Advances in the Separation of Graphite from Lithium Iron Phosphate from End-of-Life Batteries Shredded Fine Fraction Using Simple Froth Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Analyses
3. Results
3.1. Black Mass Characterization
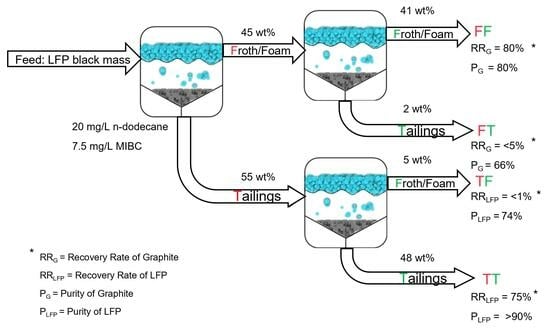
3.2. Froth Flotation
3.2.1. Influence of Chemical Agents
3.2.2. Pre-Treatment
3.2.3. Multi-Step Flotation
3.2.4. Validation of the Optimized Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Battery Alliance. A Vision for a Sustainable Battery Value Chain in 2030 Unlocking the Full Potential to Power Sustainable Development and Climate Change Mitigation; World Economic Forum: Geneva, Switzerland, 2019. [Google Scholar]
- Han, X.; Ouyang, M.; Lu, L.; Li, J. A comparative study of commercial lithium ion battery cycle life in electric vehicle: Capacity loss estimation. J. Power Sources 2014, 268, 658–669. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a recycling process for Li-ion batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Examining different recycling processes for lithium-ion batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Takahashi, M.; Tobishima, S.-i.; Takei, K.; Sakurai, Y. Reaction behavior of LiFePO4 as a cathode material for rechargeable lithium batteries. Solid State Ion. 2002, 148, 283–289. [Google Scholar] [CrossRef]
- Forte, F.; Pietrantonio, M.; Pucciarmati, S.; Puzone, M.; Fontana, D. Lithium iron phosphate batteries recycling: An assessment of current status. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2232–2259. [Google Scholar] [CrossRef]
- Xu, P.; Yang, Z.; Yu, X.; Holoubek, J.; Gao, H.; Li, M.; Cai, G.; Bloom, I.; Liu, H.; Chen, Y.; et al. Design and Optimization of the Direct Recycling of Spent Li-Ion Battery Cathode Materials. ACS Sustain. Chem. Eng. 2021, 9, 4543–4553. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Xi, X.; Nie, Z.; Zhang, Y.; Wen, X.; Lyu, Z. Regeneration and characterization of LiNi0.8Co0.15Al0.05O2 cathode material from spent power lithium-ion batteries. Waste Manag. 2019, 95, 192–200. [Google Scholar] [CrossRef]
- Xu, P.; Dai, Q.; Gao, H.; Liu, H.; Zhang, M.; Li, M.; Chen, Y.; An, K.; Meng, Y.S.; Liu, P. Efficient direct recycling of lithium-ion battery cathodes by targeted healing. Joule 2020, 4, 2609–2626. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Xu, P.; Chen, Z. Enabling sustainable critical materials for battery storage through efficient recycling and improved design: A perspective. MRS Energy Sustain. 2020, 7, E27. [Google Scholar] [CrossRef]
- Gao, H.; Tran, D.; Chen, Z. Seeking direct cathode regeneration for more efficient lithium-ion battery recycling. Curr. Opin. Electrochem. 2022, 31, 100875. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Song, J.; Song, D.; Zhang, L.; Shi, X. Environmentally friendly recycling and effective repairing of cathode powders from spent LiFePO4 batteries. Green Chem. 2016, 18, 2500–2506. [Google Scholar] [CrossRef]
- Park, K.; Yu, J.; Coyle, J.; Dai, Q.; Frisco, S.; Zhou, M.; Burrell, A. Direct Cathode Recycling of End-Of-Life Li-Ion Batteries Enabled by Redox Mediation. ACS Sustain. Chem. Eng. 2021, 9, 8214–8221. [Google Scholar] [CrossRef]
- Sun, Q.; Li, X.; Zhang, H.; Song, D.; Shi, X.; Song, J.; Li, C.; Zhang, L. Resynthesizing LiFePO4/C materials from the recycled cathode via a green full-solid route. J. Alloys Compd. 2020, 818, 153292. [Google Scholar] [CrossRef]
- Tang, X.; Wang, R.; Ren, Y.; Duan, J.; Li, J.; Li, P. Effective regeneration of scrapped LiFePO4 material from spent lithium-ion batteries. J. Mater. Sci. 2020, 55, 13036–13048. [Google Scholar] [CrossRef]
- Yang, T.; Lu, Y.; Li, L.; Ge, D.; Yang, H.; Leng, W.; Zhou, H.; Han, X.; Schmidt, N.; Ellis, M.; et al. An Effective Relithiation Process for Recycling Lithium-Ion Battery Cathode Materials. Adv. Sustain. Syst. 2020, 4, 1900088. [Google Scholar] [CrossRef]
- Ong, S.P.; Wang, L.; Kang, B.; Ceder, G. Li−Fe−P−O2 Phase Diagram from First Principles Calculations. Chem. Mater. 2008, 20, 1798–1807. [Google Scholar] [CrossRef]
- Li, J.; Ma, Z.-F. Past and present of LiFePO4: From fundamental research to industrial applications. Chem 2019, 5, 3–6. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, L.; Liu, B.; Zhou, H. A facile recycling and regeneration process for spent LiFePO4 batteries. J. Mater. Sci. Mater. Electron. 2019, 30, 14580–14588. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Song, D.; Song, J.; Zhang, L. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Sources 2017, 345, 78–84. [Google Scholar] [CrossRef]
- Liang, Q.; Yue, H.; Wang, S.; Yang, S.; Lam, K.-h.; Hou, X. Recycling and crystal regeneration of commercial used LiFePO4 cathode materials. Electrochim. Acta 2020, 330, 135323. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, J.; Liu, Y.; Zhang, W.; Chen, Y.; Wang, C. Direct Regeneration of Spent LiFePO4 Cathode Material by a Green and Efficient One-Step Hydrothermal Method. ACS Sustain. Chem. Eng. 2020, 8, 17622–17628. [Google Scholar] [CrossRef]
- Ma, Z.; Zhuang, Y.; Deng, Y.; Song, X.; Zuo, X.; Xiao, X.; Nan, J. From spent graphite to amorphous sp2+sp3 carbon-coated sp2 graphite for high-performance lithium ion batteries. J. Power Sources 2018, 376, 91–99. [Google Scholar] [CrossRef]
- Niu, B.; Xiao, J.; Xu, Z. Advances and challenges in anode graphite recycling from spent lithium-ion batteries. J. Hazard. Mater. 2022, 439, 129678. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, S.; Lei, S.; Sun, W.; Hou, H.; Jiang, F.; Ji, X.; Zhao, W.; Hu, Y. A process for combination of recycling lithium and regenerating graphite from spent lithium-ion battery. Waste Manag. 2019, 85, 529–537. [Google Scholar] [CrossRef]
- Kaya, M. State-of-the-art lithium-ion battery recycling technologies. Circ. Econ. 2022, 1, 100015. [Google Scholar] [CrossRef]
- Hossain, R.; Sarkar, M.; Sahajwalla, V. Technological options and design evolution for recycling spent lithium-ion batteries: Impact, challenges, and opportunities. WIREs Energy Environ. 2023, 12, e481. [Google Scholar] [CrossRef]
- Pawlik, M. Fundamentals of froth flotation. ChemTexts 2022, 8, 19. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Sygusch, J.; Rudolph, M.; Serna-Guerrero, R. A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127111. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, Y.; Xu, Z.; Zhang, X.; Chen, Y.; Yang, E. A promising method for recovery of graphite and cathode materials from spent lithium-ion batteries. Ionics 2022, 28, 2603–2611. [Google Scholar] [CrossRef]
- Zhan, R.; Oldenburg, Z.; Pan, L. Recovery of active cathode materials from lithium-ion batteries using froth flotation. Sustain. Mater. Technol. 2018, 17, e00062. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, W.; Wang, D.; Sun, H.; Chen, K.; Yang, B. The role of surface roughness in the wettability and floatability of quartz particles. Appl. Surf. Sci. 2020, 527, 146799. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q. Hydrodynamics of froth flotation and its effects on fine and ultrafine mineral particle flotation: A literature review. Miner. Eng. 2021, 173, 107220. [Google Scholar] [CrossRef]
- Brabcová, Z.; Karapantsios, T.; Kostoglou, M.; Basařová, P.; Matis, K. Bubble–particle collision interaction in flotation systems. Colloids Surf. A Physicochem. Eng. Asp. 2015, 473, 95–103. [Google Scholar] [CrossRef]
- Zhan, R.; Yang, Z.; Bloom, I.; Pan, L. Significance of a Solid Electrolyte Interphase on Separation of Anode and Cathode Materials from Spent Li-Ion Batteries by Froth Flotation. ACS Sustain. Chem. Eng. 2021, 9, 531–540. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef]
- Nagaraj, D.R.; Farinato, R.S. Evolution of flotation chemistry and chemicals: A century of innovations and the lingering challenges. Miner. Eng. 2016, 96–97, 2–14. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Matis, K.A.; Stalidis, G.A. Parameters influencing flotation in removal of metal ions. Int. J. Environ. Stud. 1990, 35, 183–196. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Salces, A.; Ferreira, A.; Rudolph, M.; Serna-Guerrero, R. Improving Separation Efficiency in End-of-Life Lithium-Ion Batteries Flotation Using Attrition Pre-Treatment. Minerals 2022, 12, 72. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, Y.; Shen, L.; Yang, Y.; Sun, W.; Cao, X.; Tang, H. Enhancement on the selective flotation separation of carbon coated LiFePO4 and graphite electrode materials. Sep. Purif. Technol. 2023, 311, 123252. [Google Scholar] [CrossRef]
- Ruismäki, R.; Rinne, T.; Dańczak, A.; Taskinen, P.; Serna-Guerrero, R.; Jokilaakso, A. Integrating flotation and pyrometallurgy for recovering graphite and valuable metals from battery scrap. Metals 2020, 10, 680. [Google Scholar] [CrossRef]
- Rinne, T.; Araya-Gómez, N.; Serna-Guerrero, R. A Study on the Effect of Particle Size on Li-Ion Battery Recycling via Flotation and Perspectives on Selective Flocculation. Batteries 2023, 9, 68. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Feng, Y.; Wang, H.; Zhu, X. Pyrolysis-ultrasonic-assisted flotation technology for recovering graphite and LiCoO2 from spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 10896–10904. [Google Scholar] [CrossRef]
- Zhang, G.; He, Y.; Wang, H.; Feng, Y.; Xie, W.; Zhu, X. Application of mechanical crushing combined with pyrolysis-enhanced flotation technology to recover graphite and LiCoO2 from spent lithium-ion batteries. J. Clean. Prod. 2019, 231, 1418–1427. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, T.; He, Y.; Zhao, Y.; Wang, S.; Zhang, G.; Zhang, Y.; Feng, Y. Recovery of valuable materials from spent lithium-ion batteries by mechanical separation and thermal treatment. J. Clean. Prod. 2018, 185, 646–652. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Wang, F.; Zhang, G.; Zhang, W.; Wang, J. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by Fenton reagent-assisted flotation. J. Clean. Prod. 2017, 143, 319–325. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, C.; Feng, P.; Yang, X.; Yang, X. A novel pulsated pneumatic separation with variable-diameter structure and its application in the recycling spent lithium-ion batteries. Waste Manag. 2021, 131, 20–30. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Hu, T.; Bai, X.; Wang, S.; Xie, W.; Hao, J.; He, Y. Recovery of LiCoO2 and graphite from spent lithium-ion batteries by cryogenic grinding and froth flotation. Miner. Eng. 2020, 148, 106223. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef]
- Pratheeksha, P.M.; Mohan, E.H.; Sarada, B.V.; Ramakrishna, M.; Hembram, K.; Srinivas, P.V.V.; Daniel, P.J.; Rao, T.N.; Anandan, S. Development of a novel carbon-coating strategy for producing core–shell structured carbon coated LiFePO4 for an improved Li-ion battery performance. Phys. Chem. Chem. Phys. 2017, 19, 175–188. [Google Scholar] [CrossRef]
- Raj, H.; Sil, A. Effect of carbon coating on electrochemical performance of LiFePO4 cathode material for Li-ion battery. Ionics 2018, 24, 2543–2553. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Peng, C.-T.; Hung, I.-M. Effects of particle size and carbon coating on electrochemical properties of LiFePO4/C prepared by hydrothermal method. J. Mater. Sci. 2014, 49, 6907–6916. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Y.; Runge, K. Entrainment in froth flotation: The degree of entrainment and its contributing factors. Powder Technol. 2016, 288, 202–211. [Google Scholar] [CrossRef]





| Starting Material | Particle Size (µm) | Black Mass Ratio (g/L) | Impeller Speed (rpm) | Air Flow (L/min) | Frother | Frother (mg/L) | Collector | Collector (mg/L) | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Spent LFP | - | 57.1 | 1992 | - | MIBC | 7.5 | n-Dodecane | 12.5 | [44] |
| Spent LIB | 500–1250 | 40 | 1000 | 2 | MIBC | 8 | Kerosene | 150 | [45] |
| LFP/LCO | <210 | 10 | 1500–1200 | - | MIBC | 0–12 | Kerosene | 30 | [35] |
| Spent LCO | - | 40 | 850–1000 | 2 | MIBC | 8 | - | - | [46] |
| Spent LCO | <75 | 40 | 1800 | 2 | MIBC | 150 | n-Dodecane | 300 | [47] |
| LCO | - | 40 | 1800 | 2 | MIBC | 150 | n-Dodecane | 300 | [48] |
| Spent LCO | <250 | 40 | 1800 | 0.8 | MIBC | 150 | n-Dodecane | 300 | [49] |
| Spent LCO | <250 | 40 | 1800 | - | MIBC | 150 | n-Dodecane | 300 | [50] |
| Spent LFP | <250 | 40 | 1920 | 1.5 | MIBC | 150 | n-Dodecane | 250 | [51] |
| Spent LCO | <75 | 60 | 1600 | 0.7 | MIBC | 250 | n-Dodecane | 180 | [52] |
| Li | Al | Cu | Fe | P | C | Balance | |
|---|---|---|---|---|---|---|---|
| XRF | - | 0.12 ± 0.01 | 0.44 ± 0.01 | 20.50 ± 0.11 | 8.16 ± 0.15 | 44.30 a | 30.03 ± 0.19 |
| ICP | 2.20 ± 0.01 | 0.59 ± 0.11 | 1.10 ± 0.40 | 17.00 ± 0.01 | 11.00 ± 0.01 | 42.00 b | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renier, O.; Pellini, A.; Spooren, J. Advances in the Separation of Graphite from Lithium Iron Phosphate from End-of-Life Batteries Shredded Fine Fraction Using Simple Froth Flotation. Batteries 2023, 9, 589. https://doi.org/10.3390/batteries9120589
Renier O, Pellini A, Spooren J. Advances in the Separation of Graphite from Lithium Iron Phosphate from End-of-Life Batteries Shredded Fine Fraction Using Simple Froth Flotation. Batteries. 2023; 9(12):589. https://doi.org/10.3390/batteries9120589
Chicago/Turabian StyleRenier, Olivier, Andrea Pellini, and Jeroen Spooren. 2023. "Advances in the Separation of Graphite from Lithium Iron Phosphate from End-of-Life Batteries Shredded Fine Fraction Using Simple Froth Flotation" Batteries 9, no. 12: 589. https://doi.org/10.3390/batteries9120589