A Review on Increasing the Targeting of PAMAM as Carriers in Glioma Therapy
Abstract
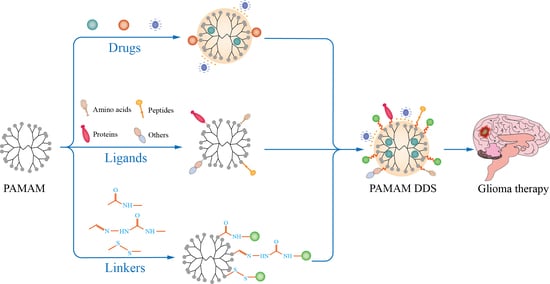
:1. Introduction
2. Brain-Targeted Drug Delivery System Based on PAMAM
3. Methods of Drug Loading
3.1. Embedded in the Cavity of PAMAM
3.2. Complexed on the Surface of PAMAM
3.3. Covalently Bound in the Arm of PAMAM
3.4. Linkers between Drug/Ligands and PAMAM
3.5. Ligands of Modified PAMAM for Brain Targeting
3.6. Amino Acids
3.7. Peptides
3.8. Proteins
3.9. Vitamins
3.10. Others
4. Strategies of PAMAM Drug Delivery Systems for Glioma Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers - Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, F.; Guzman, L. Dendrimer nanocarriers drug action: Perspective for neuronal pharmacology. Neural. Regen. Res. 2015, 10, 1029–1031. [Google Scholar] [PubMed]
- Arima, H.; Motoyama, K.; Higashi, T. Cyclodextrin/Dendrimer Conjugates as DNA and Oligonucleotide Carriers. Curr. Top. Med. Chem. 2014, 14, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.C.I.M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Al Moustafa, A.E.; Benter, I.F.; Akhtar, S. Emerging innate biological properties of nano-drug delivery systems: A focus on PAMAM dendrimers and their clinical potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef]
- Viltres, H.; Lopez, Y.C.; Leyva, C.; Gupta, N.K.; Naranjo, A.G.; Acevedo-Pena, P.; Sanchez-Diaz, A.; Bae, J.; Kim, K.S. Polyamidoamine dendrimer-based materials for environmental applications: A review. J. Mol. Liq. 2021, 334, 116017. [Google Scholar] [CrossRef]
- Bielinska, A.; Kukowskalatallo, J.; Piehler, L.T.; Yin, R.; Spindler, R.; Tomalia, D.A.; Baker, J.R. Starburst(R) Pamam Dendrimers - a Novel Synthetic Vector for the Transfection of DNA into Mammalian-Cells. Abstr. Pap. Am. Chem. S. 1995, 210, 145-Pmse. [Google Scholar]
- Liu, X.X.; Rocchi, P.; Qu, F.Q.; Zheng, S.Q.; Liang, Z.C.; Gleave, M.; Iovanna, J.; Peng, L. PAMAM Dendrimers Mediate siRNA Delivery to Target Hsp27 and Produce Potent Antiproliferative Effects on Prostate Cancer Cells. Chemmedchem 2009, 4, 1302–1310. [Google Scholar] [CrossRef]
- Oddone, N.; Lecot, N.; Fernandez, M.; Rodriguez-Haralambides, A.; Cabral, P.; Cerecetto, H.; Benech, J.C. In vitro and in vivo uptake studies of PAMAM G4.5 dendrimers in breast cancer. J. Nanobiotechnol. 2016, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Gallien, J.; Srinageshwar, B.; Gallo, K.; Holtgrefe, G.; Koneru, S.; Otero, P.S.; Bueno, C.A.; Mosher, J.; Roh, A.; Kohtz, D.S.; et al. Curcumin Loaded Dendrimers Specifically Reduce Viability of Glioblastoma Cell Lines. Molecules 2021, 26, 6050. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, L.P.; Danhier, F.; Preat, V. Drug delivery challenges and future of chemotherapeutic nanomedicine for glioblastoma treatment. J. Control. Release 2018, 281, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood-brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef] [PubMed]
- Han, S.P.; Zheng, H.Y.; Lu, Y.P.; Sun, Y.; Huang, A.H.; Fei, W.D.; Shi, X.W.; Xu, X.L.; Li, J.J.; Li, F.Z. A novel synergetic targeting strategy for glioma therapy employing borneol combination with angiopep-2-modified, DOX-loaded PAMAM dendrimer. J. Drug Target. 2018, 26, 86–94. [Google Scholar] [CrossRef]
- Huang, S.X.; Li, J.F.; Han, L.; Liu, S.H.; Ma, H.J.; Huang, R.Q.; Jiang, C. Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma. Biomaterials 2011, 32, 6832–6838. [Google Scholar] [CrossRef]
- Gao, S.; Li, J.F.; Jiang, H.; Hong, B.; Hao, B. Plasmid pORF-hTRAIL targeting to glioma using transferrin-modified polyamidoamine dendrimer. Drug Des. Dev. Ther. 2016, 10, 1–11. [Google Scholar]
- Kang, C.S.; Yuan, X.B.; Li, F.; Pu, P.Y.; Yu, S.Z.; Shen, C.H.; Zhang, Z.Y.; Zhang, Y.T. Evaluation of folate-PAMAM for the delivery of antisense oligonucleotides to rat C6 glioma cells in vitro and in vivo. J. Biomed. Mater. Res. A 2010, 93, 585–594. [Google Scholar]
- Han, L.; Zhang, A.L.; Wang, H.J.; Pu, P.Y.; Jiang, X.G.; Kang, C.S.; Chang, J. Tat-BMPs-PAMAM Conjugates Enhance Therapeutic Effect of Small Interference RNA on U251 Glioma Cells In Vitro and In Vivo. Hum. Gene Ther. 2010, 21, 417–426. [Google Scholar] [CrossRef]
- Lu, Y.P.; Han, S.P.; Zheng, H.Y.; Ma, R.; Ping, Y.T.; Zou, J.F.; Tang, H.X.; Zhang, Y.P.; Xu, X.L.; Li, F.Z. A novel RGDyC/PEG co-modified PAMAM dendrimer-loaded arsenic trioxide of glioma targeting delivery system. Int. J. Nanomed. 2018, 13, 5937–5952. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.W.; Ma, R.; Lu, Y.P.; Cheng, Y.; Fan, X.D.; Zou, J.F.; Zheng, H.Y.; Li, F.Z.; Piao, J.G. iRGD and TGN co-modified PAMAM for multi-targeted delivery of ATO to gliomas. Biochem. Biophys. Res. Commun. 2020, 527, 117–123. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Jia, X.-R.; Du, J.; Ying, X.; Lu, W.-L.; Lou, J.-N.; Wei, Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Guo, M.M.; Han, S.P.; Sun, Y.; Fei, W.D.; Xu, X.I.; Li, F.Z. Preparation and in vitro evaluation of borneol and folic acid co-modified doxorubicin loaded PAMAM drug delivery system. Yao Xue Xue Bao 2015, 50, 899–905. [Google Scholar] [PubMed]
- Waite, C.L.; Roth, C.M. PAMAM-RGD Conjugates Enhance siRNA Delivery Through a Multicellular Spheroid Model of Malignant Glioma. Bioconjugate Chem. 2009, 20, 1908–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.Z.; Li, G.; Su, Z.G.; Jiang, Z.M.; Chen, L.; Wang, J.F.; Yu, S.Z.; Liu, Z.L. Poly(amido amine) is an ideal carrier of miR-7 for enhancing gene silencing effects on the EGFR pathway in U251 glioma cells. Oncol. Rep. 2013, 29, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.; Rhim, H.S.; Lee, S.; Ko, K.S.; Han, J.; Choi, J.S. Apoptin Gene Delivery by the Functionalized Polyamidoamine Dendrimer Derivatives Induces Cell Death of U87-MG Glioblastoma Cells. J. Pharm. Sci. 2017, 106, 1618–1633. [Google Scholar] [CrossRef]
- Bae, Y.; Thuy, L.T.; Lee, Y.H.; Ko, K.S.; Han, J.; Choi, J.S. Polyplexes of Functional PAMAM Dendrimer/Apoptin Gene Induce Apoptosis of Human Primary Glioma Cells In Vitro. Polymers 2019, 11, 296. [Google Scholar] [CrossRef] [Green Version]
- Waite, C.L.; Sparks, S.M.; Uhrich, K.E.; Roth, C.M. Acetylation of PAMAM dendrimers for cellular delivery of siRNA. Bmc Biotechnol. 2009, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.R.; Kong, L.D.; Cao, X.Y.; Li, A.J.; Wei, P.; Wang, L.; Mignani, S.; Caminade, A.M.; Majoral, J.P.; Shi, X.Y. Enhanced Delivery of Therapeutic siRNA into Glioblastoma Cells Using Dendrimer-Entrapped Gold Nanoparticles Conjugated with beta-Cyclodextrin. Nanomaterials 2018, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Stenstrom, P.; Manzanares, D.; Zhang, Y.N.; Cena, V.; Malkoch, M. Evaluation of Amino-Functional Polyester Dendrimers Based on Bis-MPA as Nonviral Vectors for siRNA Delivery. Molecules 2018, 23, 2028. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.Q.; Ke, W.L.; Han, L.A.; Li, J.F.; Liu, S.H.; Jiang, C. Targeted delivery of chlorotoxin-modified DNA-loaded nanoparticles to glioma via intravenous administration. Biomaterials 2011, 32, 2399–2406. [Google Scholar] [CrossRef]
- Ren, Y.; Kang, C.S.; Yuan, X.B.; Zhou, X.; Xu, P.; Han, L.; Wang, G.X.; Jia, Z.F.; Zhong, Y.; Yu, S.Z.; et al. Co-delivery of as-miR-21 and 5-FU by Poly(amidoamine) Dendrimer Attenuates Human Glioma Cell Growth in Vitro. J. Biomat. Sci.-Polym. Ed. 2010, 21, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.M.; Shi, Z.D.; Ren, Y.; Liu, C.Y.; Ji, Y.R.; Long, L.X.; Pu, P.Y.; Sheng, J.; Yuan, X.B.; Kang, C.S. Synergistic inhibition of human glioma cell line by temozolomide and PAMAM-mediated miR-21i. J. Appl. Polym. Sci. 2013, 127, 570–576. [Google Scholar] [CrossRef]
- Bai, C.Z.; Choi, S.; Nam, K.; An, S.; Park, J.S. Arginine modified PAMAM dendrimer for interferon beta gene delivery to malignant glioma. Int. J. Pharm. 2013, 445, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Song, S.J.; Mun, J.Y.; Ko, K.S.; Han, J.; Choi, J.S. Apoptin Gene Delivery by the Functionalized Polyamidoamine (PAMAM) Dendrimer Modified with Ornithine Induces Cell Death of HepG2 Cells. Polymers 2017, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.; Lee, J.; Kho, C.; Choi, J.S.; Han, J. Apoptin gene delivery by a PAMAM dendrimer modified with a nuclear localization signal peptide as a gene carrier for brain cancer therapy. Korean J. Physiol. Pha. 2021, 25, 467–478. [Google Scholar] [CrossRef]
- Zhu, S.; Hong, M.; Tang, G.; Qian, L.; Lin, J.; Jiang, Y.; Pei, Y. Partly PEGylated polyamidoamine dendrimer for tumor-selective targeting of doxorubicin: The effects of PEGylation degree and drug conjugation style. Biomaterials 2010, 31, 1360–1371. [Google Scholar] [CrossRef]
- Uram, L.; Filipowicz, A.; Misiorek, M.; Pienkowska, N.; Markowicz, J.; Walajtys-Rode, E.; Wolowiec, S. Biotinylated PAMAM G3 dendrimer conjugated with celecoxib and/or Fmoc-L-Leucine and its cytotoxicity for normal and cancer human cell lines. Eur. J. Pharm. Sci. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Zhu, S.J.; Hong, M.H.; Zhang, L.H.; Tang, G.T.; Jiang, Y.Y.; Pei, Y.Y. PEGylated PAMAM Dendrimer-Doxorubicin Conjugates: In Vitro Evaluation and In Vivo Tumor Accumulation. Pharm. Res. 2010, 27, 161–174. [Google Scholar] [CrossRef]
- Furgeson, D.Y.; Dreher, M.R.; Chilkoti, A. Structural optimization of a "smart" doxorubicin-polypeptide conjugate for thermally targeted delivery to solid tumors. J. Control Release 2006, 110, 362–369. [Google Scholar] [CrossRef]
- Wang, X.Y.; Cai, X.P.; Hu, J.J.; Shao, N.M.; Wang, F.; Zhang, Q.; Xiao, J.R.; Cheng, Y.Y. Glutathione-Triggered "Off-On" Release of Anticancer Drugs from Dendrimer-Encapsulated Gold Nanoparticles. J. Am. Chem. Soc. 2013, 135, 9805–9810. [Google Scholar] [CrossRef]
- Wu, G.; Barth, R.F.; Yang, W.; Kawabata, S.; Zhang, L.; Green-Church, K. Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates. Mol. Cancer Ther. 2006, 5, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.J.; Wang, Y.; Ma, Z.Y.; Wang, Z.J.; Wei, Y.; Jia, X.R. A poly(amidoamine) dendrimer-based nanocarrier conjugated with Angiopep-2 for dual-targeting function in treating glioma cells. Polym. Chem. 2016, 7, 715–721. [Google Scholar] [CrossRef]
- Zhang, L.H.; Zhu, S.J.; Qian, L.L.; Pei, Y.Y.; Qiu, Y.M.; Jiang, Y.Y. RGD-modified PEG-PAMAM-DOX conjugates: In vitro and in vivo studies for glioma. Eur. J. Pharm. Biopharm. 2011, 79, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.F.; Liu, Y.; Liu, C.; Jiang, B.H.; Jiang, Y.Y. Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates. Biomaterials 2014, 35, 8735–8747. [Google Scholar] [CrossRef]
- Sk, U.H.; Dixit, D.; Sen, E. Comparative study of microtubule inhibitors - Estramustine and natural podophyllotoxin conjugated PAMAM dendrimer on glioma cell proliferation. Eur. J. Med. Chem. 2013, 68, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, H.; Jia, X.R.; Lu, W.L.; Lou, J.N.; Wei, Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials 2012, 33, 3899–3908. [Google Scholar] [CrossRef]
- An, S.; Lu, X.H.; Zhao, W.L.; Sun, T.; Zhang, Y.; Lu, Y.F.; Jiang, C. Amino Acid Metabolism Abnormity and Microenvironment Variation Mediated Targeting and Controlled Glioma Chemotherapy. Small 2016, 12, 5633–5645. [Google Scholar] [CrossRef]
- Bae, Y.; Green, E.S.; Kim, G.Y.; Song, S.J.; Mun, J.Y.; Lee, S.; Park, J.I.; Park, J.S.; Ko, K.S.; Han, J.; et al. Dipeptide-functionalized polyamidoamine dendrimer-mediated apoptin gene delivery facilitates apoptosis of human primary glioma cells. Int. J. Pharm. 2016, 515, 186–200. [Google Scholar] [CrossRef]
- Danhier, F.; Le Breton, A.; Preat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef]
- Waite, C.L.; Roth, C.M. Binding and Transport of PAMAM-RGD in a Tumor Spheroid Model: The Effect of RGD Targeting Ligand Density. Biotechnol. Bioeng. 2011, 108, 2999–3008. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zheng, X.Y.; Wan, X.; Shao, X.Y.; Liu, Q.F.; Zhang, Z.M.; Zhang, Q.Z. The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer’s disease. J. Control Release 2014, 192, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, A.L.; Wang, H.J.; Pu, P.Y.; Kang, C.S.; Chang, J. Construction of Novel Brain-Targeting Gene Delivery System by Natural Magnetic Nanoparticles. J. Appl. Polym. Sci. 2011, 121, 3446–3454. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Lv, L.; Fu, J.; Jiang, Y.; Xin, H.; Yao, Q. Glioma and microenvironment dual targeted nanocarrier for improved antiglioblastoma efficacy. Drug Deliv. 2017, 24, 1401–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Lv, L.Y.; Shi, H.H.; Hua, Y.B.; Lv, W.; Wang, X.Z.; Xin, H.L.; Xu, Q.W. PEGylated Polyamidoamine dendrimer conjugated with tumor homing peptide as a potential targeted delivery system for glioma. Colloid Surf. B 2016, 147, 242–249. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Zhang, Z.; Cui, C.; Zhou, J.; Yin, L.; Lv, H. Design, synthesis and evaluation of multi-functional tLyP-1-hyaluronic acid-paclitaxel conjugate endowed with broad anticancer scope. Carbohydr. Polym. 2017, 156, 97–107. [Google Scholar] [CrossRef]
- Jin, Z.; Piao, L.H.; Sun, G.C.; Lv, C.X.; Jing, Y.; Jin, R.H. Dual functional nanoparticles efficiently across the blood-brain barrier to combat glioblastoma via simultaneously inhibit the PI3K pathway and NKG2A axis. J. Drug. Target. 2021, 29, 323–335. [Google Scholar] [CrossRef]
- Zhao, J.J.; Zhang, B.; Shen, S.; Chen, J.; Zhang, Q.Z.; Jiang, X.G.; Pang, Z.Q. CREKA peptide-conjugated dendrimer nanoparticles for glioblastoma multiforme delivery. J. Colloid Interf Sci. 2015, 450, 396–403. [Google Scholar] [CrossRef]
- Demeule, M.; Regina, A.; Che, C.; Poirier, J.; Nguyen, T.; Gabathuler, R.; Castaigne, J.P.; Beliveau, R. Identification and design of peptides as a new drug delivery system for the brain. J. Pharmacol. Exp. Ther. 2008, 324, 1064–1072. [Google Scholar] [CrossRef] [Green Version]
- Ke, W.L.; Shao, K.; Huang, R.Q.; Han, L.; Liu, Y.; Li, J.F.; Kuang, Y.Y.; Ye, L.Y.; Lou, J.N.; Jiang, C. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 2009, 30, 6976–6985. [Google Scholar] [CrossRef]
- Demeule, M.; Currie, J.C.; Bertrand, Y.; Che, C.; Nguyen, T.; Regina, A.; Gabathuler, R.; Castaigne, J.P.; Beliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef]
- Yan, H.H.; Wang, L.; Wang, J.Y.; Weng, X.F.; Lei, H.; Wang, X.X.; Jiang, L.; Zhu, J.H.; Lu, W.Y.; Wei, X.B.; et al. Two-Order Targeted Brain Tumor Imaging by Using an Optical/Paramagnetic Nanoprobe across the Blood Brain Barrier. Acs. Nano 2012, 6, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Zarebkohan, A.; Najafi, F.; Moghimi, H.R.; Hemmati, M.; Deevband, M.R.; Kazemi, B. SRL-Coated PAMAM Dendrimer Nano-Carrier for Targeted Gene Delivery to the Glioma Cells and Competitive Inhibition by Lactoferrin. Iran. J. Pharm. Res. 2016, 15, 629–640. [Google Scholar] [PubMed]
- Wu, Y.W.; Fan, Q.; Zeng, F.; Zhu, J.Y.; Chen, J.; Fan, D.D.; Li, X.W.; Duan, W.J.; Guo, Q.H.; Cao, Z.L.; et al. Peptide-Functionalized Nanoinhibitor Restrains Brain Tumor Growth by Abrogating Mesenchymal-Epithelial Transition Factor (MET) Signaling. Nano Lett. 2018, 18, 5488–5498. [Google Scholar] [CrossRef] [PubMed]
- Markowicz, J.; Wolowiec, S.; Rode, W.; Uram, L. Synthesis and Properties of alpha-Mangostin and Vadimezan Conjugates with Glucoheptoamidated and Biotinylated 3rd Generation Poly(amidoamine) Dendrimer, and Conjugation Effect on Their Anticancer and Anti-Nematode Activities. Pharmaceutics 2022, 14, 606. [Google Scholar] [CrossRef]
- Wrobel, K.; Wolowiec, S.; Markowicz, J.; Walajtys-Rode, E.; Uram, L. Synthesis of Biotinylated PAMAM G3 Dendrimers Substituted with R-Glycidol and Celecoxib/Simvastatin as Repurposed Drugs and Evaluation of Their Increased Additive Cytotoxicity for Cancer Cell Lines. Cancers 2022, 14, 714. [Google Scholar] [CrossRef]
- Uram, L.; Misiorek, M.; Pichla, M.; Filipowicz-Rachwal, A.; Markowicz, J.; Wolowiec, S.; Walajtys-Rode, E. The Effect of Biotinylated PAMAM G3 Dendrimers Conjugated with COX-2 Inhibitor (celecoxib) and PPAR gamma Agonist (Fmoc-L-Leucine) on Human Normal Fibroblasts, Immortalized Keratinocytes and Glioma Cells in Vitro. Molecules 2019, 24, 3801. [Google Scholar] [CrossRef] [Green Version]
- Uram, L.; Markowicz, J.; Misiorek, M.; Filipowicz-Rachwal, A.; Wolowiec, S.; Walajtys-Rode, E. Celecoxib substituted biotinylated poly(amidoamine) G3 dendrimer as potential treatment for temozolomide resistant glioma therapy and anti-nematode agent. Eur. J. Pharm. Sci. 2020, 152, 105439. [Google Scholar] [CrossRef]
- Ban, J.M.; Li, S.D.; Zhan, Q.; Li, X.P.; Xing, H.K.; Chen, N.; Long, L.X.; Hou, X.; Zhao, J.; Yuan, X.B. PMPC Modified PAMAM Dendrimer Enhances Brain Tumor-Targeted Drug Delivery. Macromol. Biosci. 2021, 21, 2000392. [Google Scholar] [CrossRef]
- Sharma, R.; Liaw, K.; Sharma, A.; Jimenez, A.; Chang, M.; Salazar, S.; Amlani, I.; Kannan, S.; Kannan, R.M. Glycosylation of PAMAM dendrimers significantly improves tumor macrophage targeting and specificity in glioblastoma. J. Control Release 2021, 337, 179–192. [Google Scholar] [CrossRef]
- Czarnik-Kwaśniak, J.; Kwaśniak, K.; Tutaj, K.; Filiks, I.; Uram, Ł.; Stompor, M.; Wołowiec, S. Glucoheptoamidated polyamidoamine PAMAM G3 dendrimer as a vehicle for succinate linked doxorubicin; enhanced toxicity of DOX against grade IV glioblastoma U-118 MG cells. J. Drug Deliv. Sci. Technol. 2020, 55, 101424. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, L.; Sahu, H.; Qayum, A.; Singh, S.K.; Nakhate, K.T.; Ajazuddin; Gupta, U. Chitosan Engineered PAMAM Dendrimers as Nanoconstructs for the Enhanced Anti-Cancer Potential and Improved In vivo Brain Pharmacokinetics of Temozolomide. Pharm. Res. 2018, 35, 9. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Li, J.J.; Han, S.P.; Tao, C.H.; Fang, L.; Sun, Y.; Zhu, J.Z.; Liang, Z.H.; Li, F.Z. A novel doxorubicin loaded folic acid conjugated PAMAM modified with borneol, a nature dual-functional product of reducing PAMAM toxicity and boosting BBB penetration. Eur. J. Pharm. Sci. 2016, 88, 178–190. [Google Scholar] [CrossRef] [PubMed]




| Methods | Advantages | Disadvantages |
|---|---|---|
| Embedded in the cavity of PAMAM |
|
|
| Complexed on the surface of PAMAM |
|
|
| Covalently bound in the arm of PAMAM |
|
|
| Drugs | Carriers | Optimum Ratios (Carrier: Drugs) | Date | Reference |
|---|---|---|---|---|
| ASODNs | G5 PAMAM | 1:16 | 2009 | [17] |
| anti-GFP siRNA | G5 PAMAM-RGD | 15:1 | 2009 | [23] |
| anti-GFP siRNA | G5 PAMAM-AC(20,40,60) | 10:1 | 2009 | [27] |
| psiRNA-EGFR | G3 PAMAM -Tat-BMPs | 12.5:1 | 2010 | [18] |
| VEGF siRNA Bcl-2 siRNA | Au-G5 PAMAM-β-CD | 1:5 | 2018 | [28] |
| SiRNA | (G2-G4) PAMAM-Bis-MPA | 2.5:1 | 2018 | [29] |
| TRAIL | G5 PAMAM-PEG-CTX | 3:1 | 2010 | [30] |
| TRAIL | G5 PAMAM-PEG-Angiopep | 3:1 | 2011 | [15] |
| pORF-hTRAIL | G5 PAMAM-PEG | 3:1 | 2016 | [16] |
| as-miR-21 | G5 PAMAM-5-FU | 16:1 | 2012 | [31] |
| miR-21i | G5 PAMAM | 16:1 | 2012 | [32] |
| pORF-IFN-β plasmid | G4 PAMAM-R | 4:1 | 2013 | [33] |
| miR-7 | PAMAM-FA | 16:1 | 2013 | [24] |
| pJDK-apoptin | G4 PAMAM, G4 PAMAM-R, G4 PAMAM-H-R, G4 PAMAM-H-K | 2:1 | 2017 | [34] |
| G4 PAMAM-FHR | 2:1 | 2019 | [26] | |
| G3 PAMAM-KRRR | 4:1 | 2021 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Ta, W.; Hua, R.; Song, J.; Lu, W. A Review on Increasing the Targeting of PAMAM as Carriers in Glioma Therapy. Biomedicines 2022, 10, 2455. https://doi.org/10.3390/biomedicines10102455
Li X, Ta W, Hua R, Song J, Lu W. A Review on Increasing the Targeting of PAMAM as Carriers in Glioma Therapy. Biomedicines. 2022; 10(10):2455. https://doi.org/10.3390/biomedicines10102455
Chicago/Turabian StyleLi, Xingyue, Wenjing Ta, Ruochen Hua, Jihong Song, and Wen Lu. 2022. "A Review on Increasing the Targeting of PAMAM as Carriers in Glioma Therapy" Biomedicines 10, no. 10: 2455. https://doi.org/10.3390/biomedicines10102455