; Tanaka, T.; Yokozawa, T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J. Nutr. Sci. Vitam. 2007, 53, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, L.; Narvaez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Gonzalez-Quilen, C.; Rodriguez-Gallego, E.; Beltran-Debon, R.; Pinent, M.; Ardevol, A.; Blay, M.T.; Terra, X. Health-Promoting Properties of Proanthocyanidins for Intestinal Dysfunction. Nutrients 2020, 12, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nallathambi, R.; Poulev, A.; Zuk, J.B.; Raskin, I. Proanthocyanidin-Rich Grape Seed Extract Reduces Inflammation and Oxidative Stress and Restores Tight Junction Barrier Function in Caco-2 Colon Cells. Nutrients 2020, 12, 1623. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xue, Y.; Zhang, H.; Du, M.; Zhu, M.J. Favourable effects of grape seed extract on intestinal epithelial differentiation and barrier function in IL10-deficient mice. Br. J. Nutr. 2015, 114, 15–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, K.; Zhang, G.; Sun, M.; He, S.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020, 11, 7817–7829. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ge, B.; Yang, X.L.; Zhai, J.; Yang, L.N.; Wang, X.X.; Liu, X.; Shi, J.C.; Wu, Y.J. Proanthocyanidins from grape seeds modulates the nuclear factor-kappa B signal transduction pathways in rats with TNBS-induced recurrent ulcerative colitis. Int. Immunopharmacol. 2011, 11, 1620–1627. [Google Scholar] [CrossRef]
- Glabska, D.; Guzek, D.; Galazka, K.; Lech, G. Therapeutic Potential of Proanthocyanidins in Ulcerative Colitis in Remission. J. Clin. Med. 2020, 9, 771. [Google Scholar] [CrossRef] [Green Version]
- Subkamkaew, C.; Limtrakul Dejkriengkraikul, P.; Yodkeeree, S. Proanthocyanidin-Rich Fractions from Red Rice Extract Enhance TNF-alpha-Induced Cell Death and Suppress Invasion of Human Lung Adenocarcinoma Cell A549. Molecules 2019, 24, 3393. [Google Scholar] [CrossRef] [Green Version]
- Kangwan, N.; Pintha, K.; Preedapirom, W.; Tantipaiboonwong, P.; Chumphukam, O.; Suttajit, M. Learning and memory enhancing effects of anthocyanin in black rice extract on cerebral ischaemia in mice. ScienceAsia 2015, 41, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Bagchi, D.; Lim, P.M.; Bagchi, M.; Gross, S.M.; Kothari, S.C.; Preuss, H.G.; Stohs, S.J. Acute and long-term safety evaluation of a novel IH636 grape seed proanthocyanidin extract. Res. Commun. Mol. Pathol. Pharm. 2001, 109, 165–197. [Google Scholar]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Yeganeh, P.R.; Leahy, J.; Spahis, S.; Patey, N.; Desjardins, Y.; Roy, D.; Delvin, E.; Garofalo, C.; Leduc-Gaudet, J.-P.; St-Pierre, D.; et al. Apple peel polyphenols reduce mitochondrial dysfunction in mice with DSS-induced ulcerative colitis. J. Nutr. Biochem. 2018, 57, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Perse, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012, 2012, 718617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kangwan, N.; Kongkarnka, S.; Boonkerd, N.; Unban, K.; Shetty, K.; Khanongnuch, C. Protective Effect of Probiotics Isolated from Traditional Fermented Tea Leaves (Miang) from Northern Thailand and Role of Synbiotics in Ameliorating Experimental Ulcerative Colitis in Mice. Nutrients 2022, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Kangwan, N.; Pintha, K.; Khanaree, C.; Kongkarnka, S.; Chewonarin, T.; Suttajit, M. Anti-inflammatory effect of Perilla frutescens seed oil rich in omega-3 fatty acid on dextran sodium sulfate-induced colitis in mice. Res. Pharm. Sci. 2021, 16, 464–473. [Google Scholar] [CrossRef]
- Sun, Z.; Li, J.; Dai, Y.; Wang, W.; Shi, R.; Wang, Z.; Ding, P.; Lu, Q.; Jiang, H.; Pei, W.; et al. Indigo Naturalis Alleviates Dextran Sulfate Sodium-Induced Colitis in Rats via Altering Gut Microbiota. Front. Microbiol. 2020, 11, 731. [Google Scholar] [CrossRef]
- Kangwan, N.; Pratchayasakul, W.; Kongkaew, A.; Pintha, K.; Chattipakorn, N.; Chattipakorn, S.C. Perilla Seed Oil Alleviates Gut Dysbiosis, Intestinal Inflammation and Metabolic Disturbance in Obese-Insulin-Resistant Rats. Nutrients 2021, 13, 3141. [Google Scholar] [CrossRef]
- Pintha, K.; Yodkeeree, S.; Limtrakul, P. Proanthocyanidin in red rice inhibits MDA-MB-231 breast cancer cell invasion via the expression control of invasive proteins. Biol. Pharm. Bull. 2015, 38, 571–581. [Google Scholar] [CrossRef] [Green Version]
- Jiamyangyuen, S.; Nuengchamnong, N.; Ngamdee, P. Bioactivity and chemical components of Thai rice in five stages of grain development. J. Cereal Sci. 2017, 74, 136–144. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, F.; Chen, Y.; Huang, Y.; Beta, T.; Bao, J. Analysis of Genotype, Environment, and Their Interaction Effects on the Phytochemicals and Antioxidant Capacities of Red Rice (Oryza sativa L.). Cereal Chem. 2015, 92, 204–210. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Gelmez, E.; Lehr, K.; Kershaw, O.; Frentzel, S.; Vilchez-Vargas, R.; Bank, U.; Link, A.; Schüler, T.; Jeron, A.; Bruder, D. Characterization of Maladaptive Processes in Acute, Chronic and Remission Phases of Experimental Colitis in C57BL/6 Mice. Biomedicines 2022, 10, 1903. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.N.; Wang, M.; Guo, J.; Wang, J.P. Influences of probiotics combined with sulfasalazine on rats with ulcerative colitis via the Wnt/beta-catenin signaling pathway. Eur Rev. Med. Pharm. Sci 2019, 23, 6371–6378. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yang, X.L.; Wang, L.; Cui, M.X.; Cai, Y.Q.; Li, X.L.; Wu, Y.J. Effects of proanthocyanidins from grape seed on treatment of recurrent ulcerative colitis in rats. Can. J. Physiol. Pharm. 2010, 88, 888–898. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; Comitato, R.; Gines, I.; Ardevol, A.; Pinent, M.; Virgili, F.; Terra, X.; Blay, M. Protective Effect of Proanthocyanidins in a Rat Model of Mild Intestinal Inflammation and Impaired Intestinal Permeability Induced by LPS. Mol. Nutr. Food Res. 2019, 63, e1800720. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Pierre, J.F.; Heneghan, A.F.; Feliciano, R.P.; Shanmuganayagam, D.; Roenneburg, D.A.; Krueger, C.G.; Reed, J.D.; Kudsk, K.A. Cranberry proanthocyanidins improve the gut mucous layer morphology and function in mice receiving elemental enteral nutrition. JPEN J. Parenter Enter. Nutr. 2013, 37, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, R.; Koide, T.; Tokoro, C.; Yamamoto, T.; Godai, T.; Morohashi, T.; Fujita, Y.; Takahashi, D.; Kawana, I.; Suzuki, S.; et al. Quantitive cytokine mRNA expression profiles in the colonic mucosa of patients with steroid naive ulcerative colitis during active and quiescent disease. Inflamm. Bowel Diseases 2009, 15, 328–334. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef] [Green Version]
- Gentile, C.; Perrone, A.; Attanzio, A.; Tesoriere, L.; Livrea, M.A. Sicilian pistachio (Pistacia vera L.) nut inhibits expression and release of inflammatory mediators and reverts the increase of paracellular permeability in IL-1beta-exposed human intestinal epithelial cells. Eur. J. Nutr. 2015, 54, 811–821. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Cai, Y.; Qin, H.; Wang, L.; Wang, Y.; Huang, Y.; Wang, X.; Yan, S.; Wang, L.; et al. Proanthocyanidins from Grape Seeds Modulate the NF-κB Signal Transduction Pathways in Rats with TNBS-Induced Ulcerative Colitis. Molecules 2011, 16, 6721–6731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Y.; Liu, G.; Hao, S.; Wang, C.; Wang, Y. Black rice anthocyanin-rich extract and rosmarinic acid, alone and in combination, protect against DSS-induced colitis in mice. Food Funct. 2018, 9, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, T.; Wu, B.; Fu, W.; Xu, B.; Pamuru, R.R.; Kennett, M.; Vanamala, J.K.P.; Reddivari, L. Anthocyanin-containing purple potatoes ameliorate DSS-induced colitis in mice. J. Nutr. Biochem. 2021, 93, 108616. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Stalmach, A.; Mullen, W.; Steiling, H.; Williamson, G.; Lean, M.E.; Crozier, A. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol. Nutr. Food Res. 2010, 54, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.R.; Kim, K.J.; Kim, S.H.; Kim, S.J.; Seo, B.I.; An, H.J.; Roh, S.S. Comparative Evaluation between Sulfasalazine Alone and in Combination with Herbal Medicine on DSS-Induced Ulcerative Colitis Mice. Biomed. Res. Int. 2017, 2017, 6742652. [Google Scholar] [CrossRef]






| Score | Weight Loss (%) | Stool Consistency | Occult Blood |
|---|---|---|---|
| 0 | None | Normal stool, well form pellets | No rectal bleeding |
| 1 | 1.0–5.0 | - | - |
| 2 | 5.1–10.0 | Loose stools, pasty stools that do not stick to the anus | Hemoccult positive |
| 3 | 10.1–15.0 | - | - |
| 4 | >15.0 | Diarrhea, liquid stools that stick to the anus | Visible gross bleeding |
| Fractionated Extracts | TPC | TPAC |
|---|---|---|
| Ethanol | 56.44 ± 2.48 | 20.64 ± 2.39 |
| Hexane | 29.91 ± 1.08 | 0.30 ± 0.10 |
| Dichloromethane | 30.78 ± 1.91 | 0.34 ± 0.12 |
| Ethyl acetate | 100.75 ± 3.60 | 0.83 ± 0.15 |
| Aqueous | 136.69 ± 1.83 | 38.42 ± 1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kangwan, N.; Kongkarnka, S.; Pintha, K.; Saenjum, C.; Suttajit, M. Protective Effect of Red Rice Extract Rich in Proanthocyanidins in a Murine Colitis Model. Biomedicines 2023, 11, 265. https://doi.org/10.3390/biomedicines11020265
Kangwan N, Kongkarnka S, Pintha K, Saenjum C, Suttajit M. Protective Effect of Red Rice Extract Rich in Proanthocyanidins in a Murine Colitis Model. Biomedicines. 2023; 11(2):265. https://doi.org/10.3390/biomedicines11020265
Chicago/Turabian StyleKangwan, Napapan, Sarawut Kongkarnka, Komsak Pintha, Chalermpong Saenjum, and Maitree Suttajit. 2023. "Protective Effect of Red Rice Extract Rich in Proanthocyanidins in a Murine Colitis Model" Biomedicines 11, no. 2: 265. https://doi.org/10.3390/biomedicines11020265