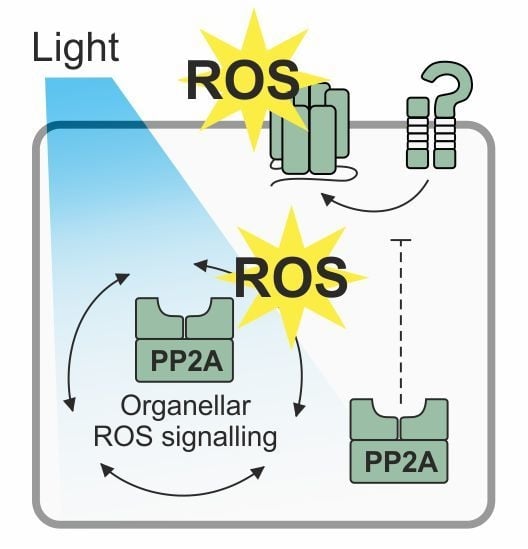
PP2A Phosphatase as a Regulator of ROS Signaling in Plants
Abstract
:1. Introduction
2. PP2A Phosphatase as a Regulatory Enzyme in Plant Stress
3. PP2A-B′γ as a Regulator of ROS Signaling and Cell Death
4. PP2A as a Regulator of Antioxidant Activities
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| AOX | alternative oxidase |
| APX | ascorbate peroxidases |
| CAT | catalase |
| CDPK, CPK | calcium-dependent protein kinase |
| CSD | copper/zinc superoxide dismutase |
| ETI | effector-triggered immunity |
| H2O2 | hydrogen peroxide |
| HSF | heat shock factor |
| HSP | heat shock protein |
| JA | jasmonic acid |
| MDAR | monodehydroascorbate reductase |
| MPK | mitogen activated protein kinase |
| PAP | phosphoadenosine 5′-phosphate |
| PP2A | Protein Phosphatase 2A |
| PRX | peroxiredoxin |
| ROS | Reactive oxygen species |
| SA | salicylic acid |
| SOD | superoxide dismutase |
| TRX | thioredoxin |
References
- Mullineaux, P.; Ball, L.; Escobar, C.; Karpinska, B.; Creissen, G.; Karpinski, S. Are diverse signaling pathways integrated in the regulation of Arabidopsis antioxidant defence gene expression in response to excess excitation energy? Phil. Trans. R. Soc. B 2000, 355, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Sierla, M.; Rahikainen, M.; Salojärvi, J.; Kangasjärvi, J.; Kangasjärvi, S. Apoplastic and chloroplastic redox signaling networks in plant stress responses. Antioxid. Redox Signal. 2013, 18, 2220–2239. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyeva, Y.; Battchikova, N.; Brosché, M.; Fujii, H.; Kangasjärvi, S.; Mulo, P.; Mähönen, A.P.; Nieminen, K.; Overmyer, K.; Salojärvi, J.; et al. Integration of photosynthesis, development and stress as an opportunity for plant biology. New Phytol. 2015, 208, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Shao, F.; Li, Y.; Cui, H.; Chen, L.; Li, H.; Zou, Y.; Long, C.; Lan, L.; Chai, J.; Chen, S.; Tang, X.; Zhou, J.M. A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host. Microb. 2007, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Savatin, D.V.; Bisceglia, N.G.; Marti, L.; Fabbri, C.; Cervone, F.; de Lorenzo, G. The Arabidopsis NUCLEUS-AND PHRAGMOPLAST-LOCALIZED KINASE1-related protein kinases are required for elicitor-induced oxidative burst and immunity. Plant Physiol. 2014, 165, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Hideg, É.; Kálai, T.; Vass, I. Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochemistry 1998, 37, 11405–11411. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox sensing and signaling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Karpinski, S.; Szechynska-Hebda, M. Cellular light memory, photo-electrochemical and redox retrograde signaling in plants. Biotechnologia 2012, 93, 27–39. [Google Scholar] [CrossRef]
- Karpinski, S.; Szechyńska-Hebda, M.; Wituszyńska, W.; Burdiak, P. Light acclimation, retrograde signaling, cell death and immune defences in plants. Plant. Cell Environ. 2013, 36, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Suorsa, M.; Järvi, S.; Grieco, M.; Nurmi, M.; Pietrzykowska, M.; Rantala, M.; Kangasjärvi, S.; Paakkarinen, V.; Tikkanen, M.; Jansson, S.; et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 2012, 24, 2934–2948. [Google Scholar] [CrossRef] [PubMed]
- Tyystjärvi, E. Photoinhibition of Photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar] [PubMed]
- Karpinski, S.; Reynolds, H.; Karpinska, B.; Wingsle, G.; Creissen, G.; Mullineaux, P.M. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science 1999, 284, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Karpinski, S.; Gabrys, H.; Mateo, A.; Karpinska, B.; Mullineaux, P.M. Light perception in plant disease defence signaling. Curr. Opin. Plant Biol. 2003, 6, 390–396. [Google Scholar] [CrossRef]
- Pogson, B.J.; Woo, N.S.; Förster, B.; Small, I.D. Plastid signaling to the nucleus and beyond. Trends Plant Sci. 2008, 13, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Kangasjärvi, S.; Tikkanen, M.; Durian, G.; Aro, E.-M. Photosynthetic light reactions—An adjustable hub in basic production and plant immunity signaling. Plant Physiol. Biochem. 2014, 81, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Rahikainen, M.; Konert, G.; Finazzi, G.; Kangasjärvi, S. Signalling crosstalk in light stress and immune reactions in plants. Phil. Trans. R. Soc. B 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Stael, S.; Kmiecik, P.; Willems, P.; van der Kelen, K.; Coll, N.S.; Teige, M.; van Breusegem, F. Plant innate immunity—Sunny side up? Trends Plant Sci. 2015, 20, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signaling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef] [PubMed]
- Caplan, J.L.; Kumar, A.S.; Park, E.; Padmanabhan, M.S.; Hoban, K.; Modla, S.; Czymmek, K.; Dinesh-Kumar, S.P. Chloroplast stromules function during innate immunity. Dev. Cell 2015, 34, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Zabala, M.; Littlejohn, G.; Jayaraman, S.; Studholme, D.; Bailey, D.; Lawson, T.; Tillich, M.; Licht, D.; Bölter, B.; Delfino, L.; et al. Chloroplasts play a central role in plant defence and are targeted by pathogen effectors. Nat. Plants 2015, 1, 15074. [Google Scholar] [CrossRef] [Green Version]
- Mullineaux, P.M.; Baker, N.R. Oxidative stress: Antagonistic signaling for acclimation or cell death? Plant Physiol. 2010, 154, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Lepistö, A.; Rintamäki, E. Coordination of plastid and light signaling pathways upon development of Arabidopsis leaves under various photoperiods. Mol. Plant 2012, 5, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, S.; Queval, G.; Vanderauwera, S.; Mhamdi, A.; Vandorpe, M.; Langlois-Meurinne, M.; Van Breusegem, F.; Saindrenan, P.; Noctor, G. Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength-related manner. Plant Physiol. 2010, 153, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Queval, G.; Issakidis-Bourguet, E.; Hoeberichts, F.A.; Vandorpe, M.; Gakière, B.; Vanacker, H.; Miginiac-Maslow, M.; van Breusegem, F.; Noctor, G. Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J. 2007, 52, 640–657. [Google Scholar] [PubMed]
- Perez-Perez, J.M.; Esteve-Bruna, D.; Gonzalez-Bayon, R.; Kangasjärvi, S.; Caldana, C.; Hannah, M.A.; Willmitzer, L.; Ponce, M.R.; Micol, J.L. Functional redundancy and divergence within the Arabidopsis RETICULATA-RELATED gene family. Plant Physiol. 2013, 162, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Mühlenbock, P.; Szechynska-Hebda, M.; Plaszczyca, M.; Baudo, M.; Mateo, A.; Mullineaux, P.M.; Parker, J.E.; Karpinska, B.; Karpinski, S. Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 2008, 20, 2339–2356. [Google Scholar] [CrossRef] [PubMed]
- Rasool, B.; Karpinska, B.; Konert, G.; Durian, G.; Denessiouk, K.; Kangasjärvi, S.; Foyer, C.H. Effects of light and the regulatory B-subunit composition of protein phosphatase 2A on the susceptibility of Arabidopsis thaliana to aphid (Myzus persicae) infestation. Front. Plant Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Bostock, R.M. Signal crosstalk and induced resistance: Straddling the line between cost and benefit. Annu. Rev. Phytopathol. 2005, 43, 545–580. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Sign. 2009, 11, 861–905. [Google Scholar] [CrossRef] [PubMed]
- Boudsocq, M.; Willmann, M.R.; McCormack, M.; Lee, H.; Shan, L.; He, P.; Bush, J.; Cheng, S.H.; Sheen, J. Differential innate immune signaling via Ca2+ sensor protein kinases. Nature 2010, 464, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.O.; Moore, M.; König, K.; Pecher, P.; Alsharafa, K.; Lee, J.; Dietz, K.J. Fast retrograde signaling in response to high light involves metabolite export, MITOGEN-ACTIVATED PROTEIN KINASE6, and AP2/ERF transcription factors in Arabidopsis. Plant Cell 2014, 26, 1151–1165. [Google Scholar] [CrossRef] [PubMed]
- Uhrig, R.G.; Labandera, A.-M.; Moorhead, G.B. Arabidopsis PPP family of serine/threonine protein phosphatases: Many targets but few engines. Trends Plant Sci. 2013, 18, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Janssens, V.; Longin, S.; Goris, J. PP2A holoenzyme assembly: In cauda venenum (the sting is in the tail). Trends Biochem. Sci. 2008, 33, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Farkas, I.; Dombradi, V.; Miskei, M.; Szabados, L.; Koncz, C. Arabidopsis PPP family of serine/threonine phosphatases. Trends Plant Sci. 2007, 12, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wang, X.; Li, X.; Kamiya, Y.; Otegui, M.S.; Chory, J. Methylation of a phosphatase specifies dephosphorylation and degradation of activated brassinosteroid receptors. Sci. Signal. 2011. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhu, Y.; Shen, G.; Zhang, H. TAP46 plays a positive role in the ABSCISIC ACID INSENSITIVE5-regulated gene expression in Arabidopsis. Plant Physiol. 2014, 164, 721–734. [Google Scholar] [CrossRef] [PubMed]
- País, S.M.; González, M.A.; Téllez-Iñón, M.T.; Capiati, D.A. Characterization of potato (Solanum tuberosum) and tomato (Solanum lycopersicum) protein phosphatases type 2A catalytic subunits and their involvement in stress responses. Planta 2009, 230, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.M.; Zhou, Y.; Xu, Z.F.; Chye, M.L.; Kong, R.Y. Two genes encoding protein phosphatase 2A catalytic subunits are differentially expressed in rice. Plant Mol. Biol. 2003, 51, 295–311. [Google Scholar] [PubMed]
- Yu, R.M.; Wong, M.M.; Jack, R.W.; Kong, R.Y. Structure, evolution and expression of a second subfamily of protein phosphatase 2A catalytic subunit genes in the rice plant (Oryza sativa L.). Planta 2005, 222, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Jing, R.; Mao, X.; Jia, X.; Chang, X. A wheat (Triticum aestivum) protein phosphatase 2A catalytic subunit gene provides enhanced drought tolerance in tobacco. Ann. Bot. 2007, 99, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, A.; Mao, X.; Jing, R. Cloning and characterization of TaPP2AbB′′-α, a member of the PP2A regulatory subunit in wheat. PLoS ONE 2014, 9, e94430. [Google Scholar] [CrossRef] [PubMed]
- Pernas, M.; Garcia-Casado, G.; Rojo, E.; Solano, R.; Sanchez-Serrano, J.J. A protein phosphatase 2A catalytic subunit is a negative regulator of abscisic acid signaling. Plant. J. 2007, 51, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Blakeslee, J.J.; Zhou, H.-W.; Heath, J.T.; Skottke, K.R.; Barrios, J.A.; Liu, S.Y.; DeLong, A. Specificity of RCN1-mediated protein phosphatase 2A regulation in meristem organization and stress response in roots. Plant Physiol. 2008, 146, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.H.; Shen, G.X.; Yan, J.Q.; He, C.X.; Zhang, H. AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment. Plant J. 2006, 46, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Leivar, P.; Antolin-Llovera, M.; Ferrero, S.; Closa, M.; Arro, M.; Ferrer, A.; Boronat, A.; Camposa, N. Multilevel control of Arabidopsis 3-hydroxy-3-methylglutaryl coenzyme A reductase by protein phosphatase 2A. Plant Cell 2011, 23, 1494–1511. [Google Scholar] [CrossRef] [PubMed]
- Latorre, K.; Harris, D.M.; Rundle, S.J. Differential expression of three Arabidopsis genes encoding the B′ regulatory subunit of protein phosphatase 2A. Eur. J. Biochem. 1997, 245, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Konert, G.; Rahikainen, M.; Trotta, A.; Durian, G.; Salojärvi, J.; Khorobrykh, S.; Tyystjärvi, E.; Kangasjärvi, S. Subunits B′γ and B′ζ of protein phosphatase 2A regulate photo-oxidative stress responses and growth in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 2641–2651. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Anderson, J.C.; del Pozo, O.; Gu, Y.Q.; Tang, X.; Martin, G.B. Silencing of subfamily I of protein phosphatase 2A catalytic subunits results in activation of plant defense responses and localized cell death. Plant J. 2004, 38, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Trotta, A.; Wrzaczek, M.; Scharte, J.; Tikkanen, M.; Konert, G.; Rahikainen, M.; Holmström, M.; Hiltunen, H.M.; Rips, S.; Sipari, N.; et al. Regulatory subunit B′γ of protein phosphatase 2A prevents unnecessary defense reactions under low light in Arabidopsis. Plant Physiol. 2011, 156, 1464–1480. [Google Scholar] [CrossRef] [PubMed]
- Kataya, A.R.A.; Behzad, H.; Cathrine, L. Protein phosphatase 2A regulatory subunits affecting plant innate immunity, energy metabolism, and flowering time—Joint functions among B′η subfamily members. Plant Signal. Behav. 2015, 10, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Segonzac, C.; Macho, A.P.; Sanmartín, M.; Ntoukakis, V.; Sánchez-Serrano, J.J.; Zipfel, C. Negative control of BAK1 by protein phosphatase 2A during plant innate immunity. EMBO J. 2014, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-J.; Zhou, Y.-H.; Shi, K.; Zhou, J.; Foyer, C.H.; Yu, J.-Q. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. J. Exp. Bot. 2015, 66, 2839–2856. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Mhamdi, A.; Trotta, A.; Kangasjärvi, S.; Noctor, G. The protein phosphatase subunit PP2A-B′γ is required to suppress day length-dependent pathogenesis responses triggered by intracellular oxidative stress. New Phytol. 2014, 202, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Sirichandra, C.; Gu, D.; Hu, H.-C.; Davanture, M.; Lee, S.; Djaoui, M.; Valot, B.; Zivy, M.; Leung, J.; Merlot, S.; et al. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett. 2009, 583, 2982–2986. [Google Scholar] [CrossRef] [PubMed]
- Waadt, R.; Manalansan, B.; Rauniyar, N.; Munemasa, S.; Booker, M.A.; Brandt, B.; Waadt, C.; Nusinow, D.A.; Kay, S.A.; Kunz, H.-H.; et al. Identification of open stomata1-interacting proteins reveals interactions with sucrose non-fermenting1-related protein kinases2 and with type 2A protein phosphatases that function in abscisic acid responses. Plant Physiol. 2015, 169, 760–779. [Google Scholar] [CrossRef] [PubMed]
- Konert, G.; Trotta, A.; Kouvonen, P.; Rahikainen, M.; Durian, G.; Blokhina, O.; Fagerstedt, K.; Muth, D.; Corthals, G.L.; Kangasjärvi, S. Protein phosphatase 2A (PP2A) regulatory subunit B’ gamma interacts with cytoplasmic ACONITASE 3 and modulates the abundance of AOX1A and AOX1D in Arabidopsis thaliana. New Phytol. 2015, 205, 1250–1263. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Strodtkoetter, I.; Padmasree, K.; Dinakar, C.; Speth, B.; Niazi, P.S.; Wojtera, J.; Voss, I.; Do, P.T.; Nunes-Nesi, A.; Fernie, A.R.; et al. Induction of the aox1d isoform of alternative oxidase in A. thaliana T-DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with antimycin A. Mol. Plant 2009, 2, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, S.; Vanderauwera, S.; Vuylsteke, M.; Rombauts, S.; Langebartels, C.; Seidlitz, H.K.; Zabeau, M.; van Montagu, M.; Inzé, D.; van Breusegem, F. Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J. 2004, 39, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Queval, G.; Neukermans, J.; Vanderauwera, S.; van Breusegem, F.; Noctor, G. Day length is a key regulator of transcriptomic responses to both CO2 and H2O2 in Arabidopsis. Plant Cell Environ. 2012, 35, 374–387. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, I.; Vermeirssen, V.; van Aken, O.; Vandepoele, K.; Murcha, M.W.; Law, S.R.; Inzé, A.; Ng, S.; Ivanova, A.; Rombaut, D.; et al. The membrane-bound NAC transcription factor ANAC013 functions in mitochondrial retrograde regulation of the oxidative stress response in Arabidopsis. Plant Cell 2013, 25, 3472–3490. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Ho, L.H.; Clifton, R.; Carroll, A.; Estavillo, G.; Tan, Y.F.; Howell, K.A.; Ivanova, A.; Pogson, B.J.; Millar, A.H.; Whelan, J. The absence of alternative oxidase1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol. 2008, 147, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Fryer, M.J.; Ball, L.; Oxborough, K.; Karpinski, S.; Mullineaux, P.M.; Baker, N.R. Control of Ascorbate Peroxidase 2 expression by hydrogen peroxide and leaf water status during excess light stress reveals a functional organisation of Arabidopsis leaves. Plant J. 2003, 33, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Estavillo, G.M.; Crisp, P.; Pornsiriwong, W.; Wirtz, M.; Collinge, D.; Carrie, C.; Giraud, E.; Whelan, J.; David, P.; Javot, H.; et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell 2011, 23, 3992–4012. [Google Scholar] [CrossRef] [PubMed]
- Rossel, J.B.; Wilson, I.W.; Pogson, B.J. Global changes in gene expression in response to high light in Arabidopsis. Plant Physiol. 2012, 130, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-S.; Crisp, P.A.; Estavillo, G.M.; Cole, B.; Hong, F.; Mockler, T.C.; Pogson, B.J.; Chory, J. Subset of heat-shock transcription factors required for the early response of Arabidopsis to excess light. Proc. Natl. Acad. Sci. USA 2013, 110, 14474–14479. [Google Scholar] [CrossRef] [PubMed]
- Volkov, R.; Panchuk, I.I.; Mullineaux, P.M.; Schöffl, F. Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. Plant Mol. Biol. 2006, 61, 733–746. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahikainen, M.; Pascual, J.; Alegre, S.; Durian, G.; Kangasjärvi, S. PP2A Phosphatase as a Regulator of ROS Signaling in Plants. Antioxidants 2016, 5, 8. https://doi.org/10.3390/antiox5010008
Rahikainen M, Pascual J, Alegre S, Durian G, Kangasjärvi S. PP2A Phosphatase as a Regulator of ROS Signaling in Plants. Antioxidants. 2016; 5(1):8. https://doi.org/10.3390/antiox5010008
Chicago/Turabian StyleRahikainen, Moona, Jesús Pascual, Sara Alegre, Guido Durian, and Saijaliisa Kangasjärvi. 2016. "PP2A Phosphatase as a Regulator of ROS Signaling in Plants" Antioxidants 5, no. 1: 8. https://doi.org/10.3390/antiox5010008