Paraoxonase/Arylesterase Activity of Serum Paraoxonase-1 and Schizophrenia: A Systematic Review and Meta-Analysis
Abstract
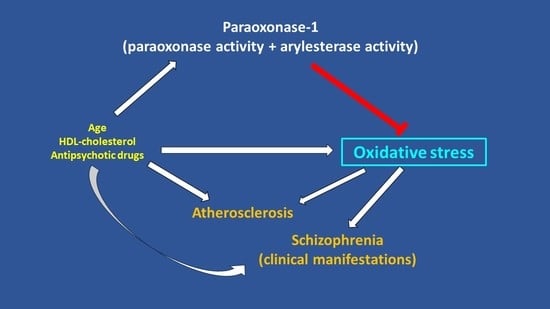
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Statistical Analysis
3. Results
3.1. Systematic Search
3.2. Paraoxonase Activity
3.3. Arylesterase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Upthegrove, R.; Khandaker, G.M. Cytokines, Oxidative Stress and Cellular Markers of Inflammation in Schizophrenia. Curr. Top. Behav. Neurosci. 2020, 44, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Rogers, J.C.; Katshu, M.; Liddle, P.F.; Upthegrove, R. Oxidative Stress and the Pathophysiology and Symptom Profile of Schizophrenia Spectrum Disorders. Front. Psychiatry 2021, 12, 703452. [Google Scholar] [CrossRef] [PubMed]
- Rambaud, V.; Marzo, A.; Chaumette, B. Oxidative Stress and Emergence of Psychosis. Antioxidants 2022, 11, 1870. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.K.; Gaur, S.; Roy, R.G.; Samkaria, A.; Ingole, R.; Goel, A. Schizophrenia, Bipolar and Major Depressive Disorders: Overview of Clinical Features, Neurotransmitter Alterations, Pharmacological Interventions, and Impact of Oxidative Stress in the Disease Process. ACS Chem. Neurosci. 2022, 13, 2784–2802. [Google Scholar] [CrossRef]
- Fisar, Z. Biological hypotheses, risk factors, and biomarkers of schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2023, 120, 110626. [Google Scholar] [CrossRef]
- Vallee, A. Neuroinflammation in Schizophrenia: The Key Role of the WNT/beta-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 2810. [Google Scholar] [CrossRef]
- Abruzzo, P.M.; Panisi, C.; Marini, M. The Alteration of Chloride Homeostasis/GABAergic Signaling in Brain Disorders: Could Oxidative Stress Play a Role? Antioxidants 2021, 10, 1316. [Google Scholar] [CrossRef]
- Morris, G.; Walker, A.J.; Walder, K.; Berk, M.; Marx, W.; Carvalho, A.F.; Maes, M.; Puri, B.K. Increasing Nrf2 Activity as a Treatment Approach in Neuropsychiatry. Mol. Neurobiol. 2021, 58, 2158–2182. [Google Scholar] [CrossRef]
- Juchnowicz, D.; Dzikowski, M.; Rog, J.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M.; Karakula-Juchnowicz, H. Oxidative Stress Biomarkers as a Predictor of Stage Illness and Clinical Course of Schizophrenia. Front. Psychiatry 2021, 12, 728986. [Google Scholar] [CrossRef]
- Lafeuille, M.H.; Dean, J.; Fastenau, J.; Panish, J.; Olson, W.; Markowitz, M.; Duh, M.S.; Lefebvre, P. Burden of schizophrenia on selected comorbidity costs. Expert Rev. Pharm. Outcomes Res. 2014, 14, 259–267. [Google Scholar] [CrossRef]
- Mizuki, Y.; Sakamoto, S.; Okahisa, Y.; Yada, Y.; Hashimoto, N.; Takaki, M.; Yamada, N. Mechanisms Underlying the Comorbidity of Schizophrenia and Type 2 Diabetes Mellitus. Int. J. Neuropsychopharmacol. 2021, 24, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Jin, D.; Palmer, N.; Fox, K.; Kohane, I.S.; Smoller, J.W.; Yu, K.H. Large-scale real-world data analysis identifies comorbidity patterns in schizophrenia. Transl. Psychiatry 2022, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tanaka, A.; Kishi, T.; Li, Y.; Matsunaga, M.; Tanihara, S.; Iwata, N.; Ota, A. Recent findings on subjective well-being and physical, psychiatric, and social comorbidities in individuals with schizophrenia: A literature review. Neuropsychopharmacol. Rep. 2022, 42, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa-Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017, 16, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Hennekens, C.H.; Hennekens, A.R.; Hollar, D.; Casey, D.E. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J. 2005, 150, 1115–1121. [Google Scholar] [CrossRef]
- Rossom, R.C.; Hooker, S.A.; O’Connor, P.J.; Crain, A.L.; Sperl-Hillen, J.M. Cardiovascular Risk for Patients with and without Schizophrenia, Schizoaffective Disorder, or Bipolar Disorder. J. Am. Heart Assoc. 2022, 11, e021444. [Google Scholar] [CrossRef]
- Nielsen, R.E.; Banner, J.; Jensen, S.E. Cardiovascular disease in patients with severe mental illness. Nat. Rev. Cardiol. 2021, 18, 136–145. [Google Scholar] [CrossRef]
- Marche, J.C.; Bannay, A.; Baillot, S.; Dauriac-Le Masson, V.; Leveque, P.; Schmitt, C.; Laprevote, V.; Schwan, R.; Dobre, D. Prevalence of severe cardiovascular disease in patients with schizophrenia. Encephale 2022, 48, 125–131. [Google Scholar] [CrossRef]
- Fraguas, D.; Diaz-Caneja, C.M.; Ayora, M.; Hernandez-Alvarez, F.; Rodriguez-Quiroga, A.; Recio, S.; Leza, J.C.; Arango, C. Oxidative Stress and Inflammation in First-Episode Psychosis: A Systematic Review and Meta-analysis. Schizophr. Bull. 2019, 45, 742–751. [Google Scholar] [CrossRef]
- Panda, P.; Verma, H.K.; Lakkakula, S.; Merchant, N.; Kadir, F.; Rahman, S.; Jeffree, M.S.; Lakkakula, B.; Rao, P.V. Biomarkers of Oxidative Stress Tethered to Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 9154295. [Google Scholar] [CrossRef]
- Caruso, G.; Grasso, M.; Fidilio, A.; Tascedda, F.; Drago, F.; Caraci, F. Antioxidant Properties of Second-Generation Antipsychotics: Focus on Microglia. Pharmaceuticals 2020, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Dietrich-Muszalska, A.; Kolodziejczyk-Czepas, J.; Nowak, P. Comparative Study of the Effects of Atypical Antipsychotic Drugs on Plasma and Urine Biomarkers of Oxidative Stress in Schizophrenic Patients. Neuropsychiatr. Dis. Treat. 2021, 17, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Mangoni, A.A. A Systematic Review and Meta-Analysis of the Effect of Statins on Glutathione Peroxidase, Superoxide Dismutase, and Catalase. Antioxidants 2021, 10, 1841. [Google Scholar] [CrossRef]
- Zinellu, A.; Paliogiannis, P.; Usai, M.F.; Carru, C.; Mangoni, A.A. Effect of statin treatment on circulating malondialdehyde concentrations: A systematic review and meta-analysis. Ther. Adv. Chronic Dis. 2019, 10, 2040622319862714. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Mangoni, A.A.; Sanna, M.; Satta, A.E.; Carru, C. Impact of cholesterol lowering treatment on plasma kynurenine and tryptophan concentrations in chronic kidney disease: Relationship with oxidative stress improvement. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Mackness, B.; Durrington, P.N.; Mackness, M.I. Human serum paraoxonase. Gen. Pharmacol. Vasc. Syst. 1998, 31, 329–336. [Google Scholar] [CrossRef]
- Deng, S.; Xu, Y.; Zheng, L. HDL Structure. Adv. Exp. Med. Biol. 2022, 1377, 1–11. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free. Radic. Biol. Med. 2004, 37, 1304–1316. [Google Scholar] [CrossRef]
- Reddy, S.T.; Wadleigh, D.J.; Grijalva, V.; Ng, C.; Hama, S.; Gangopadhyay, A.; Shih, D.M.; Lusis, A.J.; Navab, M.; Fogelman, A.M. Human paraoxonase-3 is an HDL-associated enzyme with biological activity similar to paraoxonase-1 protein but is not regulated by oxidized lipids. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 542–547. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.; Yi, X.; Xiao, S.; Wang, C.; Chen, G.; Li, Y. Substrates for Paraoxonase. Curr. Pharm. Des. 2018, 24, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, W.N. Serum esterases. II. An enzyme hydrolysing diethyl p-nitrophenyl phosphate (E600) and its identity with the A-esterase of mammalian sera. Biochem. J. 1953, 53, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Jakubowski, H. Calcium-dependent human serum homocysteine thiolactone hydrolase. A protective mechanism against protein N-homocysteinylation. J. Biol. Chem. 2000, 275, 3957–3962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthukrishnan, S.; Shete, V.S.; Sanan, T.T.; Vyas, S.; Oottikkal, S.; Porter, L.M.; Magliery, T.J.; Hadad, C.M. Mechanistic Insights into the Hydrolysis of Organophosphorus Compounds by Paraoxonase-1: Exploring the Limits of Substrate Tolerance in a Promiscuous Enzyme. J. Phys. Org. Chem. 2012, 25, 1247–1260. [Google Scholar] [CrossRef] [Green Version]
- Zinellu, A.; Fois, A.G.; Sotgia, S.; Zinellu, E.; Bifulco, F.; Pintus, G.; Mangoni, A.A.; Carru, C.; Pirina, P. Plasma protein thiols: An early marker of oxidative stress in asthma and chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2016, 46, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Fois, A.G.; Sotgia, S.; Sotgiu, E.; Zinellu, E.; Bifulco, F.; Mangoni, A.A.; Pirina, P.; Carru, C. Arginines Plasma Concentration and Oxidative Stress in Mild to Moderate COPD. PLoS ONE 2016, 11, e0160237. [Google Scholar] [CrossRef] [Green Version]
- Erre, G.L.; Bassu, S.; Giordo, R.; Mangoni, A.A.; Carru, C.; Pintus, G.; Zinellu, A. Association between Paraoxonase/Arylesterase Activity of Serum PON-1 Enzyme and Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 2317. [Google Scholar] [CrossRef]
- Pau, M.C.; Zinellu, A.; Zinellu, E.; Pintus, G.; Carru, C.; Fois, A.G.; Mangoni, A.A.; Pirina, P. Paraoxonase-1 Concentrations in Obstructive Sleep Apnoea: A Systematic Review and Meta-Analysis. Antioxidants 2022, 11, 766. [Google Scholar] [CrossRef]
- Bassu, S.; Mangoni, A.A.; Argiolas, D.; Carru, C.; Pirina, P.; Fois, A.G.; Zinellu, A. A systematic review and meta-analysis of paraoxonase-1 activity in asthma. Clin. Exp. Med. 2022. [Google Scholar] [CrossRef]
- Bassu, S.; Mangoni, A.A.; Satta, R.; Argiolas, D.; Carru, C.; Zinellu, A. Paraoxonase and arylesterase activity of serum PON-1 enzyme in psoriatic patients: A systematic review and meta-analysis. Clin. Exp. Med. 2023, 23, 301–311. [Google Scholar] [CrossRef]
- Bassu, S.; Zinellu, A.; Sotgia, S.; Mangoni, A.A.; Floris, A.; Farina, G.; Passiu, G.; Carru, C.; Erre, G.L. Oxidative Stress Biomarkers and Peripheral Endothelial Dysfunction in Rheumatoid Arthritis: A Monocentric Cross-Sectional Case-Control Study. Molecules 2020, 25, 3855. [Google Scholar] [CrossRef] [PubMed]
- Durrington, P.N.; Bashir, B.; Soran, H. Paraoxonase 1 and atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1065967. [Google Scholar] [CrossRef]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Ansell, B.J.; Fogelman, A.M. Mechanisms of disease: Proatherogenic HDL—An evolving field. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.A.; Feig, J.E.; Hewing, B.; Hazen, S.L.; Smith, J.D. High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2813–2820. [Google Scholar] [CrossRef] [Green Version]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Biological Consequences of Dysfunctional HDL. Curr. Med. Chem. 2019, 26, 1644–1664. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.; Fasciolo, G.; Tomajoli, M.T.M.; Carlucci, A.; Ascione, E.; Salvatore, A. Effects of superoxide anion attack on the lipoprotein HDL. Mol. Cell. Biochem. 2023, 478, 1059–1066. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.; James, R.W.; Dullaart, R.P. Serum paraoxonase-1 activity and risk of incident cardiovascular disease: The PREVEND study and meta-analysis of prospective population studies. Atherosclerosis 2016, 245, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Systematic reviews of etiology and risk. In Joanna Briggs Institute Reviewer’s Manual; Aromataris, E., Munn, Z., Eds.; Johanna Briggs Institute: Adelaide, Australia, 2017. [Google Scholar]
- Cohen, J. Statistical Power Analysis. Curr. Dir. Psychol. Sci. 1992, 1, 98–101. [Google Scholar] [CrossRef]
- Balshem, H.; Helfand, M.; Schunemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowden, J.; Tierney, J.F.; Copas, A.J.; Burdett, S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med. Res. Methodol. 2011, 11, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Tobias, A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech. Bull. 1999, 47, 15–17. [Google Scholar]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Sarandol, A.; Kirli, S.; Akkaya, C.; Ocak, N.; Eroz, E.; Sarandol, E. Coronary artery disease risk factors in patients with schizophrenia: Effects of short term antipsychotic treatment. J. Psychopharmacol. 2007, 21, 857–863. [Google Scholar] [CrossRef]
- Yegin, A.; Ay, N.; Aydin, O.; Yargici, N.; Eren, E.; Yilmaz, N. Increased Oxidant Stress and Inflammation in Patients with Chronic Schizophrenia. Int. J. Clin. Med. 2012, 3, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Unsal, C.; Albayrak, Y.; Albayrak, N.; Kuloglu, M.; Hashimoto, K. Reduced serum paraoxonase 1 (PON1) activity in patients with schizophrenia treated with olanzapine but not quetiapine. Neuropsychiatr. Dis. Treat. 2013, 9, 1545–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilca, M.; Piriu, G.; Gaman, L.; Delia, C.; Iosif, L.; Atanasiu, V.; Stoian, I. A study of antioxidant activity in patients with schizophrenia taking atypical antipsychotics. Psychopharmacology 2014, 231, 4703–4710. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, H.; Mechria, H.; Mechri, A.; Azizi, I.; Neffati, F.; Douki, W.; Gaha, L.; Najjar, M.F. Paraoxonase 1 activity and lipid profile in schizophrenic patients. Asian J. Psychiatry 2014, 9, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Brinholi, F.F.; Noto, C.; Maes, M.; Bonifacio, K.L.; Brietzke, E.; Ota, V.K.; Gadelha, A.; Cordeiro, Q.; Belangero, S.I.; Bressan, R.A.; et al. Lowered paraoxonase 1 (PON1) activity is associated with increased cytokine levels in drug naive first episode psychosis. Schizophr. Res. 2015, 166, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Hussein, O.; Izikson, L.; Bathish, Y.; Dabur, E.; Hanna, A.; Zidan, J. Anti-atherogenic properties of high-density lipoproteins in psychiatric patients before and after two months of atypical anti-psychotic therapy. J. Psychopharmacol. 2015, 29, 1262–1270. [Google Scholar] [CrossRef]
- Sarandol, A.; Sarandol, E.; Acikgoz, H.E.; Eker, S.S.; Akkaya, C.; Dirican, M. First-episode psychosis is associated with oxidative stress: Effects of short-term antipsychotic treatment. Psychiatry Clin. Neurosci. 2015, 69, 699–707. [Google Scholar] [CrossRef]
- Gunes, M.; Camkurt, M.A.; Bulut, M.; Demir, S.; Ibiloglu, A.O.; Kaya, M.C.; Atli, A.; Kaplan, I.; Sir, A. Evaluation of Paraoxonase, Arylesterase and Malondialdehyde Levels in Schizophrenia Patients Taking Typical, Atypical and Combined Antipsychotic Treatment. Clin. Psychopharmacol. Neurosci. 2016, 14, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Kulaksizoglu, B.; Kulaksizoglu, S. Relationship between neutrophil/lymphocyte ratio with oxidative stress and psychopathology in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 2016, 12, 1999–2005. [Google Scholar] [CrossRef] [Green Version]
- Atagun, M.I.; Tunc, S.; Alisik, M.; Erel, O. Phenotypic Variants of Paraoxonase Q192R in Bipolar Disorder, Depression and Schizophrenia. Turk. J. Psychiatry 2017, 29, 79–86. [Google Scholar] [CrossRef]
- Boll, K.M.; Noto, C.; Bonifacio, K.L.; Bortolasci, C.C.; Gadelha, A.; Bressan, R.A.; Barbosa, D.S.; Maes, M.; Moreira, E.G. Oxidative and nitrosative stress biomarkers in chronic schizophrenia. Psychiatry Res. 2017, 253, 43–48. [Google Scholar] [CrossRef]
- Paval, D.; Nemes, B.; Rusu, R.L.; Dronca, E. Genotype-phenotype Analysis of Paraoxonase 1 in Schizophrenic Patients Treated with Atypical Antipsychotics. Clin. Psychopharmacol. Neurosci. 2018, 16, 32–38. [Google Scholar] [CrossRef] [Green Version]
- Solberg, D.K.; Refsum, H.; Andreassen, O.A.; Bentsen, H. A five-year follow-up study of antioxidants, oxidative stress and polyunsaturated fatty acids in schizophrenia. Acta Neuropsychiatr. 2019, 31, 202–212. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Orr, W.C. The redox stress hypothesis of aging. Free. Radic. Biol. Med. 2012, 52, 539–555. [Google Scholar] [CrossRef] [Green Version]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Grattagliano, I.; Palmieri, V.O.; Portincasa, P.; Moschetta, A.; Palasciano, G. Oxidative stress-induced risk factors associated with the metabolic syndrome: A unifying hypothesis. J. Nutr. Biochem. 2008, 19, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Dietrich, M.; Norkus, E.P.; Morrow, J.D.; Hudes, M.; Caan, B.; Packer, L. Factors associated with oxidative stress in human populations. Am. J. Epidemiol. 2002, 156, 274–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weverling-Rijnsburger, A.W.; Jonkers, I.J.; van Exel, E.; Gussekloo, J.; Westendorp, R.G. High-density vs low-density lipoprotein cholesterol as the risk factor for coronary artery disease and stroke in old age. Arch. Intern. Med. 2003, 163, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Neumann, J.T.; Thao, L.T.P.; Callander, E.; Chowdhury, E.; Williamson, J.D.; Nelson, M.R.; Donnan, G.; Woods, R.L.; Reid, C.M.; Poppe, K.K.; et al. Cardiovascular risk prediction in healthy older people. Geroscience 2022, 44, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.J.; Velakoulis, D.; Pantelis, C. Medical comorbidity in schizophrenia. Med. J. Aust. 2003, 178, S67–S70. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Puri, B.K.; Bortolasci, C.C.; Carvalho, A.; Berk, M.; Walder, K.; Moreira, E.G.; Maes, M. The role of high-density lipoprotein cholesterol, apolipoprotein A and paraoxonase-1 in the pathophysiology of neuroprogressive disorders. Neurosci. Biobehav. Rev. 2021, 125, 244–263. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, A.; Jenkins, Z.; Castle, D.; Andrade, C. Serum lipids and suicidality among patients with schizophrenia. Schizophr. Res. 2020, 215, 479–481. [Google Scholar] [CrossRef]
- Nandeesha, H.; Keshri, N.; Rajappa, M.; Menon, V. Association of hyperglycaemia and hyperlipidaemia with cognitive dysfunction in schizophrenia spectrum disorder. Arch. Physiol. Biochem. 2023, 129, 497–504. [Google Scholar] [CrossRef]
- Huang, T.L.; Chen, J.F. Serum lipid profiles and schizophrenia: Effects of conventional or atypical antipsychotic drugs in Taiwan. Schizophr. Res. 2005, 80, 55–59. [Google Scholar] [CrossRef]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef]
- Lepping, P.; Delieu, J.; Mellor, R.; Williams, J.H.; Hudson, P.R.; Hunter-Lavin, C. Antipsychotic medication and oxidative cell stress: A systematic review. J. Clin. Psychiatry 2011, 72, 273–285. [Google Scholar] [CrossRef]
- Chen, A.T.; Nasrallah, H.A. Neuroprotective effects of the second generation antipsychotics. Schizophr. Res. 2019, 208, 1–7. [Google Scholar] [CrossRef]
- Furlong, C.E.; Cole, T.B.; Jarvik, G.P.; Costa, L.G. Pharmacogenomic considerations of the paraoxonase polymorphisms. Pharmacogenomics 2002, 3, 341–348. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, D.P.; Liang, C.C. Paraoxonase gene polymorphisms, oxidative stress, and diseases. J. Mol. Med. 2003, 81, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Moreira, E.G.; Boll, K.M.; Correia, D.G.; Soares, J.F.; Rigobello, C.; Maes, M. Why Should Psychiatrists and Neuroscientists Worry about Paraoxonase 1? Curr. Neuropharmacol. 2019, 17, 1004–1020. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.K.; Maes, M.; Supasitthumrong, T.; Maes, A.; Michelin, A.P.; de Oliveira Semeao, L.; de Lima Pedrao, J.V.; Moreira, E.G.; Kanchanatawan, B.; Barbosa, D.S. Deficit schizophrenia and its features are associated with PON1 Q192R genotypes and lowered paraoxonase 1 (PON1) enzymatic activity: Effects on bacterial translocation. CNS Spectr. 2021, 26, 406–415. [Google Scholar] [CrossRef]
- De Hert, M.; Detraux, J.; van Winkel, R.; Yu, W.; Correll, C.U. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 2011, 8, 114–126. [Google Scholar] [CrossRef]
- Correll, C.U.; Detraux, J.; De Lepeleire, J.; De Hert, M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 2015, 14, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotella, F.; Cassioli, E.; Calderani, E.; Lazzeretti, L.; Ragghianti, B.; Ricca, V.; Mannucci, E. Long-term metabolic and cardiovascular effects of antipsychotic drugs. A meta-analysis of randomized controlled trials. Eur. Neuropsychopharmacol. 2020, 32, 56–65. [Google Scholar] [CrossRef]
- Taipale, H.; Tanskanen, A.; Mehtala, J.; Vattulainen, P.; Correll, C.U.; Tiihonen, J. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry 2020, 19, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.H.; Chen, P.H.; Kuo, C.J.; Tsai, S.Y.; Huang, S.H.; Wu, W.C. Risk factors for early circulatory mortality in patients with schizophrenia. Psychiatry Res. 2018, 267, 7–11. [Google Scholar] [CrossRef]
- Davis, K.A.; Crow, J.A.; Chambers, H.W.; Meek, E.C.; Chambers, J.E. Racial differences in paraoxonase-1 (PON1): A factor in the health of southerners? Environ. Health Perspect. 2009, 117, 1226–1231. [Google Scholar] [CrossRef] [Green Version]
- Woudberg, N.J.; Goedecke, J.H.; Blackhurst, D.; Frias, M.; James, R.; Opie, L.H.; Lecour, S. Association between ethnicity and obesity with high-density lipoprotein (HDL) function and subclass distribution. Lipids Health Dis. 2016, 15, 92. [Google Scholar] [CrossRef] [Green Version]
- Thyagarajan, B.; Jacobs, D.R., Jr.; Carr, J.J.; Alozie, O.; Steffes, M.W.; Kailash, P.; Hayes, J.H.; Gross, M.D. Factors associated with paraoxonase genotypes and activity in a diverse, young, healthy population: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Clin. Chem. 2008, 54, 738–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
















| Controls | Patients with Schizophrenia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | n | Age (Years) | M/F | PON Mean ± SD (U/L) | ARE Mean ± SD (kU/L) | HDL Mean (mg/dL or mmol/L) | n | Age (Years) | M/F | PON Mean ± SD (U/L) | ARE Mean ± SD (kU/L) | HDL Mean (mg/dL or mmol/L) |
| Sarandol A. et al. (a), 2007, Turkey [61] | 35 | 34 | 17/18 | 246 ± 90 | 98 ± 37 | 1.2 | 40 | 35 | 18/22 | 199 ± 95 | 78 ± 40 | 1.2 |
| Sarandol A. et al. (b) 2007, Turkey [61] | 35 | 34 | NR | 246 ± 90 | 98 ± 37 | 1.2 | 36 | 35 | NR | 226 ± 121 | 82 ± 40 | 1.2 |
| Yegin A. et al., 2012, Turkey [62] | 30 | 38 | 30/0 | 93.7 ± 44.5 | 164 ± 66 | 45.9 | 30 | 37 | 30/0 | 99.9 ± 61.6 | 150 ± 59 | 41.5 |
| Unsal C. et al. (a), 2013, Turkey [63] | 32 | 35 | 21/11 | 222 ± 91 | NR | 40 | 29 | 42 | 14/15 | 220 ± 93 | NR | 39.5 |
| Unsal C. et al. (b), 2013, Turkey [63] | 32 | 35 | 21/11 | 222 ± 91 | NR | 40 | 35 | 38 | 15/20 | 131 ± 50 | NR | 36 |
| Gilca M. et al. (a), 2014, Romania [64] | 16 | 62 | NR | 84.0 ± 1.8 | 107.4 ± 9.6 | 37.2 | 22 | 47 | NR | 87.5 ± 3.9 | 121.0 ± 14.2 | 41.3 |
| Gilca M. et al. (b), 2014, Romania [64] | 16 | 62 | NR | 84.0 ± 1.8 | 107.4 ± 9.6 | 37.2 | 44 | 38 | NR | 90.6 ± 2.8 | 123.6 ± 12.7 | 42.8 |
| Mabrouk H. et al., 2014, Tunisia [65] | 119 | 41 | 64/55 | 290 ± 171 | NR | 1.35 | 140 | 37 | 116/24 | 239 ± 176 | NR | 0.91 |
| Brinholi F.F. et al., 2015, Brazil [66] | 61 | 26 | 32/29 | NR | 237.8 ± 58.4 | NR | 51 | 25 | 36/15 | NR | 212.2 ± 56.2 | NR |
| Hussein O. et al. (a), 2015, Israel [67] | 10 | NR | 13/6 | NR | 77.3 ± 37.7 | 1.34 | 19 | 35 | 4/6 | NR | 72 ± 22.2 | 1.2 |
| Hussein O. et al. (a), 2015, Israel [67] | 10 | NR | NR | NR | 77.3 ± 37.7 | 1.34 | 15 | NR | NR | NR | 67.1 ± 22.5 | 1.2 |
| Sarandol A. et al. (a), 2015, Turkey [68] | 25 | 24 | 10/15 | 186 ± 75 | 89.7 ± 37.2 | 48 | 26 | 26 | 10/16 | 198 ± 107 | 64.4 ± 36.7 | 45 |
| Sarandol A. et al. (b), 2015, Turkey [68] | 25 | 24 | 10/15 | 186 ± 75 | 89.7 ± 37.2 | 48 | 26 | 26 | 10/16 | 209 ± 107 | 59.9 ± 29.3 | 45 |
| Gunes M. et al., 2016, Turkey [69] | 43 | 35 | 36/7 | 66.9 ± 5.2 | 114.5 ± 24.9 | NR | 41 | 35 | 35/6 | 68.6 ± 3.9 | 135 ± 24 | NR |
| Kulaksizoglu B. et al., 2016, Turkey [70] | 61 | 41 | 33/28 | 36.1 ± 28.2 | NR | NR | 64 | 40 | 36/28 | 38.7 ± 21.0 | NR | NR |
| Boll K.M. et al., 2017, Brazil [72] | 118 | 35 | 74/44 | NR | 178.6 ± 58.6 | NR | 125 | 36 | 85/40 | NR | 181.6 ± 47.7 | NR |
| Atagun M.I. et al., 2018, Turkey [71] | 43 | 32 | 20/23 | 186 ± 75 | 215 ± 52 | 46.63 | 37 | 35 | 19/18 | 154 ± 93 | 202 ± 48 | 43.27 |
| Paval D. et al., 2018, Romania [73] | 34 | 40 | 12/22 | NR | 112 ± 51 | 1.21 | 60 | 34 | 42/18 | NR | 97 ± 26 | 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinellu, A.; Sedda, S.; Mangoni, A.A. Paraoxonase/Arylesterase Activity of Serum Paraoxonase-1 and Schizophrenia: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 1484. https://doi.org/10.3390/antiox12081484
Zinellu A, Sedda S, Mangoni AA. Paraoxonase/Arylesterase Activity of Serum Paraoxonase-1 and Schizophrenia: A Systematic Review and Meta-Analysis. Antioxidants. 2023; 12(8):1484. https://doi.org/10.3390/antiox12081484
Chicago/Turabian StyleZinellu, Angelo, Stefania Sedda, and Arduino A. Mangoni. 2023. "Paraoxonase/Arylesterase Activity of Serum Paraoxonase-1 and Schizophrenia: A Systematic Review and Meta-Analysis" Antioxidants 12, no. 8: 1484. https://doi.org/10.3390/antiox12081484