Serotonin Signalling in Flatworms: An Immunocytochemical Localisation of 5-HT7 Type of Serotonin Receptors in Opisthorchis felineus and Hymenolepis diminuta
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Fixation
2.2. Immunocytochemistry
2.3. Histochemical Staining
2.4. Confocal Laser Scanning and Fluorescent Microscopies
2.5. Bioinformatics
3. Results
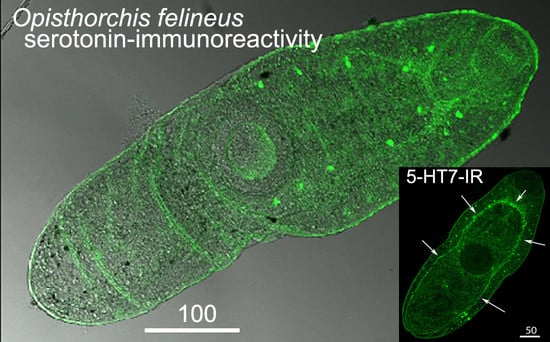
3.1. Opisthorchis felineus Metacercariae: Immuoreactivity to Serotonin and 5-HT7 Serotonin Receptor
3.2. Hymenolepis diminuta Cysticercoids, Immunoreactivity to Serotonin and 5-HT7 Serotonin Receptor
3.3. A phylogenetic Analysis of 5-HT7 Receptor
4. Discussion
4.1. Serotonin and 5-HT7-IR in Metacercariae of O. felineus
4.1.1. Ventral Sucker
4.1.2. Sensory Structures (Papillae)
4.1.3. Glandular and Excretory Cells
4.1.4. The Digestive System
4.1.5. Musculature
4.2. Serotonin and 5-HT7-IR in Cysticercoid Larvae of Hymenolepis diminuta
4.2.1. Musculature and Nerve Fibres
4.2.2. The Flame Cells
5. Conclusions
- The distribution of immunoreactivity to the 5-HT7 serotonin receptor was investigated in larvae tissues of two parasitic flatworms, the trematode Opisthorchis felineus and the cestode Hymenolepis diminuta for the first time.
- The presence of the specific serotonin 5-HT7receptor’s immunoreactivity in the studied parasitic worms has been shown. It emphasises the importance of the serotonergic signalling system for realisation of vital functions in representatives of Platyhelminthes.
- The results suggest that the 5-HT7 type of serotonin receptor can mediate the serotonin action in the studied species and is an important component of worm motor system control.
- Taking into account the important roles of 5-HT in parasite biology, the present report also suggests that the flatworm serotoninergic nervous system could be considered a target for anti-parasite drugs.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gershon, M.D. Review article: Serotonin receptors and transporters—Roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 2004, 20 (Suppl. S7), 3–14. [Google Scholar] [CrossRef]
- Martin, A.M.; Young, R.L.; Leong, L.; Rogers, G.B.; Spencer, N.J.; Jessup, C.F.; Keating, D.J. The Diverse Metabolic Roles of Peripheral Serotonin. Endocrinology 2017, 158, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.J.; Noh, H.J.; Kim, J.G.; Park, S.W.; Kim, B.; Cho, H.; Bae, Y.M. Serotonin contracts the rat mesenteric artery by inhibiting 4-aminopyridine-sensitive Kv channels via the 5-HT2A receptor and Src tyrosine kinase. Exp. Mol. Med. 2013, 45, e67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.A.; Sun, E.W.; Martin, A.M.; Keating, D.J. The ever-changing roles of serotonin. Int. J. Biochem. Cell Biol. 2020, 125, 105776. [Google Scholar] [CrossRef] [PubMed]
- Okaty, B.W.; Commons, K.G.; Dymecki, S.M. Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci. 2019, 20, 397–424. [Google Scholar] [CrossRef] [PubMed]
- Plieger, T.; Melchers, M.; Vetterlein, A.; Gortz, J.; Kuhn, S.; Ruppel, M.; Reuter, M. The serotonin transporter polymorphism (5-HTTLPR) and coping strategies influence successful emotion regulation in an acute stress situation: Physiological evidence. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2017, 114, 31–37. [Google Scholar] [CrossRef]
- Popova, N.K. From genes to aggressive behavior: The role of serotonergic system. BioEssays News Rev. Mol. Cell. Dev. Biol. 2006, 28, 495–503. [Google Scholar] [CrossRef]
- Popova, N.K.; Naumenko, V.S. Neuronal and behavioral plasticity: The role of serotonin and BDNF systems tandem. Expert Opin. Ther. Targets 2019, 23, 227–239. [Google Scholar] [CrossRef]
- Shajib, M.S.; Khan, W.I. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2015, 213, 561–574. [Google Scholar] [CrossRef]
- Wan, M.; Ding, L.; Wang, D.; Han, J.; Gao, P. Serotonin: A Potent Immune Cell Modulator in Autoimmune Diseases. Front. Immunol. 2020, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.P.; Zaide, J.V.; Yoshino, T.P. Schistosoma mansoni: Effects of serotonin and serotonin receptor antagonists on motility and length of primary sporocysts in vitro. Exp. Parasitol. 2000, 94, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Faixova, D.; Hrckova, G.; Macak Kubaskova, T.; Mudronova, D. Antiparasitic Effects of Selected Isoflavones on Flatworms. Helminthologia 2021, 58, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hrckova, G.; Kubaskova, T.M.; Reiterova, K.; Biedermann, D. Co-administration of silymarin elevates the therapeutic effect of praziquantel through modulation of specific antibody profiles, Th1/Th2/Tregs cytokines and down-regulation of fibrogenesis in mice with Mesocestoides vogae (Cestoda) infection. Exp. Parasitol. 2020, 213, 107888. [Google Scholar] [CrossRef]
- Haas, B.J.; Berriman, M.; Hirai, H.; Cerqueira, G.G.; Loverde, P.T.; El-Sayed, N.M. Schistosoma mansoni genome: Closing in on a final gene set. Exp. Parasitol. 2007, 117, 225–228. [Google Scholar] [CrossRef]
- Hu, W.; Yan, Q.; Shen, D.K.; Liu, F.; Zhu, Z.D.; Song, H.D.; Xu, X.R.; Wang, Z.J.; Rong, Y.P.; Zeng, L.C.; et al. Evolutionary and biomedical implications of a Schistosoma japonicum complementary DNA resource. Nat. Genet. 2003, 35, 139–147. [Google Scholar] [CrossRef]
- Genome, S.; Functional Analysis, C. The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature 2009, 460, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Laing, R.; Kikuchi, T.; Martinelli, A.; Tsai, I.J.; Beech, R.N.; Redman, E.; Holroyd, N.; Bartley, D.J.; Beasley, H.; Britton, C.; et al. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome Biol. 2013, 14, R88. [Google Scholar] [CrossRef] [Green Version]
- Robb, S.M.; Ross, E.; Sanchez Alvarado, A. SmedGD: The Schmidtea mediterranea genome database. Nucleic Acids Res. 2008, 36, D599–D606. [Google Scholar] [CrossRef] [Green Version]
- Tsai, I.J.; Zarowiecki, M.; Holroyd, N.; Garciarrubio, A.; Sanchez-Flores, A.; Brooks, K.L.; Tracey, A.; Bobes, R.J.; Fragoso, G.; Sciutto, E.; et al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature 2013, 496, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Chen, W.; Huang, Y.; Sun, J.; Men, J.; Liu, H.; Luo, F.; Guo, L.; Lv, X.; Deng, C.; et al. The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol. 2011, 12, R107. [Google Scholar] [CrossRef] [Green Version]
- Young, N.D.; Jex, A.R.; Li, B.; Liu, S.; Yang, L.; Xiong, Z.; Li, Y.; Cantacessi, C.; Hall, R.S.; Xu, X.; et al. Whole-genome sequence of Schistosoma haematobium. Nat. Genet. 2012, 44, 221–225. [Google Scholar] [CrossRef] [Green Version]
- Halton, D.W.; Maule, A.G. Flatworm nerve–muscle: Structural and functional analysis. Can. J. Zool. 2004, 82, 316–333. [Google Scholar] [CrossRef]
- Ribeiro, P.; El-Shehabi, F.; Patocka, N. Classical transmitters and their receptors in flatworms. Parasitology 2005, 131 (Suppl. S1), S19–S40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terenina, N.; Gustafsson, M.K.S. Neurotransmitters in Helminths; Nauka: Moscow, Russia, 2003; p. 178. [Google Scholar]
- Kreshchenko, N.; Terenina, N.; Nefedova, D.; Mochalova, N.; Voropaeva, E.; Movsesyan, S. The neuroactive substances and associated muscle system in Rhipidocotyle campanula (Digenea, Bucephalidae) from the intestine of the pike Esox lucius. J. Morphol. 2020, 281, 1047–1058. [Google Scholar] [CrossRef]
- Mair, G.R.; Halton, D.W.; Maule, A.G. The neuromuscular system of the sheep tapeworm Moniezia expansa. Invertebr. Neurosci. 2020, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Mochalova, N.V.; Terenina, N.B.; Poddubnaya, L.G.; Yashin, V.A.; Kuchin, A.V.; Kreshchenko, N.D. First evidence of serotoninergic components in the nervous system of the monogenean Chimaericola leptogaster (Chimaericolidae, Polyopisthocotylea), a gill parasite of the relict holocephalan fish. Folia Parasitol 2019, 66. [Google Scholar] [CrossRef] [PubMed]
- Terenina, N.B.; Kreshchenko, N.D.; Mochalova, N.V.; Nefedova, D.; Voropaeva, E.L.; Movsesyan, S.O.; Demiaszkiewicz, A.; Yashin, V.A.; Kuchin, A.V. The New Data on the Serotonin and FMRFamide Localization in the Nervous System of Opisthorchis felineus Metacercaria. Acta Parasitol. 2020, 65, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Tolstenkov, O.O.; Akimova, L.N.; Chrisanfova, G.G.; Terenina, N.B.; Gustafsson, M.K. The neuro-muscular system in fresh-water furcocercaria from Belarus. I Schistosomatidae. Parasitol. Res. 2012, 110, 185–193. [Google Scholar] [CrossRef]
- Tolstenkov, O.O.; Akimova, L.N.; Terenina, N.B.; Gustafsson, M.K. The neuromuscular system in freshwater furcocercaria from Belarus. II Diplostomidae, Strigeidae, and Cyathocotylidae. Parasitol. Res. 2012, 110, 583–592. [Google Scholar] [CrossRef]
- Terenina, N.B.; Gustafsson, M.K.S. The Functional Morphology of the Nrvous System of Parasitic Flatworms (Trematodes, Cestodes); KMK: Moscow, Russia, 2014; p. 296. [Google Scholar]
- Catto, B.A.; Ottesen, E.A. Serotonin uptake in schistosomules of Schistosoma mansoni. Comp. Biochem. Physiol. C Comp. Pharm. 1979, 63C, 235–242. [Google Scholar] [CrossRef]
- Chou, T.C.; Bennett, J.; Bueding, E. Occurrence and concentrations of biogenic amines in trematodes. J. Parasitol. 1972, 58, 1098–1102. [Google Scholar] [CrossRef]
- Hariri, M. Occurrence and concentration of biogenic amines in Mesocestoides corti (Cestoda). J. Parasitol. 1974, 60, 737–743. [Google Scholar] [CrossRef]
- Lee, M.B.; Bueding, E.; Schiller, E.L. The occurrence and distribution of 5-hydroxytryptamine in Hymenolepis diminuta and H. nana. J. Parasitol. 1978, 64, 257–264. [Google Scholar] [CrossRef]
- Ribeiro, P.; Webb, R.A. The occurrence, synthesis and metabolism of 5-hydroxytryptamine and 5-hydroxytryptophan in the cestode Hymenolepis diminuta: A high performance liquid chromatographic study. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1984, 79, 159–164. [Google Scholar] [CrossRef]
- Cyr, D.; Gruner, S.; Mettrick, D.F. Hymenolepis diminuta: Uptake of 5-hydroxytryptamine (serotonin), glucose, and changes in worm glycogen levels. Can. J. Zool. 1983, 61, 1469–1474. [Google Scholar] [CrossRef]
- Bennett, J.L.; Bueding, E. Uptake of 5-hydroxytryptamine by Schistosoma mansoni. Mol. Pharm. 1973, 9, 311–319. [Google Scholar]
- Boyle, J.P.; Hillyer, J.F.; Yoshino, T.P. Pharmacological and autoradiographical characterization of serotonin transporter-like activity in sporocysts of the human blood fluke, Schistosoma mansoni. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2003, 189, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Patocka, N.; Ribeiro, P. Characterization of a serotonin transporter in the parasitic flatworm, Schistosoma mansoni: Cloning, expression and functional analysis. Mol. Biochem. Parasitol. 2007, 154, 125–133. [Google Scholar] [CrossRef]
- Webb, R.A. The uptake and metabolism of 5-hydroxytryptamine by tissue slices of the cestode Hymenolepis diminuta. Comp. Biochem. Physiol. C Comp. Pharm. Toxicol. 1985, 80, 305–312. [Google Scholar] [CrossRef]
- Hamdan, F.F.; Ribeiro, P. Cloning and Characterization of a Novel Form of Tyrosine Hydroxylase from the Human Parasite, Schistosoma mansoni. J. Neurochem. 1998, 71, 1369–1380. [Google Scholar] [CrossRef] [Green Version]
- Patocka, N.; Ribeiro, P. The functional role of a serotonin transporter in Schistosoma mansoni elucidated through immunolocalization and RNA interference (RNAi). Mol. Biochem. Parasitol. 2013, 187, 32–42. [Google Scholar] [CrossRef]
- Fontana, A.C.; Sonders, M.S.; Pereira-Junior, O.S.; Knight, M.; Javitch, J.A.; Rodrigues, V.; Amara, S.G.; Mortensen, O.V. Two allelic isoforms of the serotonin transporter from Schistosoma mansoni display electrogenic transport and high selectivity for serotonin. Eur. J. Pharmacol. 2009, 616, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, F.F.; Ribeiro, P. Characterization of a stable form of tryptophan hydroxylase from the human parasite Schistosoma mansoni. J. Biol. Chem. 1999, 274, 21746–21754. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, P.; Webb, R.A. Characterization of a serotonin transporter and an adenylate cyclase-linked serotonin receptor in the cestode Hymenolepis diminuta. Life Sci. 1987, 40, 755–768. [Google Scholar] [CrossRef]
- Ribeiro, P.; Webb, R.A. The synthesis of 5-hydroxytryptamine from tryptophan and 5-hydroxytryptophan in the cestode Hymenolepis diminuta. Int. J. Parasitol. 1983, 13, 101–106. [Google Scholar] [CrossRef]
- Osloobi, N.; Webb, R.A. Localization of a sodium-dependent high-affinity serotonin transporter and recruitment of exogenous serotonin by the cestode Hymenolepis diminuta: An autoradiographic and immunohistochemical study. Can. J. Zool. 1999, 77, 1265–1277. [Google Scholar] [CrossRef]
- HrČKova, G.; VelenbnÝ, S.; Halton, D.W.; Maule, A.G. Mesocestoides corti (syn. M. vogae): Modulation of larval motility by neuropeptides, serotonin and acetylcholine. Parasitology 2002, 124, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Tolstenkov, O.O.; Prokofiev, V.V.; Pleskacheva, M.V.; Gustafsson, M.K.S.; Zhukovskaya, M.I. Age and serotonin effects on locomotion in marine trematode cercariae. J. Evol. Biochem. Physiol. 2017, 53, 135–142. [Google Scholar] [CrossRef]
- McKay, D.M.; Halton, D.W.; Allen, J.M.; Fairweather, I. The effects of cholinergic and serotoninergic drugs on motility in vitro of Haplometra cylindracea (Trematoda: Digenea). Parasitology 1989, 99 Pt 2, 241–252. [Google Scholar] [CrossRef]
- Thompson, C.S.; Mettrick, D.F. The effects of 5-hydroxytryptamine and glutamate on muscle contraction in Hymenolepis diminuta (Cestoda). Can. J. Zool. 1989, 67, 1257–1262. [Google Scholar] [CrossRef]
- Holmes, S.D.; Fairweather, I. Fasciola hepatica: The effects of neuropharmacological agents upon in vitro motility. Exp. Parasitol. 1984, 58, 194–208. [Google Scholar] [CrossRef]
- Tembe, E.A.; Holden-Dye, L.; Smith, S.W.; Jacques, P.A.; Walker, R.J. Pharmacological profile of the 5-hydroxytryptamine receptor of Fasciola hepatica body wall muscle. Parasitology 1993, 106 Pt 1, 67–73. [Google Scholar] [CrossRef]
- Maule, A.; Halton, D.; Allen, J.; Fairweather, I. Studies on motility in vitro of an ectoparasitic monogenean, Diclidophora merlangi. Parasitology 1989, 98, 85–93. [Google Scholar] [CrossRef]
- Day, T.A.; Bennett, J.L.; Pax, R.A. Serotonin and its requirement for maintenance of contractility in muscle fibres isolated from Schistosoma mansoni. Parasitology 1994, 108 Pt 4, 425–432. [Google Scholar] [CrossRef]
- Pax, R.A.; Siefker, C.; Bennett, J.L. Schistosoma mansoni: Differences in acetylcholine, dopamine, and serotonin control of circular and longitudinal parasite muscles. Exp. Parasitol. 1984, 58, 314–324. [Google Scholar] [CrossRef]
- Ribeiro, P.; Gupta, V.; El-Sakkary, N. Biogenic amines and the control of neuromuscular signaling in schistosomes. Invertebr. Neurosci. 2012, 12, 13–28. [Google Scholar] [CrossRef]
- Patocka, N.; Sharma, N.; Rashid, M.; Ribeiro, P. Serotonin signaling in Schistosoma mansoni: A serotonin-activated G protein-coupled receptor controls parasite movement. PLoS Pathog. 2014, 10, e1003878. [Google Scholar] [CrossRef] [PubMed]
- Sakharov, D.A.; Golubev, A.I.; Malyutina, L.V.; Kabotyanski, E.A.; Nezlin, L.P. Serotoninergic control of ciliary locomotion in a turbellarian flatworm. In Neurobiology of Invertebrates: Transmitters, Modulators and Receptors; Akadémiai Kiadó: Hungary, Budapest, 1988; pp. 479–491. [Google Scholar]
- Farrell, M.S.; Gilmore, K.; Raffa, R.B.; Walker, E.A. Behavioral characterization of serotonergic activation in the flatworm Planaria. Behav. Pharmacol. 2008, 19, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Moneypenny, C.G.; Kreshchenko, N.; Moffett, C.L.; Halton, D.W.; Day, T.A.; Maule, A.G. Physiological effects of FMRFamide-related peptides and classical transmitters on dispersed muscle fibres of the turbellarian, Procerodes littoralis. Parasitology 2001, 122, 447–455. [Google Scholar] [CrossRef] [Green Version]
- Herz, M.; Brehm, K. Serotonin stimulates Echinococcus multilocularis larval development. Parasites Vectors 2021, 14, 14. [Google Scholar] [CrossRef]
- Franquinet, R.; Martelly, I. Effects of serotonin and catecholamines on RNA synthesis in planarians; in vitro and in vivo studies. Cell Differ. 1981, 10, 201–209. [Google Scholar] [CrossRef]
- Sarkar, A.; Mukundan, N.; Sowndarya, S.; Dubey, V.K.; Babu, R.; Lakshmanan, V.; Rangiah, K.; Panicker, M.M.; Palakodeti, D.; Subramanian, S.P.; et al. Serotonin is essential for eye regeneration in planaria Schmidtea mediterranea. FEBS Lett. 2019, 593, 3198–3209. [Google Scholar] [CrossRef] [PubMed]
- Kreshchenko, N.D.; Grebenshchikova, E.V.; Karpov, A.N. Influence of serotonin on planarian photoreceptor’s regeneration. In Proceedings of the Theory and Practice of Parasitic Disease Control: Collection of Scientific Articles Adapted from the International Scientific Conference, Moscow, Russia, 15–17 May 2019. [Google Scholar]
- Kreshchenko, N. Institute of Cell Biophysics, Pushchino, Russia. Unpublished work. 2021. [Google Scholar]
- Maricq, A.V.; Peterson, A.S.; Brake, A.J.; Myers, R.M.; Julius, D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science 1991, 254, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Hannon, J.; Hoyer, D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008, 195, 198–213. [Google Scholar] [CrossRef]
- Gothert, M. Serotonin discovery and stepwise disclosure of 5-HT receptor complexity over four decades. Part, I. General background and discovery of serotonin as a basis for 5-HT receptor identification. Pharm. Rep. 2013, 65, 771–786. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, F.F.; Ungrin, M.D.; Abramovitz, M.; Ribeiro, P. Characterization of a novel serotonin receptor from Caenorhabditis elegans: Cloning and expression of two splice variants. J. Neurochem. 1999, 72, 1372–1383. [Google Scholar] [CrossRef]
- Huang, X.; Duran, E.; Diaz, F.; Xiao, H.; Messer, W.S., Jr.; Komuniecki, R. Alternative-splicing of serotonin receptor isoforms in the pharynx and muscle of the parasitic nematode, Ascaris suum. Mol. Biochem. Parasitol. 1999, 101, 95–106. [Google Scholar] [CrossRef]
- Olde, B.; McCombie, W.R. Molecular cloning and functional expression of a serotonin receptor from Caenorhabditis elegans. J. Mol. Neurosci. MN 1997, 8, 53–62. [Google Scholar] [CrossRef]
- Henne, S.; Sombke, A.; Schmidt-Rhaesa, A. Immunohistochemical analysis of the anterior nervous system of the free-living nematode Plectus spp. (Nematoda, Plectidae). Zoomorphology 2017, 136, 175–190. [Google Scholar] [CrossRef]
- Vleugels, R.; Verlinden, H.; Vanden Broeck, J. Serotonin, serotonin receptors and their actions in insects. Neurotransmitter 2015, 2. [Google Scholar] [CrossRef]
- Kamhi, J.F.; Arganda, S.; Moreau, C.S.; Traniello, J.F.A. Origins of Aminergic Regulation of Behavior in Complex Insect Social Systems. Front. Syst. Neurosci. 2017, 11, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivashkin, E.; Khabarova, M.; Melnikova, V.; Kharchenko, O.; Voronezhskaya, E. Local serotonin-immunoreactive plexus in the female reproductive system of hermaphroditic gastropod mollusc Lymnaea stagnalis. Invertebr. Zool. 2017, 14, 134–139. [Google Scholar] [CrossRef]
- Franquinet, R.; Le Moigne, A.; Hanoune, J. The adenylate cyclase system of planaria Polycelis tenuis: Activation by serotonin and guanine nucleotides. Biochim. Biophys. Acta 1978, 539, 88–97. [Google Scholar] [CrossRef]
- Camicia, F.; Celentano, A.M.; Johns, M.E.; Chan, J.D.; Maldonado, L.; Vaca, H.; Di Siervi, N.; Kamentezky, L.; Gamo, A.M.; Ortega-Gutierrez, S.; et al. Unique pharmacological properties of serotoninergic G-protein coupled receptors from cestodes. PLoS Negl. Trop. Dis. 2018, 12, e0006267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tierney, A.J. Structure and function of invertebrate 5-HT receptors: A review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 791–804. [Google Scholar] [CrossRef]
- Tierney, A.J. Invertebrate serotonin receptors: A molecular perspective on classification and pharmacology. J. Exp. Biol. 2018, 221. [Google Scholar] [CrossRef] [Green Version]
- Tierney, A.J. Feeding, hunger, satiety and serotonin in invertebrates. Proc. Biol. Sci./R. Soc. 2020, 287, 20201386. [Google Scholar] [CrossRef]
- Coons, A.H.; Leduc, E.H.; Connolly, J.M. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J. Exp. Med. 1955, 102, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Glaskov, G.A. Isolation of some trematode metacercariaefrom diseased fish tissue by digestion in artificial gastric juice. In Diseases and Parasites of Fish in the Litovitomsk Province (Within the USSR); Tomsk State University: Tomsk, Russia, 1979; pp. 72–82. [Google Scholar]
- Rothman, A.H. Studies on the excystment of tapeworms. Exp. Parasitol. 1959, 8, 336–364. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/protein (accessed on 12 March 2021).
- WormBase ParaSite. Available online: https://parasite.wormbase.org/index.html (accessed on 12 May 2021).
- National Center for Biotechnology Information Genome Database. Available online: https://www.ncbi.nlm.nih.gov/genome (accessed on 5 May 2021).
- Fairweather, I.; Maule, A.G.; Mitchell, S.H.; Johnston, C.F.; Halton, D.W. Immunocytochemical demonstration of 5-hydroxytryptamine (serotonin) in the nervous system of the liver fluke, Fasciola hepatica (Trematoda, Digenea). Parasitol. Res. 1987, 73, 255–258. [Google Scholar] [CrossRef]
- Fairweather, I.; McMullan, M.T.; Johnston, C.F.; Rogan, M.T.; Hanna, R.E. Serotoninergic and peptidergic nerve elements in the protoscolex of Echinococcus granulosus (Cestoda, Cyclophyllidea). Parasitol. Res. 1994, 80, 649–656. [Google Scholar] [CrossRef]
- Gustafsson, M.K. Immunocytochemical demonstration of neuropeptides and serotonin in the nervous systems of adult Schistosoma mansoni. Parasitol. Res. 1987, 74, 168–174. [Google Scholar] [CrossRef]
- Koziol, U.; Krohne, G.; Brehm, K. Anatomy and development of the larval nervous system in Echinococcus multilocularis. Front. Zool. 2013, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Maule, A.G.; Halton, D.W.; Shaw, C.; Johnston, C.F. The cholinergic, serotoninergic and peptidergic components of the nervous system of Moniezia expansa (Cestoda, Cyclophyllidea). Parasitology 1993, 106 Pt 4, 429–440. [Google Scholar] [CrossRef]
- McKay, D.M.; Halton, D.W.; Johnston, C.F.; Fairweather, I.; Shaw, C. Cytochemical demonstration of cholinergic, serotoninergic and peptidergic nerve elements in Gorgoderina vitelliloba (Trematoda: Digenea). Int. J. Parasitol. 1991, 21, 71–80. [Google Scholar] [CrossRef]
- Hrckova, G.; Halton, D.W.; Maule, A.G.; Shaw, C.; Johnston, C.F. 5-Hydroxytryptamine (serotonin)-immunoreactivity in the nervous system of Mesocestoides corti tetrathyridia (Cestoda: Cyclophyllidea). J. Parasitol. 1994, 80, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Kroeze, W.K.; Roth, B.L. Molecular Biology and Genomic Organization of G Protein-Coupled Serotonin Receptors. In The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics; Roth, B.L., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 1–38. [Google Scholar] [CrossRef]
- Mansour, T.E. Serotonin receptors in parasitic worms. Adv. Parasitol. 1984, 23, 1–36. [Google Scholar] [CrossRef]
- Cretì, P.; Capasso, A.; Grasso, M.; Parisi, E. Identification of a 5-HT1A receptor positively coupled to planarian adenylate cyclase. Cell Biol. Int. Rep. 1992, 16, 427–432. [Google Scholar] [CrossRef]
- Saitoh, O.; Yuruzume, E.; Nakata, H. Identification of planarian serotonin receptor by ligand binding and PCR studies. Neuroreport 1996, 8, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, O.; Yuruzume, E.; Watanabe, K.; Nakata, H. Molecular identification of a G protein-coupled receptor family which is expressed in planarians. Gene 1997, 195, 55–61. [Google Scholar] [CrossRef]
- Rawls, S.M.; Shah, H.; Ayoub, G.; Raffa, R.B. 5-HT(1A)-like receptor activation inhibits abstinence-induced methamphetamine withdrawal in planarians. Neurosci. Lett. 2010, 484, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishimura, K.; Unemura, K.; Tsushima, J.; Yamauchi, Y.; Otomo, J.; Taniguchi, T.; Kaneko, S.; Agata, K.; Kitamura, Y. Identification of a novel planarian G-protein-coupled receptor that responds to serotonin in Xenopus laevis oocytes. Biol. Amp Pharm. Bull. 2009, 32, 1672–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, T.E.; Mansour, J.M. Effect of some phosphodiesterase inhibitors on adenylate cyclase from the liver fluke, Fasciola hepatica. Biochem. Pharmacol. 1979, 28, 1943–1946. [Google Scholar] [CrossRef]
- Northup, J.K.; Mansour, T.E. Adenylate cyclase from Fasciola hepatica. 1. Ligand specificity of adenylate cyclase-coupled serotonin receptors. Mol. Pharm. 1978, 14, 804–819. [Google Scholar]
- McNall, S.J.; Mansour, T.E. Novel serotonin receptors in Fasciola. Characterization by studies on adenylate cyclase activation and [3H]LSD binding. Biochem. Pharmacol. 1984, 33, 2789–2797. [Google Scholar] [CrossRef]
- Campos, T.D.; Young, N.D.; Korhonen, P.K.; Hall, R.S.; Mangiola, S.; Lonie, A.; Gasser, R.B. Identification of G protein-coupled receptors in Schistosoma haematobium and Schistosoma mansoni by comparative genomics. Parasites Vectors 2014, 7, 242. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.D.; McCorvy, J.D.; Acharya, S.; Johns, M.E.; Day, T.A.; Roth, B.L.; Marchant, J.S. A Miniaturized Screen of a Schistosoma mansoni Serotonergic G Protein-Coupled Receptor Identifies Novel Classes of Parasite-Selective Inhibitors. PLoS Pathog. 2016, 12, e1005651. [Google Scholar] [CrossRef]
- Zamanian, M.; Kimber, M.J.; McVeigh, P.; Carlson, S.A.; Maule, A.G.; Day, T.A. The repertoire of G protein-coupled receptors in the human parasite Schistosoma mansoni and the model organism Schmidtea mediterranea. BMC Genom. 2011, 12, 596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamanian, M.; Agbedanu, P.N.; Wheeler, N.J.; McVeigh, P.; Kimber, M.J.; Day, T.A. Novel RNAi-mediated approach to G protein-coupled receptor deorphanization: Proof of principle and characterization of a planarian 5-HT receptor. PLoS ONE 2012, 7, e40787. [Google Scholar] [CrossRef]
- McVeigh, P.; McCammick, E.; McCusker, P.; Wells, D.; Hodgkinson, J.; Paterson, S.; Mousley, A.; Marks, N.J.; Maule, A.G. Profiling G protein-coupled receptors of Fasciola hepatica identifies orphan rhodopsins unique to phylum Platyhelminthes. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 87–103. [Google Scholar] [CrossRef]
- Marchant, J.S.; Harding, W.W.; Chan, J.D. Structure-activity profiling of alkaloid natural product pharmacophores against a Schistosoma serotonin receptor. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Tolstenkov, O.O.; Terenina, N.B.; Serbina, E.A.; Gustafsson, M.K.S. The spatial relationship between the musculature and the 5-HT and FMRFamide immunoreactivities in cercaria, metacercaria and adult Opisthorchis felineus (Digenea). Acta Parasitol. 2010, 55, 123–132. [Google Scholar] [CrossRef]
- Terenina, N.B.; Kreshchenko, N.D.; Mochalova, N.B.; Movsesyan, S.O. Serotonin and Neuropeptide FMRFamide in the Attachment Organs of Trematodes. Helminthologia 2018, 55, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Hoole, D.; Mitchell, J.B. Ultrastructural observations on the sensory papillae of juvenile and adult Gorgoderina vitelliloba (Trematoda: Gorgoderidae). Int. J. Parasitol. 1981, 11, 411–417. [Google Scholar] [CrossRef]
- Bakke, T.A.; Lien, L. The tegumental surface of Phyllodistomum conostomum (Olsson, 1876) (Digenea), revealed by scanning electron microscopy. Int. J. Parasitol. 1978, 8, 155–161. [Google Scholar] [CrossRef]
- Ibraheem, M.H. Surface ultrastructure of the plagiorchid trematode Glossidium pedatum Looss, 1899 from bagrid fish in Egypt. Acta Zool. 2007, 88, 173–178. [Google Scholar] [CrossRef]
- Bennett, C.E. Scanning electron microscopy of Fasciola hepatica L. during growth and maturation in the mouse. J. Parasitol. 1975, 61, 892–898. [Google Scholar] [CrossRef]
- Bakke, T.A. Functional morphology and surface topography of Leucochloridium sp. (Digenea), revealed by scanning electron microscopy. Z. Für Parasitenkd. 1976, 51, 115–128. [Google Scholar] [CrossRef]
- Jongsomchai, K.; Chaijaroonkhanarak, W.; Tesana, S.; Arunyanart, C.; Kanla, P.; Umka, J. Ultrastructure of tegumentary papillae of the excysted Opisthorchis viverrini metacercaria. Srinagarind Med. J. 2007, 22, 434–442. [Google Scholar]
- Bogéa, T.; Caira, J. Ultrastructure and chaetotaxy of sensory eeceptors in the cercaria of a species of Allopodocotyle Pritchard, 1966 (Digenea: Opecoelidae). Mem. Do Inst. Oswaldo Cruz 2001, 96, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Krasnodembsky, E.G. Histochemical study of glandular apparatus in marita of some trematode species. Arch. Anat. Histol. Embiology 1976, 8, 81–87. (In Russian) [Google Scholar]
- Harada, M.; Suguri, S. A histochemical study of the secretory gland cells of Cercaria shikokuensis and their role during development from cercaria to metacercaria. Parasitol. Int. 2001, 50, 149–156. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Dobrovolskij, A.A. Organization of Parthenogenetic and Hermaphroditic Generations of Trematodes. In The Biology and Evolution of Trematodes: An Essay on the Biology, Morphology, Life Cycles, Transmissions, and Evolution of Digenetic Trematodes; Galaktionov, K.V., Dobrovolskij, A.A., Fried, B., Graczyk, T.K., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 1–213. [Google Scholar] [CrossRef]
- Roser, C.; Jordan, N.; Balfanz, S.; Baumann, A.; Walz, B.; Baumann, O.; Blenau, W. Molecular and pharmacological characterization of serotonin 5-HT2alpha and 5-HT7 receptors in the salivary glands of the blowfly Calliphora vicina. PLoS ONE 2012, 7, e49459. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Sadamoto, H.; Aonuma, H. Identification and expression analysis of the genes involved in serotonin biosynthesis and transduction in the field cricket Gryllus bimaculatus. Insect Mol. Biol. 2011, 20, 619–635. [Google Scholar] [CrossRef] [Green Version]
- Pietrantonio, P.V.; Jagge, C.; McDowell, C. Cloning and expression analysis of a 5HT7-like serotonin receptor cDNA from mosquito Aedes aegypti female excretory and respiratory systems. Insect Mol. Biol. 2001, 10, 357–369. [Google Scholar] [CrossRef]
- Vanhoenacker, P.; Haegeman, G.; Leysen, J.E. 5-HT7 receptors: Current knowledge and future prospects. Trends Pharm. Sci. 2000, 21, 70–77. [Google Scholar] [CrossRef]
- Webb, R.A.; Mizukawa, K. Serotoninlike immunoreactivity in the cestode Hymenolepis diminuta. J. Comp. Neurol. 1985, 234, 431–440. [Google Scholar] [CrossRef]
- Fairweather, I.; Macartney, G.A.; Johnston, C.F.; Halton, D.W.; Buchnan, K.D. Immunocytochemical demonstration of 5-hydroxytryptamine (serotonin) and vertebrate neuropeptides in the nervous system of excysted cysticercoid larvae of the rat tapeworm, Hymenolepis diminuta (Cestoda, Cyclophyllidea). Parasitol. Res. 1988, 74, 371–379. [Google Scholar] [CrossRef]
- Rahman, M.S.; Mettrick, D.F.; Podesta, R.B. Effects of 5-hydroxytryptamine on carbohydrate metabolism in Hymenolepis diminuta (Cestoda). Can. J. Physiol. Pharmacol. 1983, 61, 137–143. [Google Scholar] [CrossRef]
- Mettrick, D.F.; Cho, C.H. Migration of Hymenolepis diminuta (Cestoda) and changes in 5-HT (serotonin) levels in the rat host following parenteral and oral 5-HT administration. Can. J. Physiol. Pharm. 1981, 59, 281–286. [Google Scholar] [CrossRef]
- Mettrick, D.F.; Podesta, R.B. Effect of gastrointestinal hormones and amines on intestinal motility and the migration of Hymenolepis diminuta in the rat small intestine. Int. J. Parasitol. 1982, 12, 151–154. [Google Scholar] [CrossRef]
- Cho, C.H.; Mettrick, D.F. Effects of 5-hydroxytryptamine and histamine on establishment, production, and reproduction by Hymenolepis diminuta in the final and intermediate hosts. Can. J. Zool. 1982, 60, 725–728. [Google Scholar] [CrossRef]
- Valverde-Islas, L.E.; Arrangoiz, E.; Vega, E.; Robert, L.; Villanueva, R.; Reynoso-Ducoing, O.; Willms, K.; Zepeda-Rodriguez, A.; Fortoul, T.I.; Ambrosio, J.R. Visualization and 3D reconstruction of flame cells of Taenia solium (cestoda). PLoS ONE 2011, 6, e14754. [Google Scholar] [CrossRef] [Green Version]
- Rohde, K.; Watson, N.A.; Roubal, F.R. Ultrastructure of the protonephridial system, of Anoplodiscus cirrusspiralis (Monogenea Monopisthocotylea). Int. J. Parasitol. 1992, 22, 443–457. [Google Scholar] [CrossRef]
- Smyth, J.D.; McManus, D.P. The Adult Cestode: Special Structural Features Relevant to Is Physiology; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
- Wahlberg, M.H. The distribution of F-actin during the development of Diphyllobothrium dendriticum (Cestoda). Cell Tissue Res. 1998, 291, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Rozario, T.; Newmark, P.A. A confocal microscopy-based atlas of tissue architecture in the tapeworm Hymenolepis diminuta. Exp. Parasitol. 2015, 158, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Arafa, S.Z.; El-Naggar, M.M.; El-Abbassy, S.A.; Stewart, M.T.; Halton, D.W. Neuromusculature of Gyrodactylus rysavyi, a monogenean gill and skin parasite of the catfish Clarias gariepinus. Parasitol. Int. 2007, 56, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Bahia, D.; Avelar, L.G.; Vigorosi, F.; Cioli, D.; Oliveira, G.C.; Mortara, R.A. The distribution of motor proteins in the muscles and flame cells of the Schistosoma mansoni miracidium and primary sporocyst. Parasitology 2006, 133, 321–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreshchenko, N.; Terenina, N.; Ermakov, A. Serotonin Signalling in Flatworms: An Immunocytochemical Localisation of 5-HT7 Type of Serotonin Receptors in Opisthorchis felineus and Hymenolepis diminuta. Biomolecules 2021, 11, 1212. https://doi.org/10.3390/biom11081212
Kreshchenko N, Terenina N, Ermakov A. Serotonin Signalling in Flatworms: An Immunocytochemical Localisation of 5-HT7 Type of Serotonin Receptors in Opisthorchis felineus and Hymenolepis diminuta. Biomolecules. 2021; 11(8):1212. https://doi.org/10.3390/biom11081212
Chicago/Turabian StyleKreshchenko, Natalia, Nadezhda Terenina, and Artem Ermakov. 2021. "Serotonin Signalling in Flatworms: An Immunocytochemical Localisation of 5-HT7 Type of Serotonin Receptors in Opisthorchis felineus and Hymenolepis diminuta" Biomolecules 11, no. 8: 1212. https://doi.org/10.3390/biom11081212