Ethyl 7-Acetyl-8a-methyl-3-(1-phenyl-1H-tetrazol-5-yl)-1,4,4a,5,6,8a-hexahydro-7H-pyrano[2,3-c]pyridazine-1-carboxylate
Abstract
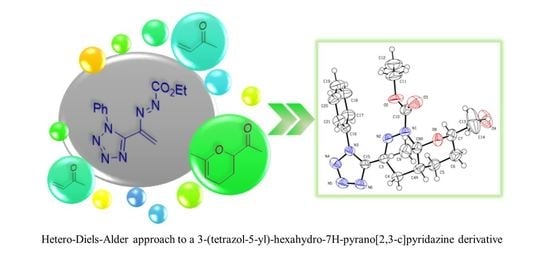
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Ethyl 7-Acetyl-8a-methyl-3-(1-phenyl-1H-tetrazol-5-yl)-1,4,4a,5,6,8a-hexahydro-7H-pyrano[2,3-c]pyridazine-1-carboxylate 7
3.3. Crystallographic Data for Ethyl 7-Acetyl-8a-methyl-3-(1-phenyl-1H-tetrazol-5-yl)-1,4,4a,5,6,8a-hexahydro-7H-pyrano[2,3-c]pyridazine-1-carboxylate 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopes, S.M.M.; Cardoso, A.L.; Lemos, A.; Pinho e Melo, T.M.V.D. Recent Advances in the Chemistry of Conjugated Nitrosoalkenes and Azoalkenes. Chem. Rev. 2018, 118, 11324–11352. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.M.M.; Lemos, A.; Pinho e Melo, T.M.V.D. A hetero-Diels-Alder approach to functionalized 1H-tetrazoles: Synthesis of tetrazolyl-1,2-oxazines, -oximes and 5-(1-aminoalkyl)-1H-tetrazoles. Tetrahedron Lett. 2010, 51, 6756–6759. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Palacios, F.; Lemos, A.; Pinho e Melo, T.M.V.D. Diels-Alder reactions of 3-(1H-tetrazol-5-yl)-nitrosoalkenes: Synthesis of functionalized 5-(substituted)-1H-tetrazoles. Tetrahedron 2011, 67, 8902–8909. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Brigas, A.F.; Palacios, F.; Lemos, A.; Pinho e Melo, T.M.V.D. [4+2] Cycloadditions of 3-Tetrazolyl-1,2-diaza-1,3-butadienes: Synthesis of 3-Tetrazolyl-1,4,5,6-tetrahydropyridazines. Eur. J. Org. Chem. 2012, 2152–2160. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Lemos, A.; Pinho e Melo, T.M.V.D. Reactivity of Dipyrromethanes towards Azoalkenes: Synthesis of Functionalized Dipyrromethanes, Calix[4] pyrroles, and Bilanes. Eur. J. Org. Chem. 2014, 7039–7048. [Google Scholar] [CrossRef]
- Nunes, S.C.C.; Lopes, S.M.M.; Gomes, C.S.B.; Lemos, A.; Pais, A.; Pinho e Melo, T.M.V.D. Reactions of Nitrosoalkenes with Dipyrromethanes and Pyrroles: Insight into the Mechanistic Pathway. J. Org. Chem. 2014, 79, 10456–10465. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Henriques, M.S.C.; Paixao, J.A.; Pinho e Melo, T.M.V.D. Exploring the Chemistry of Furans: Synthesis of Functionalized Bis(furan-2-yl)-methanes and 1,6-Dihydropyridazines. Eur. J. Org. Chem. 2015, 6146–6151. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Nunes, S.C.C.; Carato, C.C.; Pais, A.A.C.C.; Pinho e Melo, T.M.V.D. Reactivity of 1-arylnitrosoethylenes towards indole derivatives. Monatsh. Chem. 2016, 147, 1565–1573. [Google Scholar] [CrossRef]
- Alves, A.J.S.; Lopes, S.M.M.; Henriques, M.S.C.; Paixão, J.A.; Pinho e Melo, T.M.V.D. Hetero-Diels–Alder and Ring-Opening Reactions of Furans Applied to the Synthesis of Functionalized Heterocycles. Eur. J. Org. Chem. 2017, 4011–4025. [Google Scholar] [CrossRef]
- Jorda, R.; Lopes, S.M.M.; Reznickova, E.; Krystof, V.; Pinho e Melo, T.M.V.D. Biological Evaluation of Dipyrromethanes in Cancer Cell Lines: Antiproliferative and Pro-apoptotic Properties. Chem. Med. Chem. 2017, 12, 701–711. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Novais, J.S.; Costa, D.C.S.; Castro, H.C.; Figueiredo, A.M.S.; Ferreira, V.F.; Pinho e Melo, T.M.V.D.; da Silva, F.D. Hetero-Diels-Alder reactions of novel 3-triazolyl-nitrosoalkenes as an approach to functionalized 1,2,3-triazoles with antibacterial profile. Eur. J. Med. Chem. 2018, 143, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.A.M.; Lopes, S.M.M.; Lemos, A.; Pinho e Melo, T.M.V.D. On-Water Synthesis of Dipyrromethanes via Bis-Hetero-Diels-Alder Reaction of Azo- and Nitrosoalkenes with Pyrrole. Synlett 2014, 25, 423–427. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Lopes, S.M.M.; Grosso, C.; Pineiro, M.; Lemos, A.; Pinho e Melo, T.M.V.D. One-Pot Synthetic Approach to Dipyrromethanes and Bis(indolyl)methanes via Nitrosoalkene Chemistry. J. Chem. Educ. 2021, 98, 2661–2666. [Google Scholar] [CrossRef]
- Grosso, C.; Cardoso, A.L.; Lemos, A.; Varela, J.; Rodrigues, M.J.; Custódio, L.; Barreira, L.; Pinho e Melo, T.M.V.D. Novel approach to bis(indolyl)methanes: De novo synthesis of 1-hydroxyiminomethyl derivatives with anti-cancer properties. Eur. J. Med. Chem. 2015, 93, 9–15. [Google Scholar] [CrossRef]
- Grosso, C.; Cardoso, A.L.; Rodrigues, M.J.; Marques, C.; Barreira, L.; Lemos, A.; Pinho e Melo, T.M.D.V. Hetero-Diels-Alder approach to Bis(indolyl)methanes. Bioorg. Med. Chem. 2017, 25, 1122–1131. [Google Scholar] [CrossRef]
- Grosso, C.; Brigas, A.; de los Santos, J.M.; Palacios, F.; Lemos, A.; Pinho e Melo, T.M.V.D. Natural deep eutectic solvents in the hetero-Diels–Alder approach to bis(indolyl)methanes. Monatsh. Chem. 2019, 150, 1275–1288. [Google Scholar] [CrossRef]
- Grosso, C.; Lemos, A.; Pinho e Melo, T.M.V.D. Conjugate Addition of Pyrazoles to Halogenated Nitroso- and Azoalkenes: A New Entry to Novel Bis(pyrazol-1-yl)methanes. Synlett 2014, 25, 2868–2872. [Google Scholar] [CrossRef]
- Lopes, S.M.M.; Pinho e Melo, T.M.V.D. Meso-Substituted Corroles from Nitrosoalkenes and Dipyrromethanes. J. Org. Chem. 2020, 85, 3328–3335. [Google Scholar] [CrossRef]
- Mittal, R.; Awasthi, S.K. Recent Advances in the Synthesis of 5-Substituted 1H -Tetrazoles: A Complete Survey (2013–2018). Synthesis 2019, 51, 3765–3783. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Ramos, S.; Cardoso-Ortiz, J. Recent Developments in the Synthesis of Tetrazoles and their Pharmacological Relevance. Curr. Org. Chem. 2021, 25, 388–403. [Google Scholar] [CrossRef]
- Panice, M.R.; Lopes, S.M.M.; Figueiredo, M.C.; Goes Ruiz, A.L.T.; Foglio, M.A.; Nazari Formagio, A.S.; Sarragiotto, M.H.; Pinho e Melo, T.M.V.D. New 3-tetrazolyl-β-carbolines and β-carboline-3-carboxylates with anti-cancer activity. Eur. J. Med. Chem. 2019, 179, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Eggenweiler, H.-M.; Wolf, M.; Beier, N.; Leibrock, J.; Gassen, M.; Ehring, T. New 1-benzoyl-3-phenyl-tetrahydropyridazine Derivatives, Useful for Treating e.g. Allergy, Are Selective Inhibitors of Phosphodiesterase IV, and New Intermediates. German Patent DE20001064997, 23 December 2000. [Google Scholar]
- Leonhardt, S.A.; Edwards, D.P. Mechanism of Action of Progesterone Antagonists. Exp. Biol. Med. 2002, 227, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.; Campen, C.A.; Allan, G.F.; Rybczynski, P.; Haynes-Johnson, D.; Hutchins, A.; Kraft, P.; Kiddoe, M.; Lai, M.-T.; Lombardi, E.; et al. Nonsteroidal progesterone receptor ligands with unprecedented receptor selectivity. J. Steroid Biochem. Mol. Biol. 2000, 75, 33–42. [Google Scholar] [CrossRef]
- Lange, J.H.M.; den Hartog, A.P.; van der Neut, M.A.W.; van Vliet, B.J.; Kruse, C.G. Synthesis and SAR of 1,4,5,6-tetrahydropyridazines as potent cannabinoid CB1 receptor antagonists. Bioorg. Med. Chem. Lett. 2009, 19, 5675–5678. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.-G. Introduction of Bicyclic Ketal Chemistry: Synthesis and Transformation Reaction of 6,8-Dioxabicyclo[3.2.1]octane Skeletal System. Synlett 2003, 1759–1777. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, S.M.M.; Lemos, A.; Paixão, J.A.; Pinho e Melo, T.M.V.D. Ethyl 7-Acetyl-8a-methyl-3-(1-phenyl-1H-tetrazol-5-yl)-1,4,4a,5,6,8a-hexahydro-7H-pyrano[2,3-c]pyridazine-1-carboxylate. Molbank 2022, 2022, M1338. https://doi.org/10.3390/M1338
Lopes SMM, Lemos A, Paixão JA, Pinho e Melo TMVD. Ethyl 7-Acetyl-8a-methyl-3-(1-phenyl-1H-tetrazol-5-yl)-1,4,4a,5,6,8a-hexahydro-7H-pyrano[2,3-c]pyridazine-1-carboxylate. Molbank. 2022; 2022(1):M1338. https://doi.org/10.3390/M1338
Chicago/Turabian StyleLopes, Susana M. M., Américo Lemos, José A. Paixão, and Teresa M. V. D. Pinho e Melo. 2022. "Ethyl 7-Acetyl-8a-methyl-3-(1-phenyl-1H-tetrazol-5-yl)-1,4,4a,5,6,8a-hexahydro-7H-pyrano[2,3-c]pyridazine-1-carboxylate" Molbank 2022, no. 1: M1338. https://doi.org/10.3390/M1338