Heavy Metals in Crop Plants: Transport and Redistribution Processes on the Whole Plant Level
Abstract
:1. Heavy Metals: Micronutrients or Pollutants?

2. Transport with the Transpiration Stream in the Xylem


3. Redistribution via the Phloem

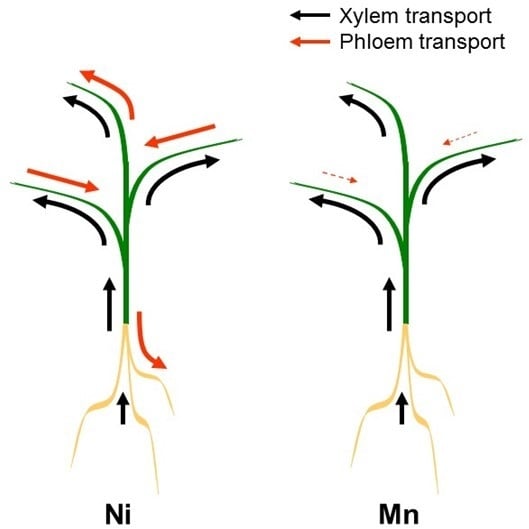
4. Xylem/Phloem Interactions
5. Selective Accumulation of Heavy Metals in Harvested Plant Parts
6. Relevance of Long-Distance Transport for Phytoremediation
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995; p. 38. [Google Scholar]
- Grotz, N.; Guerinot, M.L. Molecular aspects of Cu, Fe and Zn homeostasis in plants. Biochim. Biophys. Acta-Mol. Cell Res. 2006, 1763, 595–608. [Google Scholar] [CrossRef]
- Prasad, M.N.V. Cadmium toxicity and tolerance in vascular plants. Environ. Exp. Bot. 1995, 35, 525–545. [Google Scholar] [CrossRef]
- Pal, M.; Horvath, E.; Janda, T.; Paldi, E.; Szalai, G. Physiological changes and defense mechanisms induced by cadmium stress in maize. J. Plant Nutr. Soil Sci. 2006, 169, 239–246. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.S.; Li, P.J.; Zhou, Q.X.; Xie, L.J.; Han, Y.P. Risk assessment of cadmium-contaminated soil on plant DNA damage using RAPD and physiological indices. J. Hazard. Mater. 2009, 161, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Vecchia, F.D.; La Rocca, N.; Barbato, R.; Pagliano, C.; Raviolo, M.; Gonnelli, C.; Gabbrielli, R. Metal accumulation and damage in rice (cv. Vialone nano) seedlings exposed to cadmium. Environ. Exp. Bot. 2008, 62, 267–278. [Google Scholar] [CrossRef]
- Singh, S.; Sinha, S.; Saxena, R.; Pandey, K.; Bhatt, K. Translocation of metals and its effects in the tomato plants grown on various amendments of tannery waste: Evidence for involvement of antioxidants. Chemosphere 2004, 57, 91–99. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Zhao, S.; Wang, S.J.; Yang, Z.M. Biological detection and analysis of mercury toxicity to alfalfa (Medicago sativa) plants. Chemosphere 2008, 70, 1500–1509. [Google Scholar] [CrossRef]
- Cenkci, S.; Cigerci, I.H.; Yildiz, M.; Ozay, C.; Bozdag, A.; Terzi, H. Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environ. Exp. Bot. 2010, 67, 467–473. [Google Scholar] [CrossRef]
- Pinho, S.; Ladeiro, B. Phytotoxicity by lead as heavy metal focus on oxidative stress. J. Bot. 2012, 2012. [Google Scholar] [CrossRef]
- Sharma, D.C.; Sharma, C.; Tripathi, R.D. Phytotoxic lesions of chromium in maize. Chemosphere 2003, 51, 63–68. [Google Scholar] [CrossRef]
- Welch, R.M. Micronutrient nutrition of plants. Crit. Rev. Plant Sci. 1995, 14, 49–82. [Google Scholar] [CrossRef]
- Campbell, W.H. Nitrate reductase structure, function and regulation: Bridging the gap between biochemistry and physiology. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 277–303. [Google Scholar] [CrossRef]
- Prescott, A.G.; John, P. Dioxygenases: Molecular structure and role in plant metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 245–271. [Google Scholar] [CrossRef]
- Siedow, J.N. Plant lipoxygenase—Structure and function. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 145–188. [Google Scholar] [CrossRef]
- Briat, J.F.; Lobreaux, S. Iron transport and storage in plants. Trends Plant Sci. 1997, 2, 187–193. [Google Scholar] [CrossRef]
- Briat, J.F.; Lobreaux, S.; Grignon, N.; Vansuyt, G. Regulation of plant ferritin synthesis: How and why. Cell. Mol. Life Sci. 1999, 56, 155–166. [Google Scholar] [CrossRef]
- Ravet, K.; Touraine, B.; Boucherez, J.; Briat, J.F.; Gaymard, F.; Cellier, F. Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J. 2009, 57, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.C.; Barreiro, M.G.; Ramalho, J.C. Manganese accumulation in rice: Implications for photosynthetic functioning. J. Plant Physiol. 2004, 161, 1235–1244. [Google Scholar] [CrossRef]
- Lanquar, V.; Ramos, M.S.; Lelievre, S.; Barbier-Brygoo, H.; Krieger-Liszkay, A.; Kramer, U.; Thomine, S. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol. 2010, 152, 1986–1999. [Google Scholar] [CrossRef]
- Filiz, E.; Tombuloglu, H. Genome-wide distribution of superoxide dismutase (SOD) gene families in Sorghum bicolor. Turk. J. Biol. 2015, 39, 49–59. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef]
- Redinbo, M.R.; Yeates, T.O.; Merchant, S. Plastocyanin—Structural and functional analysis. J. Bioenerg. Biomembr. 1994, 26, 49–66. [Google Scholar] [CrossRef]
- Bueno, P.; Varela, J.; Gimenezgallego, G.; Delrio, L.A. Peroxisomal copper,zinc-superoxide dismutase—Characterization of the isoenzyme from watermelon cotyledons. Plant Physiol. 1995, 108, 1151–1160. [Google Scholar] [CrossRef]
- Yruela, I. Transition metals in plant photosynthesis. Metallomics 2013, 5, 1090–1109. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Dixit, A.; Ray, M.; Sabat, S.C. Mechanistic study of CuZn-SOD from Ipomoea carnea mutated at dimer interface: Enhancement of peroxidase activity upon monomerization. Biochimie 2014, 97, 181–193. [Google Scholar] [CrossRef]
- Hacisalihoglu, G.; Hart, J.J.; Wang, Y.H.; Cakmak, I.; Kochian, L.V. Zinc efficiency is correlated with enhanced expression and activity of zinc-requiring enzymes in wheat. Plant Physiol. 2003, 131, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Delorme, V.G.R.; McCabe, P.F.; Kim, D.J.; Leaver, C.J. A matrix metalloproteinase gene is expressed at the boundary of senescence and programmed cell death in cucumber. Plant Physiol. 2000, 123, 917–927. [Google Scholar] [CrossRef]
- Takatsuji, H. Zinc-finger transcription factors in plants. Cell. Mol. Life Sci. 1998, 54, 582–596. [Google Scholar] [CrossRef]
- Witte, C.P. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef]
- Sirko, A.; Brodzik, R. Plant ureases: Roles and regulation. Acta Biochim. Pol. 2000, 47, 1189–1195. [Google Scholar]
- Polacco, J.C.; Freyermuth, S.K.; Gerendas, J.; Cianzio, S.R. Soybean genes involved in nickel insertion into urease. J. Exp. Bot. 1999, 50, 1149–1156. [Google Scholar] [CrossRef]
- Psaras, G.K.; Constantinidis, T.; Cotsopoulos, B.; Manetas, Y. Relative abundance of nickel in the leaf epidermis of eight hyperaccumulators: Evidence that the metal is excluded from both guard cells and trichomes. Ann. Bot. 2000, 86, 73–78. [Google Scholar] [CrossRef]
- Mendel, R.R.; Schwarz, G. Molybdoenzymes and molybdenum cofactor in plants. Crit. Rev. Plant Sci. 1999, 18, 33–69. [Google Scholar]
- Mendel, R.R. Biology of the molybdenum cofactor. J. Exp. Bot. 2007, 58, 2289–2296. [Google Scholar] [CrossRef]
- Schwarz, G.; Boxer, D.H.; Mendel, R.R. Molybdenum cofactor biosynthesis—The plant protein Cnx1 binds molybdopterin with high affinity. J. Biol. Chem. 1997, 272, 26811–26814. [Google Scholar] [CrossRef]
- O’Hara, G.W. Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: A review. Aust. J. Exp. Agric. 2001, 41, 417–433. [Google Scholar] [CrossRef]
- Jayakumar, K.; Vijayarengan, P.; Changxing, Z.; Gomathinayagam, M.; Jaleel, C.A. Soil applied cobalt alters the nodulation, leg-haemoglobin content and antioxidant status of Glycine max (L.) Merr. Colloid Surf. B-Biointerfaces 2008, 67, 272–275. [Google Scholar] [CrossRef]
- Long, X.X.; Yang, X.E.; Ni, W.Z.; Ye, Z.Q.; He, Z.L.; Calvert, D.V.; Stoffella, J.P. Assessing zinc thresholds for phytotoxicity and potential dietary toxicity in selected vegetable crops. Commun. Soil Sci. Plant Anal. 2003, 34, 1421–1434. [Google Scholar] [CrossRef]
- Gupta, U.C.; Gupta, S.C. Trace element toxicity relationships to crop production and livestock and human health: Implications for management. Commun. Soil Sci. Plant Anal. 1998, 29, 1491–1522. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant. 1994, 92, 696–717. [Google Scholar] [CrossRef]
- Dat, J.; Vandenabeele, S.; Vranova, E.; Van Montagu, M.; Inze, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Xu, X.Y.; Shi, G.X.; Wang, J.; Zhang, L.L.; Kang, Y.N. Copper-induced oxidative stress in Alternanthera philoxeroides callus. Plant Cell Tissue Organ Cult. 2011, 106, 243–251. [Google Scholar] [CrossRef]
- Rodriguez-Serrano, M.; Romero-Puertas, M.C.; Zabalza, A.; Corpas, F.J.; Bomet, M.; del Rio, L.A.; Sandalio, L.M. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ. 2006, 29, 1532–1544. [Google Scholar] [CrossRef]
- Tewari, P.K.; Kumar, P.; Sharma, P.N. Antioxidant responses to enhanced generation of superoxide anion radical and hydrogen peroxide in the copper-stressed mulberry plants. Planta 2006, 223, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.B.; Korpelainen, H.; Li, C.Y. Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere 2007, 68, 686–694. [Google Scholar] [CrossRef]
- Kumar, P.; Tewari, P.K.; Sharma, P.N. Modulation of copper toxicity-induced oxidative damage by excess supply of iron in maize plants. Plant Cell Rep. 2008, 27, 399–409. [Google Scholar] [CrossRef]
- Gajewska, E.; Sklodowska, M. Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 2007, 20, 27–36. [Google Scholar] [CrossRef]
- Shi, Q.H.; Zhu, Z.J.; Xu, M.; Qian, Q.Q.; Yu, J.Q. Effect of excess manganese on the antioxidant system in Cucumis sativus L. under two light intensities. Environ. Exp. Bot. 2006, 58, 197–205. [Google Scholar] [CrossRef]
- Demirevska-Kepova, K.; Simova-Stoilova, L.; Stoyanova, Z.; Holzer, R.; Feller, U. Biochemical changes in barley plants after excessive supply of copper and manganese. Environ. Exp. Bot. 2004, 52, 253–266. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Huseinova, I.M.; Aliyeva, D.R.; Aliyev, J.A. Subcellular localization and responses of superoxide dismutase isoforms in local wheat varieties subjected to continuous soil drought. Plant Physiol. Biochem. 2014, 81, 54–60. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Zhang, J.H. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef]
- Kosegarten, H.; Koyro, H.W. Apoplastic accumulation of iron in the epidermis of maize (Zea mays) roots grown in calcareous soil. Physiol. Plant. 2001, 113, 515–522. [Google Scholar] [CrossRef]
- Bravin, M.N.; Travassac, F.; Le Floch, M.; Hinsinger, P.; Garnier, J.M. Oxygen input controls the spatial and temporal dynamics of arsenic at the surface of a flooded paddy soil and in the rhizosphere of lowland rice (Oryza sativa L.): A microcosm study. Plant Soil 2008, 312, 207–218. [Google Scholar] [CrossRef]
- Yang, X.; Li, T.Q.; Yang, J.C.; He, Z.L.; Lu, L.L.; Memg, F.H. Zinc compartmentation in root, transport into xylem, and absorption into leaf cells in the hyperaccumulating species of Sedum alfredii Hance. Planta 2006, 224, 185–195. [Google Scholar] [CrossRef]
- Richau, K.H.; Kozhevnikova, A.D.; Seregin, I.V.; Voojis, R.; Koevoets, P.L.M.; Snith, J.A.C.; Ivanov, V.B.; Schat, H. Chelation by histidine inhibits the vacuolar sequestration of nickel in roots of the hyperaccumulator Thlaspi caerulescens. New Phytol. 2009, 183, 106–116. [Google Scholar] [CrossRef]
- Page, V.; Weisskopf, L.; Feller, U. Heavy metals in white lupin: Uptake, root-to-shoot transfer and redistribution within the plant. New Phytol. 2006, 171, 329–341. [Google Scholar] [CrossRef]
- Page, V.; Feller, U. Selective transport of zinc, manganese, nickel, cobalt and cadmium in the root system and transfer to the leaves in young wheat plants. Ann. Bot. 2005, 96, 425–434. [Google Scholar] [CrossRef]
- Bhatia, N.P.; Walsh, K.B.; Baker, A.J.M. Detection and quantification of ligands involved in nickel detoxification in a herbaceous Ni hyperaccumulator Stackhousia tryonii Bailey. J. Exp. Bot. 2005, 56, 1343–1349. [Google Scholar] [CrossRef]
- Sagardoy, R.; Morales, F.; Rellen-Alvarez, R.; Abadia, A.; Abadia, J.; Lopez-Millan, A.F. Carboxylate metabolism in sugar beet plants grown with excess Zn. J. Plant Physiol. 2011, 168, 730–733. [Google Scholar] [CrossRef]
- Stolt, J.P.; Sneller, F.E.C.; Bryngellson, T.; Lundborg, T.; Schat, H. Phytochelatin and cadmium accumulation in wheat. Environ. Exp. Bot. 2003, 49, 21–28. [Google Scholar] [CrossRef]
- Mari, S.; Gendre, D.; Pianelli, K.; Ouerdane, L.; Lobinski, R.; Briat, J.-F. Root-to-shoot long-distance circulation of nicotianamine and nicotianamine-nickel chelates in the metal hyperaccumulator Thlaspi caerulescens. J. Exp. Bot. 2006, 57, 4111–4122. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P-1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef]
- Page, V.; Blösch, R.M.; Feller, U. Regulation of shoot growth, root development and manganese allocation in wheat (Triticum aestivum) genotypes by light intensity. Plant Growth Regul. 2012, 67, 209–215. [Google Scholar] [CrossRef]
- Herren, T.; Feller, U. Transfer of zinc from xylem to phloem in the peduncle of wheat. J. Plant Nutr. 1994, 17, 1587–1598. [Google Scholar] [CrossRef]
- Riesen, O.; Feller, U. Redistribution of nickel, cobalt, manganese, zinc and cadmium via the phloem in young and maturing wheat. J. Plant Nutr. 2005, 28, 421–430. [Google Scholar] [CrossRef]
- Stieger, P.A.; Feller, U. Nutrient accumulation and translocation in maturing wheat plants grown on waterlogged soil. Plant Soil 1994, 160, 87–95. [Google Scholar] [CrossRef]
- Van Bel, A.J.E. The phloem, a miracle of ingenuity. Plant Cell Environ. 2003, 26, 125–149. [Google Scholar] [CrossRef]
- Van Bel, A.J.E.; Gamalei, Y.V. Ecophysiology of phloem loading in source leaves. Plant Cell Environ. 1992, 15, 265–270. [Google Scholar]
- Turgeon, R.; Wolf, S. Phloem transport: Cellular pathways and molecular trafficking. Annu. Rev. Plant Biol. 2009, 60, 207–221. [Google Scholar] [CrossRef]
- Zeller, S.; Feller, U. Long-distance transport of cobalt and nickel in maturing wheat. Eur. J. Agron. 1999, 10, 91–98. [Google Scholar] [CrossRef]
- Stephan, U.W.; Scholz, G. Nicotianamine—Mediator of transport of iron in the phloem. Physiol. Plant. 1993, 88, 522–529. [Google Scholar] [CrossRef]
- Hazama, K.; Nagata, S.; Fujimori, T.; Yanagisawa, S.; Yoeneyama, T. Concentrations of metals and potential metal-binding compounds and speciation of Cd, Zn and Cu in phloem and xylem saps from castor bean plants (Ricinus communis) treated with four levels of cadmium. Physiol. Plant. 2015, 154, 243–255. [Google Scholar] [CrossRef]
- Mendoza-Cozatl, D.G.; Butko, E.; Springer, F.; Torpey, J.W.; Komives, E.A.; Kehr, J.; Schroeder, J.I. Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation. Plant J. 2008, 54, 249–259. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.Q.; Yamaji, N.; Yokosho, K.; Ma, J.F. YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice. Plant Cell 2012, 24, 3767–3782. [Google Scholar] [CrossRef]
- Herren, T.; Feller, U. Effect of locally increased zinc contents on zinc transport from the flag leaf lamina to the maturing grains of wheat. J. Plant Nutr. 1996, 19, 379–387. [Google Scholar] [CrossRef]
- Zeller, S.; Feller, U. Redistribution of cobalt and nickel in detached wheat shoots: effects of steam-girdling and of cobalt and nickel supply. Biol. Plant. 1998, 41, 427–434. [Google Scholar] [CrossRef]
- Herren, T.; Feller, U. Influence of increased zinc levels on phloem transport in wheat shoots. J. Plant Physiol. 1997, 150, 228–231. [Google Scholar]
- Fahn, A. Plant Anatomy, 3rd ed.; Pergamon Press: Oxford, UK, 1982; pp. 46–73. [Google Scholar]
- Van Be, A.J.E. Xylem-phloem exchange via the rays—The undervalued route of transport. J. Exp. Bot. 1990, 41, 631–644. [Google Scholar] [CrossRef]
- McNeil, D.L.; Atkins, C.A.; Pate, J.S. Uptake and utilization of xylem-borne amino-compounds by shoot organs of a legume. Plant Physiol. 1979, 63, 1076–1081. [Google Scholar] [CrossRef]
- Offler, C.E.; McCurdy, D.W.; Patrick, J.W.; Talbot, M.J. Transfer cells: Cells specialized for a special purpose. Annu. Rev. Plant Biol. 2003, 54, 431–454. [Google Scholar] [CrossRef]
- Feller, U.; Anders, I.; Wei, S. Effects of PEG-Induced Water Deficit in Solanum nigrum on Zn and Ni Uptake and Translocation in Split Root Systems. Plants 2015, 4, 284–297. [Google Scholar] [CrossRef]
- Graham, R.D.; Knez, M.; Welch, R.M. How much nutritional iron deficiency in humans globally is due to an underlying zinc deficiency? Adv. Agron. 2012, 115, 1–40. [Google Scholar] [CrossRef]
- Bouis, H.E.; Welch, R.M. Biofortification-A sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010, 50, S20–S32. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Agriculture: The real nexus for enhancing bioavailable micronutrients in food crops. J. Trace Elem. Med. Biol. 2005, 18, 299–307. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Khan, M.A.; Castro-Guerrero, N.; Medoza-Cozatl, D.G. Moving toward a precise nutrition: Preferential loading of seeds with essential nutrients over non-essential toxic elements. Front. Plant Sci. 2014, 5, 51. [Google Scholar] [CrossRef]
- Stasinos, S.; Nasopoulou, C.; Tsikrika, C.; Zabetakis, I. The bioaccumulation and physiological effects of heavy metals in carrots, onions, and potatoes and dietary implications for Cr and Ni: A review. J. Food Sci. 2014, 79, R765–R780. [Google Scholar] [CrossRef]
- Demirezen, D.; Aksoy, A. Heavy metal levels in vegetables in Turkey are within safe limits for Cu, Zn, Ni and exceeded for Cd and Pb. J. Food Qual. 2006, 29, 252–265. [Google Scholar] [CrossRef]
- Murtaza, G.; Ghafoor, A.; Qadir, M.; Owens, G.; Aziz, M.A.; Zia, M.H.; Saifullah. Disposal and use of sewage on agricultural lands in Pakistan: A review. Pedosphere 2010, 20, 23–34. [Google Scholar]
- Jamil, M.; Zia, M.S.; Qasim, M. Contamination of agro-ecosystem and human health hazards from wastewater used for irrigation. J. Chem. Soc. Pak. 2010, 32, 370–378. [Google Scholar]
- He, Z.L.L.; Yang, X.E.; Stoffella, P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef]
- Albering, H.J.; van Leusen, S.M.; Moonen, E.J.C.; Hoogewerff, J.A.; Kleinjans, J.C.S. Human health risk assessment: A case study involving heavy metal soil contamination after the flooding of the river Meuse during the winter of 1993–1994. Environ. Health Perspect. 1999, 107, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kramer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Memon, A.R.; Schroder, P. Implications of metal accumulation mechanisms to phytoremediation. Environ. Sci. Pollut. Res. 2009, 16, 162–175. [Google Scholar] [CrossRef]
- Salt, D.E.; Smith, R.D.; Raskin, I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Wei, S.; Anders, I.; Feller, U. Selective uptake, distribution, and redistribution of Cd-109, Co-57, Zn-65, Ni-63, and Cs-134 via xylem and phloem in the heavy metal hyperaccumulator Solanum nigrum L. Environ. Sci. Pollut. Res. 2014, 21, 7624–7630. [Google Scholar] [CrossRef]
- Kupper, H.; Lombi, E.; Zhao, F.J.; McGrath, S.P. Cellular compartmentation of cadmium and zinc in relation to other elements in the hyperaccumulator Arabidopsis halleri. Planta 2000, 212, 75–84. [Google Scholar] [CrossRef]
- Kramer, U.; Chardonnens, A.N. The use of transgenic plants in the bioremediation of soils contaminated with trace elements. Appl. Microbiol. Biotechnol. 2001, 55, 661–672. [Google Scholar]
- Ferraz, P.; Fidalgo, F.; Almeida, A.; Teixeira, J. Phytostabilization of nickel by the zinc and cadmium hyperaccumulator Solanum nigrum L. Are metallothioneins involved? Plant Physiol. Biochem. 2012, 57, 254–260. [Google Scholar] [CrossRef]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef]
- Pence, N.S.; Larsen, P.B.; Ebbs, S.D.; Letham, D.L.D.; Lasat, M.M.; Garvin, D.F.; Eide, D.; Kochian, L.V. The molecular physiology of heavy metal transport in the Zn/Cd hyperaccumulator Thlaspi caerulescens. Proc. Natl. Acad. Sci. USA 2000, 97, 4956–4960. [Google Scholar] [CrossRef]
- Yamaji, N.; Xia, J.X.; Mitani-Ueno, N.; Yokosho, K.; Ma, J.F. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ita, R.N.; Nishizawa, N.K. Iron deficiency responses in rice roots. Rice 2014, 7, 27. [Google Scholar] [CrossRef]
- Colangelo, E.P.; Guerinot, M.L. Put the metal to the petal: Metal uptake and transport throughout plants. Curr. Opin. Plant Biol. 2006, 9, 322–330. [Google Scholar] [CrossRef]
- Feller, U.; Vaseva, I.I. Extreme climatic events: Impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2014, 2, 39. [Google Scholar] [CrossRef]
- Sharma, R.K.; Agrawal, M. Biological effects of heavy metals: An overview. J. Environ. Biol. 2005, 26, 301–313. [Google Scholar]
- Welch, R.M.; Graham, R.D. Breeding crops for enhanced micronutrient content. Plant Soil 2002, 245, 205–214. [Google Scholar] [CrossRef]
- Yang, X.; Feng, Y.; He, Z.L.; Stoffella, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 339–353. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Page, V.; Feller, U. Heavy Metals in Crop Plants: Transport and Redistribution Processes on the Whole Plant Level. Agronomy 2015, 5, 447-463. https://doi.org/10.3390/agronomy5030447
Page V, Feller U. Heavy Metals in Crop Plants: Transport and Redistribution Processes on the Whole Plant Level. Agronomy. 2015; 5(3):447-463. https://doi.org/10.3390/agronomy5030447
Chicago/Turabian StylePage, Valérie, and Urs Feller. 2015. "Heavy Metals in Crop Plants: Transport and Redistribution Processes on the Whole Plant Level" Agronomy 5, no. 3: 447-463. https://doi.org/10.3390/agronomy5030447