Near-Infrared Photoimmunotherapy for Thoracic Cancers: A Translational Perspective
Abstract
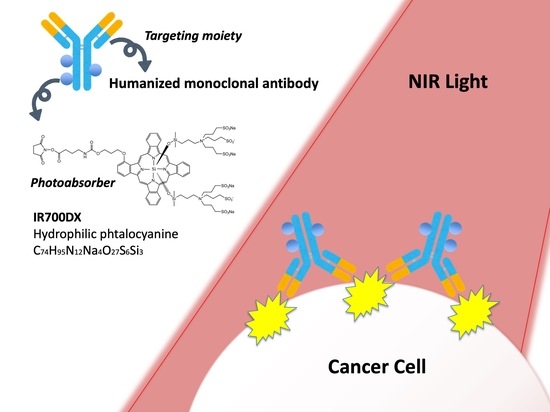
:1. Introduction
2. Summary
3. SUPR Effect
4. The Versatile Targets: EGFR, HER2, CD44, and CEA
4.1. EGFR
4.2. HER2
4.3. CD44
4.4. CEA
5. Exploring Other Targets to Detect More Specific and Effective Markers for Lung NIR-PIT: PDPN, MSLN, GPR87, and DLL3
5.1. PDPN
5.2. MSLN
5.3. GPR87
5.4. DLL3
5.5. CD26
5.6. CDH3
5.7. TROP2
5.8. XAGE1
6. Immunogenic Targets to Detect More Specific and Effective Markers for Lung NIR-PIT: CD25, PD-L1, CTLA4
6.1. CD25
6.2. PD-L1
6.3. CTLA4
7. NIR Light Irradiating Devices
8. Imaging Modality
9. Perspective on ADCs (Antibody–Drug Conjugates)

10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bibby, A.C.; Tsim, S.; Kanellakis, N.; Ball, H.; Talbot, D.C.; Blyth, K.G.; Maskell, N.A.; Psallidas, I. Malignant Pleural Mesothelioma: An Update on Investigation, Diagnosis and Treatment. Eur. Respir. Rev. 2016, 25, 472–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of Wavelength and Beam Width on Penetration in Light-Tissue Interaction Using Computational Methods. Lasers Med. Sci. 2017, 32, 1909. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, J.; Weissleder, R.; Ntziachristos, V. Would Near-Infrared Fluorescence Signals Propagate through Large Human Organs for Clinical Studies?–Errata. Opt. Lett. 2002, 27, 1652. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, X.; Xu, W.; Wang, Y.; Song, Y.; Garg, S.; Luan, Y. Engineering of a Dual-Modal Phototherapeutic Nanoplatform for Single NIR Laser-Triggered Tumor Therapy. J. Colloid Interface Sci. 2021, 594, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ren, X.; Hou, Z.; Wang, N.; Jiang, Y.; Luan, Y. Engineering a Photosensitizer Nanoplatform for Amplified Photodynamic Immunotherapy via Tumor Microenvironment Modulation. Nanoscale Horiz. 2021, 6, 120–131. [Google Scholar] [CrossRef]
- Moghissi, K.; Dixon, K. Update on the Current Indications, Practice and Results of Photodynamic Therapy (PDT) in Early Central Lung Cancer (ECLC). Photodiagnosis Photodyn. Ther. 2008, 5, 10–18. [Google Scholar] [CrossRef]
- Balchum, O.J.; Doiron, D.R.; Huth, G.C. Photoradiation Therapy of Endobronchial Lung Cancers Employing the Photodynamic Action of Hematoporphyrin Derivative. Lasers Surg. Med. 1984, 4, 13–30. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, acscentsci.8b00565. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Okada, R.; Goto, Y.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Daar, D.; Turkbey, B.; Choyke, P.L.; et al. Electron Donors Rather Than Reactive Oxygen Species Needed for Therapeutic Photochemical Reaction of Near-Infrared Photoimmunotherapy. ACS Pharmacol. Transl. Sci. 2021, 4, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.-J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic Cancer Cell Death Selectively Induced by near Infrared Photoimmunotherapy Initiates Host Tumor Immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular Effectors of Multiple Cell Death Pathways Initiated by Photodynamic Therapy. Biochim. Biophys. Acta Rev. Cancer 2007, 1776, 86–107. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA Exonuclease Trex1 Regulates Radiotherapy-Induced Tumour Immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F.; AI, M.; IF, T. Drug Penetration in Solid Tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Hambley, T.W. Is Anticancer Drug Development Heading in the Right Direction? Cancer Res. 2009, 69, 1259–1262. [Google Scholar] [CrossRef] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Maeda, H.; Matsumura, Y. EPR Effect Based Drug Design and Clinical Outlook for Enhanced Cancer Chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 129–130. [Google Scholar] [CrossRef]
- Forssen, E.A.; Coulter, D.M.; Proffitt, R.T. Selective in Vivo Localization of Daunorubicin Small Unilamellar Vesicles in Solid Tumors. Cancer Res. 1992, 52, 3255–3261. [Google Scholar] [PubMed]
- Presant, C.A.; Scolaro, M.; Kennedy, P.; Blayney, D.W.; Flanagan, B.; Lisak, J.; Presant, J. Liposomal Daunorubicin Treatment of HIV-Associated Kaposi’s Sarcoma. Lancet 1993, 341, 1242–1243. [Google Scholar] [CrossRef]
- Sano, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Markedly Enhanced Permeability and Retention Effects Induced by Photo-Immunotherapy of Tumors. ACS Nano 2012, 7, 717–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Choyke, P.L. Super Enhanced Permeability and Retention (SUPR) Effects in Tumors Following near Infrared Photoimmunotherapy. Nanoscale 2016, 8, 12504–12509. [Google Scholar] [CrossRef] [Green Version]
- Roskoski, R. The ErbB/HER Family of Protein-Tyrosine Kinases and Cancer. Pharmacol. Res. 2014, 79, 34–74. [Google Scholar] [CrossRef]
- Nicholson, R.; Gee, J.M.; Harper, M. EGFR and Cancer Prognosis. Eur. J. Cancer 2001, 37, 9–15. [Google Scholar] [CrossRef]
- Nagaya, T.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Knapp, D.W.; Karagiannis, S.N.; Fazekas-Singer, J.; Choyke, P.L.; LeBlanc, A.K.; Jensen-Jarolim, E.; et al. Near Infrared Photoimmunotherapy Targeting Bladder Cancer with a Canine Anti-Epidermal Growth Factor Receptor (EGFR) Antibody. Oncotarget 2018, 9, 19026–19038. [Google Scholar] [CrossRef] [Green Version]
- Nagaya, T.; Sato, K.; Harada, T.; Nakamura, Y.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy Targeting EGFR Positive Triple Negative Breast Cancer: Optimizing the Conjugate-Light Regimen. PLoS ONE 2015, 10, e0136829. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Railkar, R.; Sanford, T.; Crooks, D.R.; Eckhaus, M.A.; Haines, D.; Choyke, P.L.; Kobayashi, H.; Agarwal, P.K. Targeting Epidermal Growth Factor Receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (HER2) Expressing Bladder Cancer Using Combination Photoimmunotherapy (PIT). Sci. Rep. 2019, 9, 2084. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer Cell-Selective in Vivo near Infrared Photoimmunotherapy Targeting Specific Membrane Molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, T.; Sato, K.; Hanaoka, H.; Watanabe, R.; Harada, T.; Choyke, P.L.; Kobayashi, H. The Effects of Conjugate and Light Dose on Photo-Immunotherapy Induced Cytotoxicity. BMC Cancer 2014, 14, 389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Selective Cell Elimination in Vitro and in Vivo from Tissues and Tumors Using Antibodies Conjugated with a near Infrared Phthalocyanine. RSC Adv. 2015, 5, 25105–25114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Watanabe, R.; Hanaoka, H.; Harada, T.; Nakajima, T.; Kim, I.; Paik, C.H.; Choyke, P.L.; Kobayashi, H. Photoimmunotherapy: Comparative Effectiveness of Two Monoclonal Antibodies Targeting the Epidermal Growth Factor Receptor. Mol. Oncol. 2014, 8, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Burley, T.A.; Mączyńska, J.; Shah, A.; Szopa, W.; Harrington, K.J.; Boult, J.K.R.; Mrozek-Wilczkiewicz, A.; Vinci, M.; Bamber, J.C.; Kaspera, W.; et al. Near-Infrared Photoimmunotherapy Targeting EGFR-Shedding New Light on Glioblastoma Treatment. Int. J. Cancer 2018, 142, 2363–2374. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Ohler, Z.W.; Householder, D.; Nagaya, T.; Sato, K.; Okuyama, S.; Ogata, F.; Daar, D.; Hoa, T.; Choyke, P.L.; et al. Near Infrared Photoimmunotherapy in a Transgenic Mouse Model of Spontaneous Epidermal Growth Factor Receptor (EGFR)-Expressing Lung Cancer. Mol. Cancer Ther. 2017, 16, 408–414. [Google Scholar] [CrossRef] [Green Version]
- Rakuten Medical, Inc. Study of RM-1929 and Photoimmunotherapy in Patients with Recurrent Head and Neck Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02422979 (accessed on 9 July 2022).
- Cognetti, D.M.; Johnson, J.M.; Curry, J.M.; Kochuparambil, S.T.; McDonald, D.; Mott, F.; Fidler, M.J.; Stenson, K.; Vasan, N.R.; Razaq, M.A.; et al. Phase 1/2a, Open-Label, Multicenter Study of RM-1929 Photoimmunotherapy in Patients with Locoregional, Recurrent Head and Neck Squamous Cell Carcinoma. Head Neck 2021, 43, 3875–3887. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurti, U.; Silverman, J.F. HER2 in Breast Cancer. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef]
- Sato, K.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy in the Treatment of Pleural Disseminated NSCLC: Preclinical Experience. Theranostics 2015, 5, 698–709. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Nagaya, T.; Mitsunaga, M.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy for Lung Metastases. Cancer Lett. 2015, 365, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Nagaya, T.; Nakamura, Y.; Harada, T.; Choyke, P.L.; Kobayashi, H. Near Infrared Photoimmunotherapy Prevents Lung Cancer Metastases in a Murine Model. Oncotarget 2015, 6, 19747–19758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Taki, S.; Yasui, H.; Nishinaga, Y.; Isobe, Y.; Matsui, T.; Shimizu, M.; Koike, C.; Sato, K. HER2 Targeting Near-infrared Photoimmunotherapy for a CDDP-resistant Small-cell Lung Cancer. Cancer Med. 2021, 10, 8808–8819. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The Biology and Role of CD44 in Cancer Progression: Therapeutic Implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; He, J.L.; Qiu, Z.X.; Chen, N.Y.; Luo, Z.; Chen, B.J.; Li, W.M. Prognostic Value of CD44 Variant Exon 6 Expression in Non-Small Cell Lung Cancer: A Meta-Analysis. Asian Pacific J. Cancer Prev. 2014, 15, 6761–6766. [Google Scholar] [CrossRef] [Green Version]
- Zöller, M. CD44: Can a Cancer-Initiating Cell Profit from an Abundantly Expressed Molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef]
- Yan, Y.; Zuo, X.; Wei, D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. Stem Cells Transl. Med. 2015, 4, 1033–1043. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Allen, C.; Kobayashi, H. Syngeneic Mouse Models of Oral Cancer Are Effectively Targeted by Anti-CD44-Based NIR-PIT. Mol. Cancer Res. 2017, 15, 1667–1677. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Krishnamachary, B.; Mironchik, Y.; Kobayashi, H.; Bhujwalla, Z.M. Phototheranostics of CD44-Positive Cell Populations in Triple Negative Breast Cancer. Sci. Rep. 2016, 6, 27871. [Google Scholar] [CrossRef] [Green Version]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Interleukin-15 after Near-Infrared Photoimmunotherapy (NIR-PIT) Enhances T Cell Response against Syngeneic Mouse Tumors. Cancers 2020, 12, 2575. [Google Scholar] [CrossRef]
- Nagaya, T.; Friedman, J.; Maruoka, Y.; Ogata, F.; Okuyama, S.; Clavijo, P.E.; Choyke, P.L.; Allen, C.; Kobayashi, H. Host Immunity Following Near-Infrared Photoimmunotherapy Is Enhanced with PD-1 Checkpoint Blockade to Eradicate Established Antigenic Tumors. Cancer Immunol. Res. 2019, 7, 401–413. [Google Scholar] [CrossRef]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Fujimura, D.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Combined with CTLA4 Checkpoint Blockade in Syngeneic Mouse Cancer Models. Vaccines 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Campos-da-Paz, M.; Dórea, J.G.; Galdino, A.S.; Lacava, Z.G.M.; de Fatima Menezes Almeida Santos, M. Carcinoembryonic Antigen (CEA) and Hepatic Metastasis in Colorectal Cancer: Update on Biomarker for Clinical and Biotechnological Approaches. Recent Pat. Biotechnol. 2018, 12, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Grunnet, M.; Sorensen, J.B.B. Carcinoembryonic Antigen (CEA) as Tumor Marker in Lung Cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef]
- Hammarström, S. The Carcinoembryonic Antigen (CEA) Family: Structures, Suggested Functions and Expression in Normal and Malignant Tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Shirasu, N.; Yamada, H.; Shibaguchi, H.; Kuroki, M.M.; Kuroki, M.M. Potent and Specific Antitumor Effect of CEA-Targeted Photoimmunotherapy. Int. J. Cancer 2014, 135, 2697–2710. [Google Scholar] [CrossRef] [PubMed]
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Heim, R.; Makings, L.; Garcia-Guzman, M.; Luiken, G.A.; Kobayashi, H.; Hoffman, R.M.; Bouvet, M. Near Infra-Red Photoimmunotherapy with Anti-CEA-IR700 Results in Extensive Tumor Lysis and a Significant Decrease in Tumor Burden in Orthotopic Mouse Models of Pancreatic Cancer. PLoS ONE 2015, 10, e0121989. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Maawy, A.; Zhang, Y.; Guzman, M.G.; Heim, R.; Makings, L.; Luiken, G.A.; Kobayashi, H.; Tanaka, K.; Endo, I.; et al. Photoimmunotherapy Inhibits Tumor Recurrence After Surgical Resection on a Pancreatic Cancer Patient-Derived Orthotopic Xenograft (PDOX) Nude Mouse Model. Ann. Surg. Oncol. 2015, 22, 1469–1474. [Google Scholar] [CrossRef]
- Maawy, A.A.; Hiroshima, Y.; Zhang, Y.; Garcia-Guzman, M.; Luiken, G.A.; Kobayashi, H.; Hoffman, R.M.; Bouvet, M. Photoimmunotherapy Lowers Recurrence after Pancreatic Cancer Surgery in Orthotopic Nude Mouse Models. J. Surg. Res. 2015, 197, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martín-Villar, E. Podoplanin in Inflammation and Cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef] [Green Version]
- Schacht, V.; Ramirez, M.I.; Hong, Y.-K.K.; Hirakawa, S.; Feng, D.; Harvey, N.; Williams, M.; Dvorak, A.M.; Dvorak, H.F.; Oliver, G.; et al. T1α/Podoplanin Deficiency Disrupts Normal Lymphatic Vasculature Formation and Causes Lymphedema. EMBO J. 2003, 22, 3546–3556. [Google Scholar] [CrossRef]
- Wicki, A.; Lehembre, F.; Wick, N.; Hantusch, B.; Kerjaschki, D.; Christofori, G. Tumor Invasion in the Absence of Epithelial-Mesenchymal Transition: Podoplanin-Mediated Remodeling of the Actin Cytoskeleton. Cancer Cell 2006, 9, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Wicki, A.; Christofori, G. The Potential Role of Podoplanin in Tumour Invasion. Br. J. Cancer 2007, 96, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nishinaga, Y.; Sato, K.; Yasui, H.; Taki, S.; Takahashi, K.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; Nakamura, S.; et al. Targeted Phototherapy for Malignant Pleural Mesothelioma: Near-Infrared Photoimmunotherapy Targeting Podoplanin. Cells 2020, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Nishinaga, Y.; Taki, S.; Takahashi, K.; Isobe, Y.; Sato, K. Near Infrared Photoimmunotherapy for Mouse Models of Pleural Dissemination. J. Vis. Exp. 2021, 2021, e61593. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Application of Mesothelin Immunostaining in Tumor Diagnosis. Am. J. Surg. Pathol. 2003, 27, 1418–1428. [Google Scholar] [CrossRef]
- Vizcaya, D.; Farahmand, B.; Walter, A.O.; Kneip, C.; Jöhrens, K.; Tukiainen, M.; Schmitz, A.A. Prognosis of Patients with Malignant Mesothelioma by Expression of Programmed Cell Death 1 Ligand 1 and Mesothelin in a Contemporary Cohort in Finland. Cancer Treat. Res. Commun. 2020, 25, 100260. [Google Scholar] [CrossRef]
- Feng, F.; Zhang, H.; Zhang, Y.; Wang, H. Level of Mesothelin Expression Can Indicate the Prognosis of Malignant Pleural Mesothelioma. Transl. Cancer Res. 2020, 9, 7479–7485. [Google Scholar] [CrossRef]
- He, X.; Wang, L.; Riedel, H.; Wang, K.; Yang, Y.; Dinu, C.Z.; Rojanasakul, Y. Mesothelin Promotes Epithelial-to-Mesenchymal Transition and Tumorigenicity of Human Lung Cancer and Mesothelioma Cells. Mol. Cancer 2017, 16, 63. [Google Scholar] [CrossRef] [Green Version]
- Kaneko, O.; Gong, L.; Zhang, J.; Hansen, J.K.; Hassan, R.; Lee, B.; Ho, M. A Binding Domain on Mesothelin for CA125/MUC16. J. Biol. Chem. 2009, 284, 3739–3749. [Google Scholar] [CrossRef] [Green Version]
- Weidemann, S.; Gagelmann, P.; Gorbokon, N.; Lennartz, M.; Menz, A.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Blessin, N.C.; Fraune, C.; et al. Mesothelin Expression in Human Tumors: A Tissue Microarray Study on 12,679 Tumors. Biomedicines 2021, 9, 397. [Google Scholar] [CrossRef]
- Yeo, D.; Castelletti, L.; van Zandwijk, N.; Rasko, J.E.J. Hitting the Bull’s-Eye: Mesothelin’s Role as a Biomarker and Therapeutic Target for Malignant Pleural Mesothelioma. Cancers 2021, 13, 3932. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Zhang, Y.-F.; Ni, M.; Choyke, P.L.; Ho, M.; Kobayashi, H. Near Infrared Photoimmunotherapy with an Anti-Mesothelin Antibody. Oncotarget 2016, 7, 23361–23369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, A.S.; Khalil, F.K.; Welsh, E.A.; Schabath, M.B.; Enkemann, S.A.; Davis, A.; Zhou, J.-M.; Boulware, D.C.; Kim, J.; Haura, E.B.; et al. Cell-Surface Marker Discovery for Lung Cancer. Oncotarget 2017, 8, 113373–113402. [Google Scholar] [CrossRef] [Green Version]
- Gugger, M.; White, R.; Song, S.; Waser, B.; Cescato, R.; Rivière, P.; Reubi, J.C. GPR87 Is an Overexpressed G-Protein Coupled Receptor in Squamous Cell Carcinoma of the Lung. Dis. Markers 2008, 24, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Nii, K.; Tokunaga, Y.; Liu, D.; Zhang, X.; Nakano, J.; Ishikawa, S.; Kakehi, Y.; Haba, R.; Yokomise, H. Overexpression of G Protein-Coupled Receptor 87 Correlates with Poorer Tumor Differentiation and Higher Tumor Proliferation in Non-Small-Cell Lung Cancer. Mol. Clin. Oncol. 2014, 2, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhou, W.; Zhong, Y.; Huo, Y.; Fan, P.; Zhan, S.; Xiao, J.; Jin, X.; Gou, S.; Yin, T.; et al. Overexpression of G Protein-Coupled Receptor GPR87 Promotes Pancreatic Cancer Aggressiveness and Activates NF-ΚB Signaling Pathway. Mol. Cancer 2017, 16, 61. [Google Scholar] [CrossRef] [Green Version]
- Yasui, H.; Nishinaga, Y.; Taki, S.; Takahashi, K.; Isobe, Y.; Shimizu, M.; Koike, C.; Taki, T.; Sakamoto, A.; Katsumi, K.; et al. Near-Infrared Photoimmunotherapy Targeting GPR87: Development of a Humanised Anti-GPR87 MAb and Therapeutic Efficacy on a Lung Cancer Mouse Model. EBioMedicine 2021, 67, 103372. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Precision Medicine for Human Cancers with Notch Signaling Dysregulation (Review). Int. J. Mol. Med. 2020, 45, 279–297. [Google Scholar] [CrossRef] [Green Version]
- Owen, D.H.; Giffin, M.J.; Bailis, J.M.; Smit, M.-A.D.; Carbone, D.P.; He, K. DLL3: An Emerging Target in Small Cell Lung Cancer. J. Hematol. Oncol. 2019, 12, 61. [Google Scholar] [CrossRef]
- Rudin, C.M.; Pietanza, M.C.; Bauer, T.M.; Ready, N.; Morgensztern, D.; Glisson, B.S.; Byers, L.A.; Johnson, M.L.; Burris, H.A.; Robert, F.; et al. Rovalpituzumab Tesirine, a DLL3-Targeted Antibody-Drug Conjugate, in Recurrent Small-Cell Lung Cancer: A First-in-Human, First-in-Class, Open-Label, Phase 1 Study. Lancet Oncol. 2017, 18, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Isobe, Y.; Sato, K.; Nishinaga, Y.; Takahashi, K.; Taki, S.; Yasui, H.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; et al. Near Infrared Photoimmunotherapy Targeting DLL3 for Small Cell Lung Cancer. EBioMedicine 2020, 52, 102632. [Google Scholar] [CrossRef] [PubMed]
- De Zutter, A.; Van Damme, J.; Struyf, S. The Role of Post-Translational Modifications of Chemokines by CD26 in Cancer. Cancers 2021, 13, 4247. [Google Scholar] [CrossRef]
- Enz, N.; Vliegen, G.; De Meester, I.; Jungraithmayr, W. CD26/DPP4—A Potential Biomarker and Target for Cancer Therapy. Pharmacol. Ther. 2019, 198, 135–159. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, T.; Yamada, T.; Ohnuma, K.; Kina, S.; Takahashi, N.; Yamochi, T.; Inamoto, S.; Katsuoka, Y.; Hosono, O.; Tanaka, H.; et al. Humanized Anti-CD26 Monoclonal Antibody as a Treatment for Malignant Mesothelioma Tumors. Clin. Cancer Res. 2007, 13, 4191–4200. [Google Scholar] [CrossRef] [Green Version]
- Angevin, E.; Isambert, N.; Trillet-Lenoir, V.; You, B.; Alexandre, J.; Zalcman, G.; Vielh, P.; Farace, F.; Valleix, F.; Podoll, T.; et al. First-in-Human Phase 1 of YS110, a Monoclonal Antibody Directed against CD26 in Advanced CD26-Expressing Cancers. Br. J. Cancer 2017, 116, 1126–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, Q.; D’Alessio, J.A.; Menezes, D.L.; Karim, C.; Tang, Y.; Tam, A.; Clark, S.; Ying, C.; Connor, A.; Mansfield, K.G.; et al. PCA062, a P-Cadherin Targeting Antibody–Drug Conjugate, Displays Potent Antitumor Activity against P-Cadherin–Expressing Malignancies. Mol. Cancer Ther. 2021, 20, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, T.F.; Wang, C.L.; Wu, Y.C.; Feng, H.P.; Chiu, Y.C.; Lin, H.Y.; Liu, K.J.; Chang, G.C.; Chien, K.Y.; Yu, J.S.; et al. Integrative Omics Analysis Reveals Soluble Cadherin-3 as a Survival Predictor and an Early Monitoring Marker of EGFR Tyrosine Kinase Inhibitor Therapy in Lung Cancer. Clin. Cancer Res. 2020, 26, 3220–3229. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.; Kobayashi, M.; Takasaki, C.; Ishibashi, H.; Okubo, K. High Expression of P-Cadherin Is Significantly Associated with Poor Prognosis in Patients with Non-Small-Cell Lung Cancer. Lung Cancer 2018, 118, 13–19. [Google Scholar] [CrossRef]
- Lenárt, S.; Lenárt, P.; Šmarda, J.; Remšík, J.; Souček, K.; Beneš, P. Trop2: Jack of All Trades, Master of None. Cancers 2020, 12, 3328. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, M.B.; Zhou, L.N.; Tang, M.; Liu, C.Y.; Lu, P.H. Impact of TROP2 Expression on Prognosis in Solid Tumors: A Systematic Review and Meta-Analysis. Sci. Rep. 2016, 6, 33658. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, Y.; Berenguer-Pina, J.J.; Mahgoub, T. The Rise of the TROP2-Targeting Agents in NSCLC: New Options on the Horizon. Oncology 2021, 99, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, M.J.; Gure, A.O.; Jungbluth, A.A.; Old, L.J.; Chen, Y.T. Cancer/Testis Antigens: An Expanding Family of Targets for Cancer Immunotherapy. Immunol. Rev. 2002, 188, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Kurose, K.; Sakaeda, K.; Abo, H.; Atarashi, Y.; Ide, N.; Sato, T.; Kanda, E.; Fukuda, M.; Oga, T.; et al. A Novel Automated Immunoassay for Serum NY-ESO-1 and XAGE1 Antibodies in Combinatory Prediction of Response to Anti-Programmed Cell Death-1 Therapy in Non-Small-Cell Lung Cancer. Clin. Chim. Acta 2021, 519, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ohue, Y.; Kurose, K.; Mizote, Y.; Matsumoto, H.; Nishio, Y.; Isobe, M.; Fukuda, M.; Uenaka, A.; Oka, M.; Nakayama, E. Prolongation of Overall Survival in Advanced Lung Adenocarcinoma Patients with the XAGE1 (GAGED2a) Antibody. Clin. Cancer Res. 2014, 20, 5052–5063. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, K.; Noguchi, Y.; Uenaka, A.; Sato, S.; Okumura, H.; Tanaka, M.; Shimono, M.; Eldib, A.M.A.; Ono, T.; Ohara, N.; et al. XAGE-1 Expression in Non–Small Cell Lung Cancer and Antibody Response in Patients. Clin. Cancer Res. 2005, 11, 5496–5503. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Sato, N.; Xu, B.; Nakamura, Y.; Nagaya, T.; Choyke, P.L.; Hasegawa, Y.; Kobayashi, H. Spatially Selective Depletion of Tumor-Associated Regulatory T Cells with near-Infrared Photoimmunotherapy. Sci. Transl. Med. 2016, 8, 352.ra110. [Google Scholar] [CrossRef] [Green Version]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and Regulation of IL-2: From Molecular Mechanisms to Human Therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef]
- Lu, J.; Chen, X.M.; Huang, H.R.; Zhao, F.P.; Wang, F.; Liu, X.; Li, X.P. Detailed Analysis of Inflammatory Cell Infiltration and the Prognostic Impact on Nasopharyngeal Carcinoma. Head Neck 2018, 40, 1245–1253. [Google Scholar] [CrossRef]
- Zou, W. Regulatory T Cells, Tumour Immunity and Immunotherapy. Nat. Rev. Immunol. 2006, 6, 295–307. [Google Scholar] [CrossRef]
- Maruoka, Y.; Furusawa, A.; Okada, R.; Inagaki, F.; Fujimura, D.; Wakiyama, H.; Kato, T.; Nagaya, T.; Choyke, P.L.; Kobayashi, H.; et al. Combined CD44- and CD25-Targeted Near-Infrared Photoimmunotherapy Selectively Kills Cancer and Regulatory T Cells in Syngeneic Mouse Cancer Models. Cancer Immunol. 2020, 8, 345–355. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The Function of Programmed Cell Death 1 and Its Ligands in Regulating Autoimmunity and Infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Kurzrock, R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol. Cancer Ther. 2015, 14, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taki, S.; Matsuoka, K.; Nishinaga, Y.; Takahashi, K.; Yasui, H.; Koike, C.; Shimizu, M.; Sato, M.; Sato, K. Spatiotemporal Depletion of Tumor-Associated Immune Checkpoint PD-L1 with near-Infrared Photoimmunotherapy Promotes Antitumor Immunity. J. Immunother. Cancer 2021, 9, e003036. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Sato, K.; Harada, T.; Choyke, P.L.; Hodge, J.W.; Schlom, J.; Kobayashi, H. Near Infrared Photoimmunotherapy with Avelumab, an Anti-Programmed Death-Ligand 1 (PD-L1) Antibody. Oncotarget 2017, 8, 8807–8817. [Google Scholar] [CrossRef] [Green Version]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A Moving Target in Immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Walker, L.S.K.; Sansom, D.M. The Emerging Role of CTLA4 as a Cell-Extrinsic Regulator of T Cell Responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef]
- Okada, R.; Kato, T.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Choyke, P.L.; Kobayashi, H. Local Depletion of Immune Checkpoint Ligand CTLA4 Expressing Cells in Tumor Beds Enhances Antitumor Host Immunity. Adv. Ther. 2021, 4, 2000269. [Google Scholar] [CrossRef]
- Kato, T.; Okada, R.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Okuyama, S.; Fukushima, H.; Choyke, P.L.; Kobayashi, H. Simultaneously Combined Cancer Cell- and CTLA4-Targeted NIR-PIT Causes a Synergistic Treatment Effect in Syngeneic Mouse Models. Mol. Cancer Ther. 2021, 20, 2262–2273. [Google Scholar] [CrossRef]
- Hirata, H.; Kuwatani, M.; Nakajima, K.; Kodama, Y.; Yoshikawa, Y.; Ogawa, M.; Sakamoto, N. Near-Infrared Photoimmunotherapy (NIR-PIT) on Cholangiocarcinoma Using a Novel Catheter Device with Light Emitting Diodes. Cancer Sci. 2021, 112, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Watanabe, R.; Hanaoka, H.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Comparative Effectiveness of Light Emitting Diodes (LEDs) and Lasers in near Infrared Photoimmunotherapy. Oncotarget 2016, 7, 14324–14335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, S.; Nagaya, T.; Sato, K.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Interstitial Near-Infrared Photoimmunotherapy: Effective Treatment Areas and Light Doses Needed for Use with Fiber Optic Diffusers. Oncotarget 2018, 9, 11159–11169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, R.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Kato, T.; Okuyama, S.; Furumoto, H.; Fukushima, H.; Choyke, P.L.; Kobayashi, H.; et al. Endoscopic Near-Infrared Photoimmunotherapy in an Orthotopic Head and Neck Cancer Model. Cancer Sci. 2021, 112, 3041–3049. [Google Scholar] [CrossRef]
- Zhong, W.; Celli, J.P.; Rizvi, I.; Mai, Z.; Spring, B.Q.; Yun, S.H.; Hasan, T. In Vivo High-Resolution Fluorescence Microendoscopy for Ovarian Cancer Detection and Treatment Monitoring. Br. J. Cancer 2009, 101, 2015–2022. [Google Scholar] [CrossRef] [Green Version]
- Henderson, T.A.; Morries, L.D.; TA, H.; LD, M.; Henderson, T.A.; Morries, L.D. Near-Infrared Photonic Energy Penetration: Can Infrared Phototherapy Effectively Reach the Human Brain? Neuropsychiatr. Dis. Treat. 2015, 11, 2191–2208. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, K.; Kimura, T.; Takakura, H.; Yoshikawa, Y.; Kameda, A.; Shindo, T.; Sato, K.; Kobayashi, H.; Ogawa, M. Implantable Wireless Powered Light Emitting Diode (LED) for near-Infrared Photoimmunotherapy: Device Development and Experimental Assessment in Vitro and in Vivo. Oncotarget 2018, 9, 20048–20057. [Google Scholar] [CrossRef] [Green Version]
- Ogata, F.; Nagaya, T.; Nakamura, Y.; Sato, K.; Okuyama, S.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H.; Ogata, F.; Nagaya, T.; et al. Near-Infrared Photoimmunotherapy: A Comparison of Light Dosing Schedules. Oncotarget 2017, 8, 35069–35075. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, K.; Sato, M.; Sato, K. Hurdles for the Wide Implementation of Photoimmunotherapy. Immunotherapy 2021, 13, 1427–1438. [Google Scholar] [CrossRef]
- Okuyama, S.; Nagaya, T.; Ogata, F.; Maruoka, Y.; Sato, K.; Nakamura, Y.; Choyke, P.L.; Kobayashi, H. Avoiding Thermal Injury during Near-Infrared Photoimmunotherapy (NIR-PIT): The Importance of NIR Light Power Density. Oncotarget 2017, 8, 113194–113201. [Google Scholar] [CrossRef] [Green Version]
- Furumoto, H.; Kato, T.; Wakiyama, H.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Endoscopic Applications of Near-Infrared Photoimmunotherapy (NIR-PIT) in Cancers of the Digestive and Respiratory Tracts. Biomedicines 2022, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, Y.; Nagaya, T.; Nakamura, Y.; Sato, K.; Ogata, F.; Okuyama, S.; Choyke, P.L.; Kobayashi, H. Evaluation of Early Therapeutic Effects after Near-Infrared Photoimmunotherapy (NIR-PIT) Using Luciferase-Luciferin Photon-Counting and Fluorescence Imaging. Mol. Pharm. 2017, 14, 4628–4635. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, F.F.; Fujimura, D.; Furusawa, A.; Okada, R.; Wakiyama, H.; Kato, T.; Choyke, P.L.; Kobayashi, H. Fluorescence Imaging of Tumor-Accumulating Antibody-IR700 Conjugates Prior to Near-Infrared Photoimmunotherapy (NIR-PIT) Using a Commercially Available Camera Designed for Indocyanine Green. Mol. Pharm. 2021, 18, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Fujimura, D.; Inagaki, F.; Okada, R.; Maruoka, Y.; Wakiyama, H.; Kato, T.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Real-Time IR700 Fluorescence Imaging During Near-Infrared Photoimmunotherapy Using a Clinically-Approved Camera for Indocyanine Green. Cancer Diagn. Progn. 2021, 1, 29–34. [Google Scholar] [CrossRef]
- Ali, T.; Nakajima, T.; Sano, K.; Sato, K.; Choyke, P.L.; Kobayashi, H. Dynamic Fluorescent Imaging with Indocyanine Green for Monitoring the Therapeutic Effects of Photoimmunotherapy. Contrast Media Mol. Imaging 2014, 9, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, F.F.; Fujimura, D.; Furusawa, A.; Okada, R.; Wakiyama, H.; Kato, T.; Choyke, P.L.; Kobayashi, H. Diagnostic Imaging in Near-infrared Photoimmunotherapy Using a Commercially Available Camera for Indocyanine Green. Cancer Sci. 2021, 112, 1326–1330. [Google Scholar] [CrossRef]
- Nakamura, Y.; Bernardo, M.; Nagaya, T.; Sato, K.; Harada, T.; Choyke, P.L.; Kobayashi, H. MR Imaging Biomarkers for Evaluating Therapeutic Effects Shortly after near Infrared Photoimmunotherapy. Oncotarget 2016, 7, 17254. [Google Scholar] [CrossRef] [Green Version]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg Effect: Historical Dogma versus Current Understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar]
- Vaupel, P.; Schmidberger, H.; Mayer, A. The Warburg Effect: Essential Part of Metabolic Reprogramming and Central Contributor to Cancer Progression. Int. J. Radiat. Biol. 2019, 95, 912–919. [Google Scholar] [CrossRef]
- Van der Vos, C.S.; Koopman, D.; Rijnsdorp, S.; Arends, A.J.; Boellaard, R.; van Dalen, J.A.; Lubberink, M.; Willemsen, A.T.M.; Visser, E.P. Quantification, Improvement, and Harmonization of Small Lesion Detection with State-of-the-Art PET. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 4–16. [Google Scholar] [CrossRef]
- Aarntzen, E.H.J.G.; Heijmen, L.; Oyen, W.J.G. 18F-FDG PET/CT in Local Ablative Therapies: A Systematic Review. J. Nucl. Med. 2018, 59, 551–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Higuchi, T.; Achmad, A.; Bhattarai, A.; Tomonaga, H.; Thu, H.N.; Yamaguchi, A.; Hirasawa, H.; Taketomi-Takahashi, A.; Tsushima, Y. Can 18F-Fluorodeoxyglucose Positron Emission Tomography Predict the Response to Radioactive Iodine Therapy in Metastatic Differentiated Thyroid Carcinoma? Eur. J. Hybrid Imaging 2018, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Sano, K.; Mitsunaga, M.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Acute Cytotoxic Effects of Photoimmunotherapy Assessed by 18F-FDG PET. J. Nucl. Med. 2013, 54, 770–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Mitsunaga, M.; Nishimura, T.; Saruta, M.; Iwamoto, T.; Kobayashi, H.; Tajiri, H. Near-Infrared Photochemoimmunotherapy by Photoactivatable Bifunctional Antibody–Drug Conjugates Targeting Human Epidermal Growth Factor Receptor 2 Positive Cancer. Bioconjug. Chem. 2017, 28, 1458. [Google Scholar] [CrossRef] [PubMed]
- Nani, R.R.; Gorka, A.P.; Nagaya, T.; Yamamoto, T.; Ivanic, J.; Kobayashi, H.; Schnermann, M.J. In Vivo Activation of Duocarmycin-Antibody Conjugates by Near-Infrared Light. ACS Cent. Sci. 2017, 3, 329–337. [Google Scholar] [CrossRef]
- Nagaya, T.; Gorka, A.P.; Nani, R.R.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Schnermann, M.J.; Kobayashi, H. Molecularly Targeted Cancer Combination Therapy with Near-Infrared Photoimmunotherapy and Near-Infrared Photorelease with Duocarmycin–Antibody Conjugate. Mol. Cancer Ther. 2018, 17, 661–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Target Antigens | |
|---|---|
| Versatile targets | EGFR CD44 CEA HER2 GPR87 |
| Exploring targets | PDPN MSLN GPR87 DLL3 CD26 CDH TROP2 XAGE1 |
| Immune targets | CD25 PD-L1 CTLA4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuoka, K.; Yamada, M.; Sato, M.; Sato, K. Near-Infrared Photoimmunotherapy for Thoracic Cancers: A Translational Perspective. Biomedicines 2022, 10, 1662. https://doi.org/10.3390/biomedicines10071662
Matsuoka K, Yamada M, Sato M, Sato K. Near-Infrared Photoimmunotherapy for Thoracic Cancers: A Translational Perspective. Biomedicines. 2022; 10(7):1662. https://doi.org/10.3390/biomedicines10071662
Chicago/Turabian StyleMatsuoka, Kohei, Mizuki Yamada, Mitsuo Sato, and Kazuhide Sato. 2022. "Near-Infrared Photoimmunotherapy for Thoracic Cancers: A Translational Perspective" Biomedicines 10, no. 7: 1662. https://doi.org/10.3390/biomedicines10071662