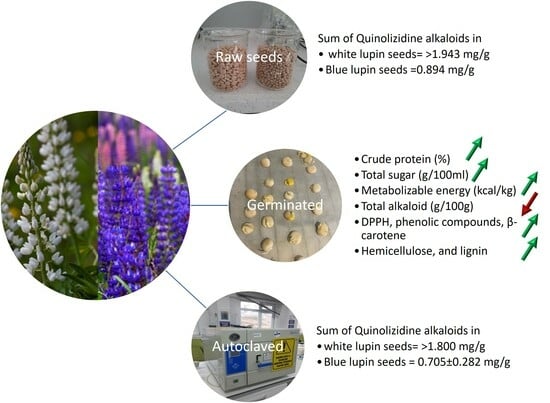
Impacts of Different Processes on the Nutritional and Antinutritional Contents of White and Blue Lupin Seeds and Usage Possibilities for Sustainable Poultry Production
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preliminary Experiment for Autoclave Process Times
2.3. Processes
2.4. Laboratory Methods
2.5. Statistical Design
3. Results
Nutrient Ingredient Changes in White and Blue Lupin Seeds via Processing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leeson, S.; Summers, J.D. Commercial Poultry Nutrition, 3rd ed.; Nottingham University Press: Nottingham, UK, 2005; p. 398. ISBN 978-1-904761-78-5. [Google Scholar]
- Karadağ, E.; Ağma Okur, A. Nutrient contents of some food industry by-products and their usage possibilities as alternative feed raw materials in animal nutrition. Turk. J. Agric. Food Sci. Technol. 2022, 10, 2180–2187. [Google Scholar] [CrossRef]
- Şengül, A.Y.; İnci, H.; Şengül, Ö. Using Possibilities of Chickpeas (Cicer arietinum L.) in Poultry Diets. Atatürk Univ. J. Agric. Fac. 2019, 50, 305–311. [Google Scholar] [CrossRef]
- Tüzün, A.E. An alternative source of protein lupin (Lupinus L.) use of broiler nutrition. J. Anim. Prod. 2013, 54, 50–54. [Google Scholar]
- Hejdysz, M.; Kaczmarek, S.A.; Rogiewicz, A.; Rutkowski, A. Influence of graded dietary levels of meals from three lupin species on the excreta dry matter, intestinal viscosity, excretion of total and free sialic acids, and intestinal morphology of broiler chickens. Anim. Feed Sci. Technol. 2018, 241, 223–232. [Google Scholar] [CrossRef]
- Silva, G. Feeding the World in 2050 and Beyond—Part 1: Productivity Challenges. Michigan State University Extension. 2018. Available online: https://www.canr.msu.edu/news/feeding-the-world-in-2050-and-beyond-part-1 (accessed on 23 February 2022).
- Bai, Y.; Alemu, R.; Block, S.A.; Headey, D.; Masters, W.A. Cost and affordability of nutritious diets at retail prices: Evidence from 177 countries. Food Policy 2021, 99, 101983. [Google Scholar] [CrossRef]
- Pereira, A.; Ramos, F.; Sanches Silva, A. Lupin (Lupinus albus L.) seeds: Balancing the good and the bad and addressing future challenges. Molecules 2022, 27, 8557. [Google Scholar] [CrossRef]
- Ruel, M.T.; Fanzo, J. Nutrition and climate change shifting to sustainable healthy diets. In 2022 Global Food Policy Report: Climate Change and Food Systems; International Food Policy Research Institute: Washington, WA, USA, 2022; Available online: https://ebrary.ifpri.org/utils/getfile/collection/p15738coll2/id/135886/filename/136096.pdf (accessed on 12 September 2023).
- Vos, R.; Martin, W.; Resnick, D. Repurposing agricultural support creating food systems incentives to address climate change. In 2022 Global Food Policy Report: Climate Change and Food Systems; International Food Policy Research Institute: Washington, WA, USA, 2022; Chapter 2; Available online: https://ebrary.ifpri.org/utils/getfile/collection/p15738coll2/id/135888/filename/136108.pdf (accessed on 12 September 2023).
- Agma Okur, A. Nutrient Contents and Anti-Nutrition. In 6th International New York Conference on Evolving Trends in Interdisciplinary Research & Practices; Proceedings Book: Manhattan, NY, USA, 2022; pp. 244–250. ISBN 978-1-955094-20-7. [Google Scholar]
- Yorgancılar, M.; Başarı, D.; Atalay, E.; Tanur Erkoyuncu, M.A. Functional Food: Lupin. J. Bahri Dagdas Crop Res. 2020, 9, 89–101. [Google Scholar]
- Hama, J.R.; Strobel, B.W. Natural alkaloids from narrow-leaf and yellow lupins transfer to soil and soil solution in agricultural fields. Environ. Sci. Eur. 2020, 32, 126. [Google Scholar] [CrossRef]
- Yeşil, E. Determination of Protein and Amino Acid Content of Some Protein Supplements Used in Animal Nutrition in Our Country. Master’s Thesis, Selçuk University, Konya, Turkey, 2010; p. 82. [Google Scholar]
- Balcıoğlu, A.; Orak, A. The Effects of Plant Growth Regulators on Yield and Yield Components in White Lupin (Lupinus albus L.) Genotypes. J. Bahri Dagdas Crop Res. 2020, 9, 197–211. [Google Scholar]
- Çetiner, M.; Ersus Bilek, S. Plant protein sources. Çukurova J. Agric. Food Sci. 2018, 33, 111–126. [Google Scholar]
- Yıldız, M. A Research on Gluten-Free Cookie Production with Usage of Buckwheat (Fagopyrum esculentum Moench.) and Lupine (Lupinus albus L.) Flours. Master’s Thesis, Selçuk University, Konya, Turkey, 2012; p. 88. [Google Scholar]
- Kaya, İ.; Yalçın, S. Grain legumes and Usage in Ruminant Rations. Livest. Stud. 1999, 39, 101–114. [Google Scholar]
- Güner, M. Phytochemical Studies of Chronanhtus Orientalis (Lois) Heywood & Frodin (Fabaceae) One of Our Endemic Plants. Master’s Thesis, Ege University, İzmir, Turkey, 2011; p. 66. [Google Scholar]
- Ertaş, N.; Bilgiçli, N. Effect of different debittering processes on mineral and phytic acid content of lupin (Lupinus albus L.) seeds. J. Food Sci. Technol. 2014, 51, 3348–3354. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A. Optimization of Debittering Process of Lupin (Lupinus albus L.) by Ultrasound. Master’s Thesis, Niğde Ömer Halisdemir University, Niğde, Turkey, 2019; p. 107. [Google Scholar]
- Schouten, M.A.; Fryganas, C.; Tappi, S.; Romani, S.; Fogliano, V. Influence of lupin and chickpea flours on acrylamide formation and quality characteristics of biscuits. Food Chem. 2023, 402, 134221. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; Volume 1. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Akyıldız, A.R. Yemlerin kimyasal yollarla incelenmesi (Examination of feeds by chemical analysis). In Yemler Bilgisi Laboratuvar Kılavuzu, 2nd ed.; Ankara University, Agricultural Faculty Publications: Ankara, Turkey, 1984; Volume 895, pp. 79–195. [Google Scholar]
- Cemeroğlu, B.S. Food Analysis, 3rd ed.; Bizim Grup Basımevi: Ankara, Turkey, 2013. [Google Scholar]
- Carpenter, K.J.; Clegg, K.M. The metabolizable energy of poultry feeding stuffs in relation to their chemical composition. J. Sci. Food Agric. 1956, 7, 45–51. [Google Scholar] [CrossRef]
- Garces, R.; Mancha, M. One step lipid extraction and fatty acid methyl esters preparation from fresh plant tissues. Anal. Biochem. 1993, 211, 139–143. [Google Scholar] [CrossRef]
- Çelik, O. Effects of Ozone Usage on Microbial Properties and Nutrient Composition of Broiler Feeds. Master’s Thesis, Namık Kemal University, Tekirdağ, Turkey, 2019; p. 101. [Google Scholar]
- Tırpancı Sivri, G. Production of Cheese with High CLA Content by Propionibacterium Isolated from Mihaliç Cheese. Ph.D. Thesis, Namık Kemal University, Tekirdağ, Turkey, 2020; p. 214. [Google Scholar]
- Chuah, A.M.; Lee, Y.-C.; Yamaguchi, T.; Takamura, H.; Yin, L.-J.; Matoba, T. Effect of cooking on the antioxidant properties of coloured peppers. Food Chem. 2008, 111, 20–28. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol. 1999, 299, 152–178. [Google Scholar]
- Dorman, H.J.D.; Peltoketo, A.; Hiltunen, R.; Tikkanen, M.J. Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamiaceae herbs. Food Chem. 2003, 83, 255–262. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice, E.C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Şenol, O. Determination of Antioxidant Activity/Capacity of Some Pharmaceutical Formulation with Several Methods and Classify Pharmaceuticals by Chemometric Calculations. Ph.D. Thesis, Atatürk University, Erzurum, Turkey, 2015; p. 99. [Google Scholar]
- Kaczmarek, S.A.; Kasprowicz-Potocka, M.; Hejdysz, M.; Mikuła, R.; Rutkowski, A. The nutritional value of narrow-leafed lupin (Lupinus angustifolius) for broilers. J. Anim. Feed Sci. 2014, 23, 160–166. [Google Scholar] [CrossRef]
- Kaczmarek, S.A.; Hejdysz, M.; Kubiś, M.; Kasprowicz-Potocka, M.; Rutkowski, A. The nutritional value of yellow lupin (Lupinus luteus L.) for broilers. Anim. Feed Sci. Technol. 2016, 222, 43–53. [Google Scholar] [CrossRef]
- Şamlı, H.E.; Şenköylü, N.; Özdüven, M.L.; Ağma, A. An in vitro assessment of nutritional and physical characteristics of wheat varieties obtained from thrace and aegean regions for poultry. Pak. J. Nutr. 2006, 5, 83–85. [Google Scholar]
- Konieczka, P.; Smulikowska, S. Viscosity negatively affects the nutritional value of blue lupin seeds for broilers. Animal 2018, 12, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Kılınçer, F.N. A study on Nutritional and Functional Properties of Germinated Some Cereals and Legumes. Master’s Thesis, Necmettin Erbakan University, Konya, Turkey, 2018; p. 88. [Google Scholar]
- Von Baer, D.; Reimerdes, E.H.; Feldheim, W. Methoden zur bestimmung der chinolizidin alkaloide in Lupinus mutabilis. Z. Lebensm. Unters. Forsch. 1979, 169, 27–31. [Google Scholar] [CrossRef]
- SPSS. PASW® Statistics 18, Statistical Package for the Social Sciencesi; SPSS Inc.: Chicago, IL, USA, 2018. [Google Scholar]
- Agma Okur, A. Lupines (Lupinus spp.) as a sustainable and alternative feedstuff in poultry diets. In Climate Change and Soil-Plant-Environment Interactions; Bellitürk, K., Çelik, A., Kılıç, M., Büyükfiliz, F., Eds.; Iksad Publications: Ankara, Turkey, 2023; Chapter 9; pp. 259–302. ISBN 978-625-367-101-3. [Google Scholar]
- Desbruslais, A.; Wealleans, A.; Gonzalez-Sanchez, D.; di Benedetto, M. Dietary fibre in laying hens: A revies of effects on performance, gut health and feather pecking. World’s Poult. Sci. J. 2021, 77, 797–823. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Bedford, M.R.; Morgan, N.K. Importance of considering non-starch polysaccharide content of poultry diets. World’s Poult. Sci. J. 2021, 77, 619–637. [Google Scholar] [CrossRef]
- Pilegaard, K.; Gry, J. Alkaloids in Edible Lupin Seeds, A Toxicological Review and Recommendations; Norden, Nordic Council of Ministers: Copenhagen, Denmark, 2008; p. 71, ISBN 978-92-893-1802-0; Available online: https://www.norden.org/da/node/56520 (accessed on 5 May 2023).
- Boschin, G.; Tesio, E.; Arnoldi, A. A field case of pig poisoning by accidental feed contamination by alkaloid-rich lupin seeds. J. Appl. Anim. Res. 2022, 50, 725–731. [Google Scholar] [CrossRef]
- Keuth, O.; Humpf, H.U.; Fürst, P. Quinolizidine alkaloids in lupine flour and lupine products from the German retail market and risk assessment of the results regarding human health. Food Addit. Contam. Part A 2023, 40, 667–674. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Lee, K.J.; Kim, G.-H.; Lee, G.-A.; Lee, J.-R.; Cho, G.-T.; Ma, K.-H.; Lee, S. Antioxidant Activities and Total Phenolic Contents of Three Legumes. Korean J. Plant Res. 2021, 34, 527–535. [Google Scholar] [CrossRef]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Estivi, L.; Buratti, S.; Fusi, D.; Benedetti, S.; Rodríguez, G.; Brandolini, A.; Hidalgo, A. Alkaloid content and taste profile assessed by electronic tongue of Lupinus albus seeds debittered by different methods. J. Food Compos. Anal. 2022, 114, 104810. [Google Scholar] [CrossRef]
- INRAE. Charts of Amino Acid (g/16g N) Contents of Some Feedstuffs. 2023. Available online: https://www.feedtables.com/charts/amino-acids?feed_ch_id%5B%5D=12370&feed_ch_id%5B%5D=12442&feed_ch_id%5B%5D=12363&feed_ch_id%5B%5D=12453 (accessed on 15 September 2023).
- von Baer, D.; Perez, I. Quality standard propositions for commercial grain of white lupin (Lupinus albus). In Proceedings of the 6th International Lupin Conference, Temuco-Pucón, Chile, 25–30 November 1990; pp. 158–167. [Google Scholar]
- Boschin, G.; Annicchiarico, P.; Resta, D.; D’Agostina, A.; Arnoldi, A. Quinolizidine alkaloids in seeds of lupin genotypes of different origins. J. Agric. Food Chem. 2008, 56, 3657–3663. [Google Scholar] [CrossRef]
- Rutkowski, A.; Kaczmarek, S.A.; Hejdysz, M.; Jamroz, D. Effect of extrusion on nutrients digestibility, metabolizable energy and nutritional value of yellow lupine seeds for broiler chickens. Ann. Anim. Sci. 2016, 16, 1059–1072. [Google Scholar] [CrossRef]
- Hejdysz, M.; Kaczmarek, S.A.; Rogiewicz, A.; Rutkowski, A. Influence of graded levels of meals from three lupin species on growth performance and nutrient digestibility in broiler chickens. Br. Poult. Sci. 2019, 60, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Kubiś, M.; Kaczmarek, S.; Hejdysz, M.; Mikula, R.; Wiśniewska, Z.; Pruszyńska-Oszmałek, E.; Kołodziejski, P.; Sassek, M.; Rutkowski, A. Microbial phytase improves performance and bone traits in broilers fed diets based on soybean meal and white lupin (Lupinus albus) meal. Ann. Anim. Sci. 2020, 20, 1379–1394. [Google Scholar] [CrossRef]
- Smulikowska, S.; Konieczka, P.; Czerwinski, J.; Mieczkowska, A.; Jankowiak, J. Feeding broiler chickens with practical diets containing lupin seeds (L. angustifolius or L. luteus): Effects of incorporation level and mannanase supplementation on growth performance, digesta viscosity, microbial fermentation and gut morphology. J. Anim. Feed Sci. 2014, 23, 64–72. [Google Scholar] [CrossRef]
- Brenes, A.; Marquardt, R.R.; Guenter, W.; Rotter, B.A. Effect of enzyme supplementation on the nutritional value of raw, autoclaved, and dehulled lupins (Lupinus albus) in chicken diets. Poult. Sci. 1993, 72, 2281–2293. [Google Scholar] [CrossRef]
- Bekrić, B.; Božović, I.; Pavlovski, Z.; Masić, B. Lupine, field pea, horse bean and soya-bean in combination with maize as feed for 21 to 52 days old broilers. In L’aviculture en Méditerranée; Options Méditerranéennes, Série A, Séminaires Méditenanéens; No: 7; Sauveur, B., Ed.; CIHEAM: Montpellier, France, 1990; pp. 103–106. [Google Scholar]
- Castanon, J.I.R.; Perez-Lanzac, J. Substitution of fixed amounts of soyabean meal for field beans (Vicia faba), sweet lupins (Lupinus albus), cull peas (Pisum sativum) and vetchs (Vicia saliva) in diets for high performance laying Leghorn hens. Br. Poult. Sci. 1990, 31, 173–180. [Google Scholar] [CrossRef]
- Watkins, B.A.; Manning, B.; Al-Athari, A.K. The effects of Lupinus albus cultivar Ultra on broiler performance. Nutr. Rep. Int. 1988, 38, 173–181. [Google Scholar]
- Buraczewska, L.; Pastuszewska, B.; Smulikowska, S.; Wasilewko, J. Response of pigs, rats and chickens to dietary level of alkaloids of different lupin species. In Proceedings of the 2nd International Workshop on Antinutritional Factors in Legume Seed, Wageningen, The Netherlands, 1–3 December 1993; pp. 371–376. [Google Scholar]
- Prinsloo, J.J.; Smith, G.A.; Rode, W. Sweet white Lupinus albus (cv. Buttercup) as a feedstuff for layers. Br. Poult. Sci. 1992, 33, 525–530. [Google Scholar] [CrossRef]
- Prusinski, J. White lupin (Lupinus albus L.)—Nutritional and health values in human nutrition. Czech J. Food Sci. 2017, 35, 95–105. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Schrenk, D.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.-C.; Nebbia, C.S.; et al. Scientific opinion on the risks for animal and human health related to the presence of quinolizidine alkaloids in feed and food, in particular in lupins and lupin-derived products. EFSA J. 2019, 17, e05860. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, M.; Certel, M.; Uslu, M.K. Some chemical properties of white lupine seeds (Lupinus albus L.). Food Chem. 2005, 89, 341–345. [Google Scholar] [CrossRef]
- Doxastakis, G.; Zafiriadis, I.; Irakli, M.; Marlani, H.; Tananaki, C. Lupin, soya and triticale addition to wheat flour doughs and their effect on rheological properties. Food Chem. 2002, 77, 219–227. [Google Scholar] [CrossRef]
- Çoban, D.İ. Production of Lupine (Lupinus albus L.) Added Chips Production and Determination of Quality of Final Product. Master of Science Thesis, Selçuk University, Konya, Turkey, 2018; p. 77.
- Lampart-Szczapa, E.; Korczak, J.; Nogala-Kalucka, M.; Zawirska-Wojtaskiak, R. Antioxidant properties of lupin seed products. Food Chem. 2003, 83, 279–285. [Google Scholar] [CrossRef]
- Rahman, M.H.; Hossain, M.I.; Moslehuddin. Mineral balance of rats fed on diets containing sweet lupin (Lupinus angustifolius L.) or its fractions. Anim. Feed. Sci. Technol. 1997, 65, 231–248. [Google Scholar] [CrossRef]
- Choct, M. Fibre—Chemistry and Functions in Poultry Nutrition. LII Simp. Científico De Avic. Málaga 2015, 28, 113–119. Available online: https://www.wpsa-aeca.es/aeca_imgs_docs/16478_fibra_mingan.pdf (accessed on 3 May 2023).
- Guillon, F.; Champ, M.M.-J. Carbohydrate fractions of legumes: Uses in human nutrition and potential for health. Br. J. Nutr. 2002, 88, 293–306. [Google Scholar] [CrossRef]
- Tizazu, H.; Emire, S.A. Chemical composition, physicochemical and functional properties of lupin (Lupinus albus) seeds grown in Ethiopia. Afr. J. Food Agric. Nutr. Dev. 2010, 10, 3029–3041. [Google Scholar] [CrossRef]
- Jeroch, H.; Kozłowski, K.; Schöne, F.; Zduńczyk, Z. Lupines (Lupinus spp.) as a protein feedstuff for poultry. 1) Varieties, composition and nutritional values for poultry. Europ. Poult. Sci. 2016, 80. [Google Scholar] [CrossRef]
- van de Noort, M. Lupin: An important protein and nutrient source. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA, 2017; Chapter 10; pp. 165–183. [Google Scholar] [CrossRef]
- Petterson, D.S. The use of lupins in feeding systems—Review. Asian-Australas. J. Anim. Sci. 2000, 13, 861–882. [Google Scholar] [CrossRef]
- de Cortes Sánchez, M.; Altares, P.; Pedrosa, M.M.; Burbano, C.; Cuadrado, C.; Goyoaga, C.; Muzquiz, M.; Jiménez-Martínez, C.; Dávila-Ortiz, G. Alkaloid variation during germination in different lupin species. Food Chem. 2005, 90, 347–355. [Google Scholar] [CrossRef]



| Main Effects | Treatments | DM (%) | Ash (%DM) | CP (%DM) | EE (%DM) | Starch (%) | Total Sugar (g/100 mL) | ME (kcal/kg) |
|---|---|---|---|---|---|---|---|---|
| White lupin | Raw | 94.583 c | 2.913 ab | 35.168 a | 6.997 c | 4.781 b | 0.653 d | 2212.177 b |
| Germinated | 95.356 a | 2.761 ab | 35.998 a | 8.041 b | 4.781 b | 0.845 c | 2340.372 a | |
| Autoclaved | 94.358 d | 2.871 ab | 35.196 a | 9.911 a | 4.045 b | 0.217 f | 2415.156 a | |
| Blue lupin | Raw | 93.816 e | 2.706 b | 29.329 c | 4.062 e | 6.987 a | 1.011 b | 1845.291 d |
| Germinated | 94.805 b | 2.750 ab | 32.342 b | 4.993 d | 6.251 a | 1.205 a | 2016.042 c | |
| Autoclaved | 93.996 e | 3.047 a | 29.804 c | 6.719 c | 4.413 b | 0.563 e | 1965.850 c | |
| Variety | White lupin | 94.766 A | 2.848 | 35.454 A | 8.316 A | 4.535 B | 0.572 B | 2322.568 A |
| Blue lupin | 94.206 B | 2.834 | 30.492 B | 5.258 B | 5.884 A | 0.927 A | 1942.394 B | |
| Process | Raw | 94.200 b | 2.810 | 32.249 b | 5.529 c | 5.884 a | 0.832 b | 2028.734 b |
| Germinated | 95.080 a | 2.755 | 34.170 a | 6.517 b | 5.516 a | 1.025 a | 2178.207 a | |
| Autoclaved | 94.177 b | 2.959 | 32.500 b | 8.315 a | 4.229 b | 0.390 c | 2190.503 a | |
| SEM | 0.126 | 0.043 | 0.662 | 0.467 | 0.279 | 0.078 | 50.917 | |
| p levels | ||||||||
| Variety (V) | 0.000 | 0.860 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Process (Prc) | 0.000 | 0.130 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | |
| V × Prc | 0.021 | 0.178 | 0.051 | 0.680 | 0.053 | 0.771 | 0.157 | |
| Main Effects | Treatments | CF (%DM) | NDF (g/kg DM) | ADF (g/kg DM) | ADL (g/kg DM) | Viscosity (cP = mPa.s) | Tot. Alkaloids (mg/g) |
|---|---|---|---|---|---|---|---|
| White lupin | Raw | 8.316 c | 158.482 e | 138.092 d | 8.547 d | 43.847 b | 50.796 a |
| Germinated | 11.185 b | 220.673 b | 127.389 e | 11.921 b | 43.000 b | 14.893 e | |
| Autoclaved | 8.467 c | 171.274 d | 123.382 e | 8.204 d | 43.540 b | 36.260 c | |
| Blue lupin | Raw | 11.750 b | 233.719 a | 179.349 b | 9.421 c | 43.520 b | 48.293 b |
| Germinated | 11.479 b | 237.688 a | 162.335 c | 19.349 a | 45.870 a | 9.600 f | |
| Autoclaved | 18.822 a | 195.634 c | 222.483 a | 6.664 e | 43.660 b | 27.543 d | |
| Variety | White lupin | 9.323 B | 183.476 B | 129.621 B | 9.557 B | 43.462 B | 33.983 A |
| Blue lupin | 14.017 A | 222.347 A | 188.056 A | 11.811 A | 44.350 A | 28.479 B | |
| Process | Raw | 10.033 c | 196.100 b | 158.721 b | 8.984 b | 43.683 | 49.545 a |
| Germinated | 11.332 b | 229.181 a | 144.862 c | 15.635 a | 44.435 | 12.247 c | |
| Autoclaved | 13.645 a | 183.454 c | 172.932 a | 7.434 c | 43.600 | 31.902 b | |
| SEM | 0.851 | 7.364 | 8.417 | 1.019 | 0.259 | 3.770 | |
| p levels | |||||||
| Variety (V) | 0.000 | 0.000 | 0.000 | 0.000 | 0.016 | 0.000 | |
| Process (Prc) | 0.000 | 0.000 | 0.000 | 0.0000 | 0.100 | 0.000 | |
| V × Prc | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | |
| Quinolizidine Alkaloids (QA) | Raw Seeds (mg/g) | After Germination (mg/g) | After Autoclaving (mg/g) |
|---|---|---|---|
| Lupanine | >0.5 | >0.5 | >0.5 |
| 13α- Hydroxylupanine | >0.5 | >0.5 | >0.5 |
| Cytisine | <0.01 * | <0.01 * | <0.01 * |
| Sparteine | 0.013 ± 0.0052 | <0.01 * | 0.020 ± 0.008 |
| Angustifoline | 0.260 ± 0.1 | 0.180 ± 0.072 | 0.160 ± 0.064 |
| Lupinine | <0.01 * | <0.01 * | <0.01 * |
| Multiflorine | >0.5 | >0.5 | >0.5 |
| α-Isolupanine | 0.170 ± 0.068 | 0.120 ± 0.048 | 0.120 ± 0.048 |
| Sum of all positive QA | >1.943 | >1.800 | >1.800 |
| Quinolizidine Alkaloids (QA) | Raw Seeds (mg/g) | After Germination (mg/g) | After Autoclaving (mg/g) |
|---|---|---|---|
| Lupanine | 0.490 | 0.350 ± 0.140 | 0.390 ± 0.160 |
| 13α- Hydroxylupanine | 0.260 | 0.190 ± 0.076 | 0.220 ± 0.088 |
| Cytisine | <0.01 * | <0.01 * | <0.01 * |
| Sparteine | <0.01 * | <0.01 * | <0.01 * |
| Angustifoline | 0.066 | 0.020 ± 0.0080 | 0.037 ± 0.015 |
| Lupinine | <0.01 * | <0.01 * | <0.01 * |
| Multiflorine | <0.01 * | <0.01 * | <0.01 * |
| α-Isolupanine | 0.078 | 0.049 ± 0.020 | 0.058 ± 0.023 |
| Sum of all positive QA | 0.894 | 0.609 ± 0.244 | 0.705 ± 0.282 |
| Main Effects | Treatments | ABTS (mg/mL) | DPPH (IC50 µL) | PC (mg GAE/L) | β-Carotene (mg/100 g) | Color | ||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| White lupin | Raw | 38.235 b | 132.785 cd | 1014.159 c | 2.535 d | 85.277 a | 3.223 b | 31.830 c |
| Germinated | 35.335 c | 109.704 d | 1187.809 a | 4.709 a | 84.950 b | 1.920 e | 30.933 d | |
| Autoclaved | 42.697 a | 79.386 e | 1055.429 b | 3.599 c | 82.063 c | 4.167 a | 35.373 a | |
| Blue lupin | Raw | 22.713 d | 150.066 c | 256.857 d | 2.316 d | 81.750 d | 2.150 d | 34.280 b |
| Germinated | 18.691 e | 296.534 a | 188.286 e | 4.088 b | 81.690 d | 0.453 f | 31.033 d | |
| Autoclaved | 18.245 f | 236.025 b | 209.714 e | 2.414 d | 80.063 e | 2.357 c | 31.930 c | |
| Variety | White lupin | 38.755 A | 107.292 B | 1085.799 A | 3.614 A | 84.097 A | 3.103 A | 32.712 A |
| Blue lupin | 19.883 B | 227.542 A | 218.286 B | 2.939 B | 81.168 B | 1.653 B | 32.414 B | |
| Process | Raw | 30.474 a | 141.426 b | 635.508 b | 2.425 c | 83.513 a | 2.687 b | 33.055 b |
| Germinated | 27.013 b | 203.119 a | 688.047 a | 4.399 a | 83.320 b | 1.187 c | 30.983 c | |
| Autoclaved | 30.471 a | 157.705 b | 632.571 b | 3.007 b | 81.063 c | 3.262 a | 33.652 a | |
| SEM | 2.373 | 17.971 | 99.013 | 0.226 | 0.454 | 0.278 | 0.408 | |
| p levels | ||||||||
| Variety (V) | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.014 | |
| Process (Prc) | 0.000 | 0.0001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| V × Prc | 0.000 | 0.000 | 0.000 | 0.009 | 0.000 | 0.000 | 0.000 | |
| Fatty Acids (FAs) (%) | Short Name | White Lupin | Blue Lupin | Variety (V) | Process (Prc) | p Levels | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Germinated | Autoclaved | Raw | Germinated | Autoclaved | White | Blue | Raw | Germinated | Autoclaved | SEM | V | Prc | V × Prc | ||
| Palmitic acid | C16:0 | 7.654 b | 7.605 b | 7.698 b | 10.801 a | 10.648 a | 10.638 a | 7.652 B | 10.696 A | 9.227 | 9.126 | 9.168 | 0.483 | 0.000 | 0.952 | 0.955 |
| Stearic acid | C18:0 | 2.316 b | 2.042 b | 2.334 b | 6.733 a | 5.777 a | 5.897 a | 2.231 B | 6.136 A | 4.524 | 3.910 | 4.115 | 0.557 | 0.000 | 0.185 | 0.396 |
| Oleic acid | C18:1n9c | 53.875 a | 51.331 b | 53.814 a | 38.882 c | 38.701 c | 39.444 c | 53.007 A | 39.009 B | 46.378 ab | 45.016 b | 46.629 a | 1.826 | 0.000 | 0.055 | 0.172 |
| Linoleic acid | C18:2n6c | 15.983 d | 18.561 c | 15.889 d | 37.991 a | 36.332 b | 38.638 a | 16.811 B | 37.653 A | 26.987 | 27.447 | 27.264 | 2.681 | 0.000 | 0.551 | 0.001 |
| γ-linolenic acid | C18:3n6 | 12.513 a | 12.651 a | 12.505 a | 2.805 b | 3.626 b | 2.937 b | 12.557 A | 3.122 B | 7.659 | 8.139 | 7.721 | 1.291 | 0.000 | 0.698 | 0.837 |
| Arachidic acid | C20:0 | 1.116 ab | 1.084 b | 1.143 a | 0.755 d | 0.817 c | 0.790 cd | 1.115 A | 0.787 B | 0.936 b | 0.950 ab | 0.967 a | 0.045 | 0.000 | 0.088 | 0.006 |
| Behenic acid | C22:0 | 3.793 a | 3.855 a | 3.835 a | 1.620 b | 1.711 b | 1.656 b | 3.828 A | 1.662 B | 2.706 | 2.783 | 2.746 | 0.287 | 0.000 | 0.501 | 0.960 |
| Erucic acid | C22:1n9 | 1.821 a | 1.952 a | 1.898 a | 0.000 b | 0.000 b | 0.000 b | 1.890 A | 0.000 B | 0.910 | 0.976 | 0.949 | 0.253 | 0.000 | 0.526 | 0.526 |
| DHA | C22:6n3 | 0.000 b | 0.000 b | 0.000 b | 0.000 b | 1.056 a | 0.000 b | 0.000 B | 0.352 A | 0.000 b | 0.528 a | 0.000 b | 0.116 | 0.000 | 0.000 | 0.000 |
| Lignoceric acid | C24:0 | 0.929 a | 0.920 a | 0.883 a | 0.415 b | 0.000 c | 0.000 c | 0.911 A | 0.138 B | 0.672 a | 0.460 b | 0.442 b | 0.112 | 0.000 | 0.000 | 0.000 |
| Nervonic acid | C24:1 | 0.000 b | 0.000 b | 0.000 b | 0.000 b | 1.768 a | 0.000 b | 0.000 B | 0.589 A | 0.000 b | 0.884 a | 0.000 b | 0.196 | 0.002 | 0.000 | 0.000 |
| Saturated FA (%) | 15.808 b | 15.505 b | 15.892 b | 20.323 a | 18.952 a | 18.982 a | 15.735 B | 19.419 A | 18.066 | 17.229 | 17.437 | 0.596 | 0.000 | 0.330 | 0.451 | |
| Monounsaturated FA (%) | 55.696 a | 53.282 b | 55.713 a | 38.882 c | 40.180 c | 39.444 c | 54.897 A | 39.502 B | 47.289 | 46.731 | 47.578 | 1.989 | 0.000 | 0.343 | 0.017 | |
| Polyunsaturated FA (%) | 28.496 b | 31.212 b | 28.395 b | 40.796 a | 40.868 a | 41.575 a | 29.368 B | 41.079 A | 34.646 | 36.040 | 34.985 | 1.530 | 0.000 | 0.308 | 0.188 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzun, T.; Agma Okur, A. Impacts of Different Processes on the Nutritional and Antinutritional Contents of White and Blue Lupin Seeds and Usage Possibilities for Sustainable Poultry Production. Animals 2023, 13, 3496. https://doi.org/10.3390/ani13223496
Uzun T, Agma Okur A. Impacts of Different Processes on the Nutritional and Antinutritional Contents of White and Blue Lupin Seeds and Usage Possibilities for Sustainable Poultry Production. Animals. 2023; 13(22):3496. https://doi.org/10.3390/ani13223496
Chicago/Turabian StyleUzun, Tugce, and Aylin Agma Okur. 2023. "Impacts of Different Processes on the Nutritional and Antinutritional Contents of White and Blue Lupin Seeds and Usage Possibilities for Sustainable Poultry Production" Animals 13, no. 22: 3496. https://doi.org/10.3390/ani13223496