European Society of Organ Transplantation (ESOT) Consensus Statement on Prehabilitation for Solid Organ Transplantation Candidates
- 1Section of Nursing Science, Department of Health Sciences, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 2Group Rehabilitation for Internal Disorders, Department of Rehabilitation Sciences, KU Leuven, Leuven, Belgium
- 3Nephrology and Renal Transplantation, Department of Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Belgium
- 4Laboratory of Abdominal Transplantation, Department of Microbiology, Immunology and Transplantation, KU Leuven, Leuven, Belgium
- 5Physiotherapy, Department of Health Sciences, College of Health, Medicine and Life Sciences, Brunel University London, London, United Kingdom
- 6Department of Endocrinology, University Hospitals Leuven, Leuven, Belgium
- 7Section of Hepatobiliary Surgery & Liver Transplantation, Department of Surgery, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 8Respiratory Epidemiology and Clinical Research Unit, Centre for Outcomes Research and Evaluation, Research Institute of McGill University Health Centre, Montreal, QC, Canada
- 9School of Physical and Occupational Therapy, McGill University, Montreal, QC, Canada
- 10School of Rehabilitation Therapy, Queen’s University, Kingston, ON, Canada
- 11Laboratory of Sports Medicine, Department of Physical Education and Sports Science, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 12Kidney Transplant Program, Hospital del Mar, Barcelona, Spain
- 13Clinical and Experimental Endocrinology, Department of Chronic Diseases and Metabolism, KU Leuven, Leuven, Belgium
- 14Department of Public Health and Primary Care, Academic Centre for Nursing and Midwifery, KU Leuven, Leuven, Belgium
- 15Associazione Italiana Ipertensione Polmonare (AIPI), Bologna, Italy
- 16Department of Nephrology, Transplantology and Internal Medicine, Medical University of Gdańsk, Gdańsk, Poland
- 17Liver Transplant Center, University Hospital Center Zagreb, School of Medicine, University of Zagreb, Zagreb, Croatia
- 18Diabetes Center, Institute for Clinical and Experimental Medicine, Prague, Czechia
- 19Section of Lung Transplantation, Department of Cardiology, Rigshospitalet, Copenhagen, Denmark
- 20Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
- 21Department of General, Visceral and Transplant Surgery, Hospital Clínico y Universitario Virgen de La Arrixaca, Murcia, Spain
- 22Representative of the European Kidney Patients’ Federation, Dublin, Ireland
- 23Ukrainian Transplant Coordination Center, Specialized State Institution, Kiev, Ukraine
- 24Renal Medicine and Therapies, King’s College Hospital NHS Trust, London, United Kingdom
- 25Centre for Nephrology, Urology and Transplantation, Faculty of Life Sciences and Medicine, King’s College London, London, United Kingdom
- 26Transplantoux Foundation, Leuven, Belgium
There is increasingly growing evidence and awareness that prehabilitation in waitlisted solid organ transplant candidates may benefit clinical transplant outcomes and improve the patient’s overall health and quality of life. Lifestyle changes, consisting of physical training, dietary management, and psychosocial interventions, aim to optimize the patient’s physical and mental health before undergoing surgery, so as to enhance their ability to overcome procedure-associated stress, reduce complications, and accelerate post-operative recovery. Clinical data are promising but few, and evidence-based recommendations are scarce. To address the need for clinical guidelines, The European Society of Organ Transplantation (ESOT) convened a dedicated Working Group “Prehabilitation in Solid Organ Transplant Candidates,” comprising experts in physical exercise, nutrition and psychosocial interventions, to review the literature on prehabilitation in this population, and develop recommendations. These were discussed and voted upon during the Consensus Conference in Prague, 13–15 November 2022. A high degree of consensus existed amongst all stakeholders including transplant recipients and their representatives. Ten recommendations were formulated that are a balanced representation of current published evidence and real-world practice. The findings and recommendations of the Working Group on Prehabilitation for solid organ transplant candidates are presented in this article.
Introduction
Patients who need a solid organ transplant often have a compromised overall condition due to end-stage organ failure, comorbidities, deconditioning, and treatment-related adverse effects such as dialysis in end-stage kidney disease (ESKD), left ventricular assist device (LVAD) in heart failure, and oxygen therapy in end-stage pulmonary disease (ESPD) [1–3]. Although considered a frail patient population with malnutrition, low physical fitness, fatigue, and often secondary psychological challenges, it is imperative for such patients to attain, and maintain, their optimal physical and mental wellbeing, as this will help them tolerate the waiting time and the stress of transplant surgery and expedite recovery after the transplant. The time spent on the transplant waitlist provides a window of opportunity to work towards enhancing the overall condition of such patients.
Prehabilitation refers to the optimization of patient’s overall physical and psychological condition before undergoing surgery, in order to enhance his/hers ability to overcome the stress associated with the procedure, to reduce the risk of complications and to accelerate post-operative recovery, with the ultimate goal to improve survival and quality of life [4]. The approach focuses on achieving lifestyle changes and should consist of physical training, dietary management, and psychological interventions [4]. By providing a multimodal program, the complex interaction between the physical and psychological health of a patient is addressed, which is important to maximize the outcomes of the interventions [5].
Prehabilitation has shown promising results in non-transplant patients undergoing major abdominal or orthopedic procedures [6–10], with reduced overall post-operative complications and morbidity, improved aerobic capacity, and improved functional recovery and shorter length of stay. The conclusions from two systematic reviews supported the feasibility and safety of such interventions in waitlisted solid organ transplant candidates [11, 12]. In addition, observed beneficial effects included improvements in cardiorespiratory function, exercise capacity, muscular strength, mental/physical composite scores and health-related quality of life [11, 12]. There is a growing awareness and evidence that prehabilitation may not only benefit clinical transplant outcomes, but may also improve the transplant candidate’s overall health and quality of life, through adoption of a sustainable, healthy lifestyle. Despite this growing awareness and promising data, evidence-based recommendations for physical exercise, nutritional, or psychological prehabilitation interventions in candidates for solid organ transplants are not available. With regard to exercise interventions, recommendations on the role of exercise in solid organ transplantation were made by Janaudis-Ferreira et al in a position statement paper in 2019 [13].
The limited clinical guidance on how to implement prehabilitation for solid organ transplant candidates was presented as one of the priority themes at the first European Society of Organ Transplantation (ESOT) consensus conference in November 2022. Under the oversight of the ESOT guideline taskforce, and in keeping with the procedures recently established by the ESOT Consensus Platform for Organ Transplantation, leading experts presented in-depth literature evidence and proposed recommendations, which were publicly discussed and assessed by an independent jury, and consensus was formed [14]. Participants in the consensus process included not only transplant, prehabilitation and medical specialists, but also allied health professionals, patients and patient representatives.
This document presents the 2022 ESOT consensus findings and recommendations on implementing prehabilitation in the care for solid organ transplant candidates. These guidelines and recommendations undergo continual review and will be updated to reflect new evidence as it becomes available.
Methods
The consensus development process was governed by the dedicated ESOT Guidelines Taskforce and co-organized by the ESOT sections European Liver and Intestine Transplant Association, European Kidney Transplant Association, European Pancreas and Islet Transplant Association, European Cardio Thoracic Transplant Association, European Transplant Allied Healthcare Professionals, the ESOT Education Committee and Young Professionals in Transplantation.
The consensus development process followed the methodology stipulated by the ESOT Consensus Platform as recently published in detail [14]. In brief, the subsequent steps were as follows:
i) Prehabilitation for solid organ transplant candidates was selected as a priority topic for the first ESOT Consensus Conference, as published [14].
ii) A specific steering committee was selected, consisting of experts in the topic field, members from the Centre for Evidence in Transplantation, a Young Professional in Transplantation representative, and a guideline taskforce member to liaise with ESOT.
iii) The steering committee identified key relevant questions related to prehabilitation of solid organ transplant candidates (heart, lung, liver, kidney) using to the Population, Intervention, Comparator and Outcome (PICO) methodology [15] (Table 1).
iv) The staff of the Centre for Evidence in Transplantation performed systematic literature reviews that were informed by the PICO questions and thus related to exercise, nutritional and psychological interventions in solid organ transplant candidates. The search strategy is presented in Supplementary Table S1. The PRISMA diagrams from the evidence review are shown in Figures 1–3. As the number of publications was expected to be limited, selection was not limited to randomized clinical trials but also included studies that used a pre/post or case-control design, prospective and retrospective studies (cohorts or registry), feasibility studies and pilot studies. Reviews and meta-analyses were included for hand searching of bibliographies for additional literature. Studies were included only if a minimum of 80% of study participants were formally waitlisted for a solid organ transplant. Case reports on fewer than 10 patients, conference abstracts, and letters to the editor were excluded, as was non-English literature. The literature evidence relating to the PICO questions was summarized, as shown in Tables 2–4 and Supplementary File S1.
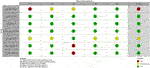
v) The steering committee integrated the literature evidence and formulated recommendations (Supplementary File S1). When proposing recommendations for each question, the quality of evidence was considered as evaluated by the GRADE approach [16]. This included risk of bias (Figures 4–6), which was assessed by two independent reviewers, and an additional third one if disagreement occurred. The strength of the individual recommendations was rated as strong or weak.
vi) Jury members, who were not part of the steering committee were selected and vetted by the guideline taskforce and were comprised of allied health professionals, patients (representatives), transplant physicians, and transplant surgeons.
vii) Consensus was generated using discussion within the entire working group and modified Delphi methodology including consensus polling, followed by jury voting of the recommendations during a session at the ESOT Consensus conference in Prague [17].
viii) A committee of validating experts validated the recommendations using the AGREE II guidelines [18].
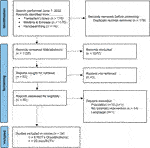
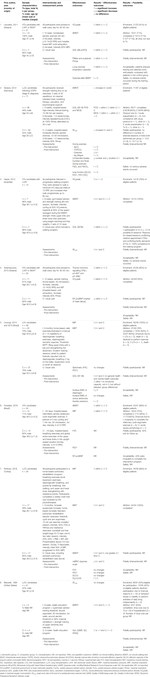
FIGURE 1. Flow chart of study selection process exercise interventions with reasons for exclusion. Note: n = number of studies. Figure adapted from: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffman TC, Mulrow CD, et al. The PRIMSA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71, doi:10.1136/bmj.n71. For more information visit http://www.prisma-statement.org/.
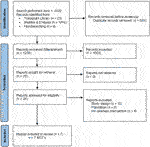
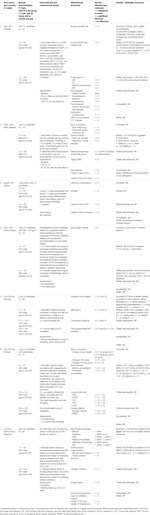
FIGURE 2. Flow chart of study selection process nutritional interventions with reasons for exclusion.
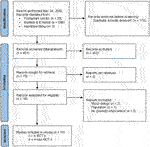
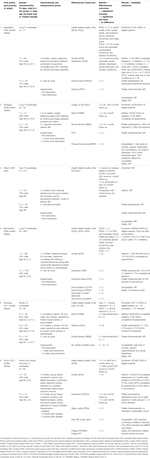
FIGURE 3. Flow chart of study selection process psychosocial interventions with reasons for exclusion.
Results
A total of 4 PICO questions were identified, along with key criteria for analysis, as presented in Table 1. The systematic review of literature yielded 34 studies on exercise, 7 on nutritional, and 10 on psychological interventions (Figures 1–3). Summaries of the literature evidence were generated and are presented in Tables 2–4 and Supplementary File S1. A total of 26 recommendations were formulated (Supplementary File S2). At the Consensus Conference, the literature summaries and recommendations were presented, discussed, and amended according to the ESOT consensus-finding process. In response to the considerations voiced during the discussion, and in an attempt to avoid overlap, the number and nature of the recommendations was revised to 10 well-defined recommendations, i.e., 4 general and 6 specific ones. In a first voting round, 100% agreement was achieved on 7 out of 10 recommendations, whereas 3 recommendations reached 86% agreement (1.2, 2.4, 2.5) due to being considered as too exclusive of certain patient groups. Consensus was reached to amend these recommendations to be more inclusive, and in a second voting round, 100% agreement was achieved on all 10 recommendations.
Recommendations
PICO Question 1
In adult candidates for lung, liver, kidney, & heart transplantation: what is the evidence for the effectiveness of pre-transplant exercise training, nutritional support and psychosocial interventions as measured by prehabilitation efficacy outcomes, clinical outcomes and patient-reported outcomes.
To date, multimodal prehabilitation programs that offer a combination of exercise, nutritional, and psychosocial interventions, have not been studied in solid organ transplant candidates. Rather, literature is limited to studies investigating a single type of intervention. Based on the committee’s literature review and analysis of the predefined criteria prehabilitation effectiveness, clinical and patient-reported outcomes, one general recommendation and two specific recommendations were made.
Recommendation 1.1
Studies are needed that evaluate multi-modal prehabilitation interventions in candidates for all types of solid organ transplantation and that focus on core outcomes and implementation. Such studies should be of high quality, and preferably–but not exclusively–adequately powered RCTs.
Quality of Evidence: not applicable.
Strength of Recommendation: Strong.
Rationale: Although supportive, the current evidence (Table 2–4) based on the effectiveness of pre-transplant exercise, nutritional, and psychosocial interventions is weak because of the limited number of randomized studies; 8 for exercise interventions [19–25], 7 for nutritional interventions [26–32], and 6 for psychosocial interventions [33–38]. In addition, 25 non-randomized studies regarding exercise [39–64], and 4 non-randomized studies on psychosocial interventions [65–68] were retrieved by literature review (Supplementary File S1). The small sample size per study and the limited size and heterogeneity of the total populations studied, the variability in interventions and outcomes measures, the generally low-to-moderate quality of the methodology, and–as a result–the inconsistency of findings across studies (Tables 2–4), warrants high-quality studies on multimodal prehabilitation before solid organ transplantation.
Recommendation 1.2
It is suggested that exercise-based interventions are included in the prehabilitation care of solid organ transplant candidates, with the objective to improve cardiorespiratory fitness and/or inspiratory muscle strength.
Quality of Evidence: Low.
Strength of Recommendation: Weak.
Rationale: Although the number and size of RCTs is limited, studies have shown that exercise training was associated with clinically meaningful improvement in cardiorespiratory fitness in heart transplant candidates [19, 21, 22, 24] and a clinically meaningful gain in inspiratory muscle strength in heart and in liver transplant candidates [19, 23, 24].
Recommendation 1.3
It is suggested that probiotic therapy be used in candidates for liver transplantation to reduce their susceptibility to post-transplant infections.
Quality of Evidence: Very low.
Strength of Recommendation: Weak.
Rationale: Two studies were identified in which pre-transplant probiotic and symbiotic therapy were associated with reduced post-transplant infection rates in recipients of a liver transplant [26, 28]. However, both studies had small sample sizes (n = 44/n = 50) and used different products.
PICO Question 2
In adult candidates for lung, liver, kidney, & heart transplantation: which type(s) of exercise, nutritional support and psychosocial interventions are recommended in the pre-transplant phase?
As there are no established prehabilitation programs for solid organ transplant candidates, evidence review was focused on studies that addressed interventions that could be of value in a multimodal prehabilitation program. One general recommendation and four specific recommendations were established.
Recommendation 2.1
Studies are needed to identify the optimal component(s) and the mode of delivery of pre-transplant multimodal prehabilitation programs in solid organ transplant candidates. Such studies should be of high quality and be preferably -but not exclusively-adequately powered RCTs.
Quality of Evidence: not applicable.
Strength of Recommendation: Strong.
Rationale: Because of the heterogeneity in the study populations and in the nature and delivery mode of the interventions described in the current literature (Tables 2–4; Supplementary File S1), it remains unclear which organ transplant candidates would benefit most from which intervention program. Most exercise intervention studies used aerobic training [19–22, 24], peripheral muscle training [41], inspiratory muscle strength training [25, 51], or a combination of these training modalities. Nutrition intervention studies mostly used nutritional support to optimize energy intake and/or obtain weight loss [29–32, 69, 70]. Whilst psychosocial interventions predominantly included cognitive behavioral therapy [33, 34, 36, 37, 65, 67], psycho-educational interventions [35, 68] and stress management and relaxation techniques [38, 67] or a combination of these interventions. Studies are needed that will help determine the modalities of the intervention, and for each modality (exercise, nutrition or psychosocial), the intervention characteristics (frequency, intensity and timing), the delivery mode (type of interventionist, level of supervision, home-based versus in- or outpatient) for each type of donor organ recipient.
Recommendation 2.2
Solid organ transplant candidates who are underweight may be offered nutritional interventions with the aim to achieve optimal target weight before the transplant.
Quality of Evidence: Very low.
Strength of Recommendation: Weak.
Rationale: Evidence from two intervention studies in lung transplant candidates [29, 30] have indicated that increased caloric intake before transplantation may allow solid organ transplant candidates, especially those who are underweight, to reach a pre-transplant target weight. However, these studies had a small sample size and were conducted in different settings (hospital vs. outpatient clinic).
Recommendation 2.3
Solid organ transplant candidates who are overweight may be offered nutritional interventions with the aim to achieve optimal target weight before the transplant.
Quality of Evidence: Very low.
Strength of Recommendation: Weak.
Rationale: One study (n = 43) [32] showed that a weight-loss program, consisting of bibliotherapy and voice call counselling by a dietician, was successful in reducing body weight in adult candidates for heart transplantation.
Recommendation 2.4
It is suggested that cognitive behavioral therapy and psychoeducational interventions are considered for solid organ transplant candidates who have symptoms of anxiety and/or depression.
Quality of Evidence: Very low.
Strength of Recommendation: Weak.
Rationale: Six studies utilized elements of cognitive behavioral therapy (CBT) and psychoeducational interventions [33, 34, 36, 37, 65, 67] of which five reported a significant decrease in symptoms of anxiety and depression or mood [33, 34, 36, 37, 65] in lung, liver, and kidney transplant candidates. However, studies differed regarding duration (8–12 weeks), modality (group vs. individual; remote vs. in person), and most studies had small sample sizes (n = 29 to n = 71) (Table 4). Only the study of Blumenthal et al (2006)) [36] had an adequate sample size (n = 328).
Recommendation 2.5
It is suggested to consider stress-reducing interventions such as mindfulness-based stress reduction or relaxation techniques in candidates for solid organ transplantation to reduce anxiety or stress levels.
Quality of Evidence: Very low.
Strength of Recommendation: Weak.
Rationale: In two studies among kidney and kidney-pancreas transplant candidates, stress-reducing interventions were associated with alleviated symptoms of anxiety [65] or depression [38, 65] directly after the intervention. However, this effect was not maintained long-term. In addition, sample sizes were small (n = 41/n = 63) and the intervention differed regarding content and interventionist.
PICO Question 3
In adult candidates for lung, liver, kidney, & heart transplantation: what are the outcomes relevant to exercise and physical activity, nutritional support and psychosocial interventions that should be measured pre-transplant?
In order to reliably assess the effects of prehabilitation interventions, it is imperative to standardize outcome measures, their definitions, and the tools to measure them. Literature was reviewed with respect to the outcomes evaluated as well as the tools to measure them. One general recommendation was formulated.
Recommendation 3.1
It is strongly recommended that a core outcome measurement set is defined for future multimodal prehabilitation studies in solid organ transplant candidates.
Quality of Evidence: not applicable.
Strength of Recommendation: Strong.
Rationale: The studies retrieved during this review varied widely with respect to the clinical and patient-reported outcomes that were utilized, and the methods to assess them (Tables 2–4). Most exercise intervention studies included cardiorespiratory fitness including peak or maximal oxygen consumption (VO2peak) and/or six-minute walking distance (6MWD)], Health-related Quality of Life (HRQoL), dyspnea, or maximal inspiratory pressures outcome measures. Nutritional intervention studies mostly monitored weight changes, infection rates, body composition and survival as either primary or secondary outcomes. The outcomes in studies that used psychosocial interventions included mostly HRQoL as well as parameters of mood, social intimacy and coping, while the use of clinical outcomes was rare. All stakeholders including solid organ transplant candidates and recipients, transplant professionals, and researchers in the field of transplantation strongly supported that a core outcome set be defined to facilitate comparative studies and give impetus to the field. A core outcomes set refers to a minimum set of outcome measures that are critical to patients, caregivers, and health professionals for decision making [71]. Selected outcomes that have so far not been considered but do carry clinical relevance during the pre-transplant waiting time are health-related physical fitness parameters such as muscular fitness, motor fitness, body composition and (cardio)metabolic health, as well as patient-reported outcomes such as fatigue, medication adherence and lifestyle, and clinical outcomes such as duration of intensive care stay, hospitalization, (re-)admissions, complications, graft function and survival, and waitlist and post-transplant mortality.
PICO Question 4
In adult candidates for lung, liver, kidney, & heart transplantation: what is the evidence for the feasibility (enrolment, retention, acceptability, fidelity, safety) of prehabilitation?
Implementation of prehabilitation in clinical practice of solid organ transplantation should be supported by evidence of feasibility. Two systematic reviews have previously concluded that exercise prehabilitation is feasible and safe for solid organ transplant candidates [11, 12]. The review by Wallen et al was performed with focus on the feasibility outcomes enrolment, retention, acceptability, fidelity and safety [11]. One general recommendation was made.
Recommendation 4.1
It is strongly recommended that future studies on multimodal prehabilitation in solid organ transplant candidates include the specific assessment of feasibility.
Quality of Evidence: Moderate.
Strength of Recommendation: Strong.
Rationale: One study was identified that was specifically designed to assess the feasibility of delivering a psychosocial prehabilitation in solid organ transplant candidates. This study showed that a stress management and relaxation training program in liver transplant candidates was efficiently deliverable and considered acceptable and tolerable by the patients [67]. However, the enrolment rate was low, (29%) and the attrition rate was moderate (68%). Amongst the remainder of the literature, most studies reported on some aspect(s) of feasibility as a secondary outcome, mainly regarding enrolment and attrition (Tables 2–4). The feasibility measures fidelity of participants and/or interventionist and safety were less reported (Tables 2–4). For the exercise intervention studies, the enrolment rate was approximately 86%, while the average attrition rate ranged between 71% and 100% [19–25]. However, drop-outs were often due to transplant surgery. In the studies on nutritional interventions, feasibility measures were poorly reported. If reported, the enrolment rate was found to be low to moderate [26, 31, 32]. Attrition rates ranged between 62% and 98% [26, 27, 29–32]. In the psychosocial intervention studies, enrolment rates ranged between 24% and 59%, attrition rates between 69% and 88%, and acceptability of the intervention was high [33, 34, 36–38]. Only two studies reported the occurrence of adverse events [27, 65], but no serious adverse events occurred.
Overall, the consensus was that these studies do support the notion that it is feasible, acceptable and safe for adults to participate in exercise, nutritional, and psychosocial interventions during the waiting-list period (Tables 2–4). Although enrolment in studies differed significantly across studies, the overall willingness to participate in studies was found to be good and the attrition rates are adequate, and few adverse events are reported. Fidelity of participants as well as the interventionist and acceptability of the intervention are less reported. Nonetheless, implementation of prehabilitation in a clinical practice has not been established so far. Future dedicated studies should focus on the feasibility of implementation in clinical practice by assessing factors related to potential implementation strategy effects (e.g., adoption, fidelity, reach, sustainability) and factors to inform the design or development of the implementation strategy (e.g., acceptability, adaptability, feasibility, compatibility, complexity, self-efficacy, context, costs) [72].
Discussion and Future Perspectives
The newly established ESOT consensus platform has proven successful in supporting the development of evidence-based consensus recommendations for prehabilitation in candidates for solid organ transplantation. Ten recommendations were formulated for which full consensus was reached within two voting rounds. This indicated that a high degree of consensus existed amongst all stakeholders from the prehabilitation, rehabilitation and transplantation fields, including transplant recipients and their representatives, and that the recommendations are a balanced representation of current published evidence and expert opinion.
Published evidence on prehabilitation before solid organ transplantation was found to be limited and consisted of studies addressing unimodal prehabilitation interventions with heterogeneous design, methodology and relatively small sample sizes. Nevertheless, by consensus and expert opinion, the available evidence on effects of prehabilitation on physical functioning, nutritional status, and psychosocial wellbeing and the evidence on safety of prehabilitation interventions was felt sufficiently strong to recommend that multimodal, patient-tailored prehabilitation should be offered as standard of care to patients awaiting solid organ transplantation. Specific recommendations included exercise-based intervention as well as psychological and stress management support for all solid organ transplant candidates, nutritional intervention for those who are over- or underweight, and probiotic supplementation for candidates for liver transplantation.
Because of the shortage in clinical evidence, however, particularly strong recommendations were formulated regarding the urgent need for high quality, but not exclusively, randomized controlled trials and implementation research studies that address the feasibility and effectiveness of pre-transplant multimodal prehabilitation. Two RCTs on multimodal prehabilitation interventions in kidney transplant candidates are currently underway: the FRAIL-MAR-study (NCT04701398) [73] and the PreCareTx-study (NCT05489432).
In addition, it was strongly recommended that priority should be given to the definition and consistent use of a Core Outcome Set to be measured by all future trials modalities, timing, duration and delivery modes of an optimal prehabilitation program.
From the in-person public discussions during the Consensus ESOT Conference, additional constructive perspectives emerged. It was advocated that clinical guidelines should be broadly applicable to transplant candidates irrespective of organ type, while leaving room for organ-specific recommendations, such as probiotics for liver transplant candidates. It was noted that intoxication-related interventions are not included in the recommendations, as intoxication (i.e., tobacco smoking or alcohol abuse) is typically addressed prior to patients joining the waitlist. The suggestion was made to formulate recommendations regarding pre-transplant peer support, however, such was considered premature as no evidence base could be found in the literature review. Lastly, the consideration was made that the designing of future studies or the future revisiting of the new guidelines may benefit of being informed by the prehabilitation literature in the broader field of surgery. However, unlike elective surgery, the waiting time is often unpredictably long while physical and mental condition may deteriorate due to the underlying disease. Therefore, prehabilitation should be offered throughout the waiting period from the moment of listing until transplantation.
These new evidence-based recommendations on prehabilitation serve to support best clinical practice in solid organ transplantation and help identify priorities for future research, thus optimizing patient health and post-transplant clinical outcome. The final recommendations will be included in the ESOT guidelines for transplant management, and under the auspices of the ESOT consensus development platform, will undergo continuous review and updating as new evidence becomes available.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
Involved in the conception or design of the work: CA, SS, EC, YO, JK, TJ-F, SM, EK, MS, PF, DM, and SG. Literature screen and review: CA, SS, EC, YO, JK, TJ-F, SM, EK, MS, CM, FD, DM, and SG. Drafted the article: CA, SS, EC, YO, CM, DM, and SG. Critically revised the article: JK, TJ-F, SM, EK, MS, FD, PF, JG, AM, PG, MP, VL-L, CW, DK, DM, and SG. Finally approved the version to be published: CA, SS, EC, YO, JK, TJ-F, SM, EK, MS, CM, FD, PF, JG, AM, PG, MP, VL-L, CW, DK, DM, and SG. All authors contributed to the article and approved the submitted version.
Funding
All costs related to taskforce and workgroup meetings were covered by ESOT, without external funding.
Conflict of Interest
DM is a senior researcher of The Research Foundation- Flanders.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This manuscript and the ESOT Consensus Working Group is a collaborative work product of ESOT and its Sections and Committees. We would like to thank Devi Mey, Justyna Klimek, Irene Garcia, Giovanna Rossi, Daniele Roppolo and the entire ESOT staff for their tireless efforts to support this endeavor. We would also like to thank Liset Pengel, the CET and the YPTs for coordinating and performing the systematic literature searches which were additionally instrumental in this endeavor. We are grateful to Anne Kaiser for the assistance in preparation of the manuscript and to An Billiau, for medical writing and editing support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11564/full#supplementary-material
Abbreviations
AGREE, Appraisal of Guidelines for Research & Evaluation; CET, Centre for Evidence in Transplantation; ESOT, European Society of Organ Transplantation; GRADE, Grading of Recommendations, Assessment, Development, and Evaluations; PICO, Population, Intervention, Comparator and Outcome; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; RCT, Randomized Controlled Trial.
References
1. Davison, SN, Levin, A, Moss, AH, Jha, V, Brown, EA, Brennan, F, et al. Executive Summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: Developing a Roadmap to Improving Quality Care. Kidney Int (2015) 88(3):447–59. doi:10.1038/ki.2015.110
2. Kilic, A, Acker, MA, and Atluri, P. Dealing With Surgical Left Ventricular Assist Device Complications. J Thorac Dis (2015) 7(12):2158–64. doi:10.3978/j.issn.2072-1439.2015.10.64
3. Rocker, G. Harms of Overoxygenation in Patients With Exacerbation of Chronic Obstructive Pulmonary Disease. CMAJ (2017) 189(22):E762–E763. doi:10.1503/cmaj.170196
4. Minnella, EM, and Carli, F. Prehabilitation and Functional Recovery for Colorectal Cancer Patients. Ejso-eur J Surg Onc (2018) 44(7):919–26. doi:10.1016/j.ejso.2018.04.016
5. Carli, F, and Scheede-Bergdahl, C. Prehabilitation to Enhance Perioperative Care. Anesthesiol Clin (2015) 33(1):17–33. doi:10.1016/j.anclin.2014.11.002
6. Heger, P, Probst, P, Wiskemann, J, Steindorf, K, Diener, MK, and Mihaljevic, AL. A Systematic Review and Meta-Analysis of Physical Exercise Prehabilitation in Major Abdominal Surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg (2020) 24(6):1375–85. doi:10.1007/s11605-019-04287-w
7. Moyer, R, Ikert, K, Long, K, and Marsh, J. The Value of Preoperative Exercise and Education for Patients Undergoing Total Hip and Knee Arthroplasty: A Systematic Review and Meta-Analysis. JBJS Rev (2017) 5(12):e2. doi:10.2106/JBJS.RVW.17.00015
8. Waterland, JL, McCourt, O, Edbrooke, L, Granger, CL, Ismail, H, Riedel, B, et al. Efficacy of Prehabilitation Including Exercise on Postoperative Outcomes Following Abdominal Cancer Surgery: A Systematic Review and Meta-Analysis. Front Surg (2021) 8:628848. doi:10.3389/fsurg.2021.628848
9. Lambert, JE, Hayes, LD, Keegan, TJ, Subar, DA, and Gaffney, CJ. The Impact of Prehabilitation on Patient Outcomes in Hepatobiliary, Colorectal, and Upper Gastrointestinal Cancer Surgery: A PRISMA-Accordant Meta-Analysis. Ann Surg (2021) 274(1):70–7. doi:10.1097/SLA.0000000000004527
10. van Wijk, L, Bongers, BC, Berkel, AEM, Buis, CI, Reudink, M, Liem, MSL, et al. Improved Preoperative Aerobic Fitness Following a Home-Based Bimodal Prehabilitation Programme in High-Risk Patients Scheduled for Liver or Pancreatic Resection. Br J Surg (2022) 109(11):1036–9. doi:10.1093/bjs/znac230
11. Wallen, MP, Skinner, TL, Pavey, TG, Hall, A, Macdonald, GA, and Coombes, JS. Safety, Adherence and Efficacy of Exercise Training in Solid-Organ Transplant Candidates: A Systematic Review. Transplant Rev (2016) 30(4):218–26. doi:10.1016/j.trre.2016.07.004
12. Pesce de Souza, F, Massierer, D, Anand Raje, U, Tansey, CM, Boruff, J, and Janaudis-Ferreira, T. Exercise Interventions in Solid Organ Transplant Candidates: A Systematic Review. Clin Transplant (2020) 34(9):e13900. doi:10.1111/ctr.13900
13. Janaudis-Ferreira, T, Mathur, S, Deliva, R, Howes, N, Patterson, C, Rakel, A, et al. Exercise for Solid Organ Transplant Candidates and Recipients: A Joint Position Statement of the Canadian Society of Transplantation and CAN-RESTORE. Transplantation (2019) 103(9):e220–e238. doi:10.1097/TP.0000000000002806
14. Cillo, U, Weissenbacher, A, Pengel, L, Jochmans, I, Roppolo, D, Amarelli, C, et al. ESOT Consensus Platform for Organ Transplantation: Setting the Stage for a Rigorous, Regularly Updated Development Process. Transpl Int (2022) 35:10915. doi:10.3389/ti.2022.10915
15. Davies, KS. Formulating the Evidence Based Practice Question: A Review of the Frameworks. Evid Based Libr Inf Pract (2011) 6(2):75–80. doi:10.18438/b8ws5n
16. Guyatt, GH, Oxman, AD, Vist, GE, Kunz, R, Falck-Ytter, Y, Alonso-Coello, P, et al. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. Br Med J (2008) 336(7650):924–6. doi:10.1136/bmj.39489.470347.AD
17.DMDIAf. investopedia.com/terms/d/delphi-method.asp (2023). Available From: https://www.investopedia.com/terms/d/delphi-method.asp (Accessed May 11th, 2023).
18. Brouwers, MC, Kho, ME, Browman, GP, Burgers, JS, Cluzeau, F, Feder, G, et al. The Global Rating Scale Complements the AGREE II in Advancing the Quality of Practice Guidelines. J Clin Epidemiol (2012) 65(5):526–34. doi:10.1016/j.jclinepi.2011.10.008
19. Laoutaris, ID, Dritsas, A, Adamopoulos, S, Manginas, A, Gouziouta, A, Kallistratos, MS, et al. Benefits of Physical Training on Exercise Capacity, Inspiratory Muscle Function, and Quality of Life in Patients With Ventricular Assist Devices Long-Term Postimplantation. Eur J Cardiovasc Prev Rehabil (2011) 18(1):33–40. doi:10.1097/HJR.0b013e32833c0320
20. Gloeckl, R, Halle, M, and Kenn, K. Interval Versus Continuous Training in Lung Transplant Candidates: A Randomized Trial. J Heart Lung Transplant (2012) 31(9):934–41. doi:10.1016/j.healun.2012.06.004
21. Hayes, K, Leet, AS, Bradley, SJ, and Holland, AE. Effects of Exercise Training on Exercise Capacity and Quality of Life in Patients With a Left Ventricular Assist Device: A Preliminary Randomized Controlled Trial. J Heart Lung Transplant (2012) 31(7):729–34. doi:10.1016/j.healun.2012.02.021
22. Adamopoulos, S, Gouziouta, A, Mantzouratou, P, Laoutaris, ID, Dritsas, A, Cokkinos, DV, et al. Thyroid Hormone Signalling Is Altered in Response to Physical Training in Patients With End-Stage Heart Failure and Mechanical Assist Devices: Potential Physiological Consequences? Interactive Cardiovasc Thorac Surg (2013) 17(4):664–8. doi:10.1093/icvts/ivt294
23. Limongi, V, Dos Santos, DC, da Silva, AMO, Ataide, EC, Mei, MFT, Udo, EY, et al. Effects of a Respiratory Physiotherapeutic Program in Liver Transplantation Candidates. Transpl Proc (2014) 46(6):1775–7. doi:10.1016/j.transproceed.2014.05.044
24. Forestieri, P, Guizilini, S, Peres, M, Bublitz, C, Bolzan, DW, Rocco, IS, et al. A Cycle Ergometer Exercise Program Improves Exercise Capacity and Inspiratory Muscle Function in Hospitalized Patients Awaiting Heart Transplantation: A Pilot Study. Braz J Cardiovasc Surg (2016) 31(5):389–95. doi:10.5935/1678-9741.20160078
25. Pehlivan, E, Mutluay, F, Balci, A, and Kilic, L. The Effects of Inspiratory Muscle Training on Exercise Capacity, Dyspnea and Respiratory Functions in Lung Transplantation Candidates: A Randomized Controlled Trial. Clin Rehabil (2018) 32(10):1328–39. doi:10.1177/0269215518777560
26. Grat, M, Wronka, KM, Lewandowski, Z, Grat, K, Krasnodebski, M, Stypulkowski, J, et al. Effects of Continuous Use of Probiotics Before Liver Transplantation: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin Nutr (2017) 36(6):1530–9. doi:10.1016/j.clnu.2017.04.021
27. Plank, LD, Mathur, S, Gane, EJ, Peng, SL, Gillanders, LK, McIlroy, K, et al. Perioperative Immunonutrition in Patients Undergoing Liver Transplantation: A Randomized Double-Blind Trial. Hepatology (2015) 61(2):639–47. doi:10.1002/hep.27433
28. Eguchi, S, Takatsuki, M, Hidaka, M, Soyama, A, Ichikawa, T, and Kanematsu, T. Perioperative Synbiotic Treatment to Prevent Infectious Complications in Patients After Elective Living Donor Liver Transplantation: A Prospective Randomized Study. Am J Surg (2011) 201(4):498–502. doi:10.1016/j.amjsurg.2010.02.013
29. Forli, L, Pedersen, JI, Bjortuft, O, Vatn, M, and Boe, J. Dietary Support to Underweight Patients With End-Stage Pulmonary Disease Assessed for Lung Transplantation. Respiration (2001) 68(1):51–7. doi:10.1159/000050463
30. Forli, L, Bjortuft, O, Vatn, M, Kofstad, J, and Boe, J. A Study of Intensified Dietary Support in Underweight Candidates for Lung Transplantation. Ann Nutr Metab (2001) 45(4):159–68. doi:10.1159/000046724
31. Le Cornu, KA, McKiernan, FJ, Kapadia, SA, and Neuberger, JM. A Prospective Randomized Study of Preoperative Nutritional Supplementation in Patients Awaiting Elective Orthotopic Liver Transplantation. Transplantation (2000) 69(7):1364–9. doi:10.1097/00007890-200004150-00026
32. Park, TL, Perri, MG, and Rodrigue, JR. Minimal Intervention Programs for Weight Loss in Heart Transplant Candidates: A Preliminary Examination. Prog Transpl (2003) 13(4):284–8. doi:10.1177/152692480301300408
33. Napolitano, MA, Babyak, MA, Palmer, S, Tapson, V, Davis, RD, Blumenthal, JA, et al. Effects of a Telephone-Based Psychosocial Intervention for Patients Awaiting Lung Transplantation. Chest (2002) 122(4):1176–84. doi:10.1378/chest.122.4.1176
34. Rodrigue, JR, Baz, MA, Widows, MR, and Ehlers, SL. A Randomized Evaluation of Quality-Of-Life Therapy With Patients Awaiting Lung Transplantation. Am J Transplant (2005) 5(10):2425–32. doi:10.1111/j.1600-6143.2005.01038.x
35. Sharif, F, Mohebbi, S, Tabatabaee, HR, Saberi-Firoozi, M, and Gholamzadeh, S. Effects of Psycho-Educational Intervention on Health-Related Quality of Life (QOL) of Patients With Chronic Liver Disease Referring to Shiraz University of Medical Sciences. Health Qual Life Outcomes (2005) 3:81. doi:10.1186/1477-7525-3-81
36. Blumenthal, JA, Babyak, MA, Keefe, FJ, Davis, RD, Lacaille, RA, Carney, RM, et al. Telephone-Based Coping Skills Training for Patients Awaiting Lung Transplantation. J Consult Clin Psychol (2006) 74(3):535–44. doi:10.1037/0022-006X.74.3.535
37. Rodrigue, JR, Mandelbrot, DA, and Pavlakis, M. A Psychological Intervention to Improve Quality of Life and Reduce Psychological Distress in Adults Awaiting Kidney Transplantation. Nephrol Dial Transplant (2011) 26(2):709–15. doi:10.1093/ndt/gfq382
38. Gross, CR, Reilly-Spong, M, Park, T, Zhao, R, Gurvich, OV, and Ibrahim, HN. Telephone-Adapted Mindfulness-Based Stress Reduction (tMBSR) for Patients Awaiting Kidney Transplantation. Contemp Clin Trials (2017) 57:37–43. doi:10.1016/j.cct.2017.03.014
39. Ben-Gal, T, Pinchas, A, Zafrir, N, Sahar, G, Berman, M, and Aravot, D. Long-Term Physical Training in Cardiac Transplant Candidates: Is it Feasible? Transpl Proc (2000) 32(4):740–2. doi:10.1016/s0041-1345(00)00964-7
40. Karapolat, H, Engin, C, Eroglu, M, Yagdi, T, Zoghi, M, Nalbantgil, S, et al. Efficacy of the Cardiac Rehabilitation Program in Patients With End-Stage Heart Failure, Heart Transplant Patients, and Left Ventricular Assist Device Recipients. Transpl Proc (2013) 45(9):3381–5. doi:10.1016/j.transproceed.2013.06.009
41. Florian, J, Rubin, A, Mattiello, R, Fontoura, FF, Camargo Jde, J, and Teixeira, PJ. Impact of Pulmonary Rehabilitation on Quality of Life and Functional Capacity in Patients on Waiting Lists for Lung Transplantation. J Bras Pneumol (2013) 39(3):349–56. doi:10.1590/S1806-37132013000300012
42. Li, M, Mathur, S, Chowdhury, NA, Helm, D, and Singer, LG. Pulmonary Rehabilitation in Lung Transplant Candidates. J Heart Lung Transpl (2013) 32(6):626–32. doi:10.1016/j.healun.2013.04.002
43. Debette-Gratien, M, Tabouret, T, Antonini, MT, Dalmay, F, Carrier, P, Legros, R, et al. Personalized Adapted Physical Activity Before Liver Transplantation: Acceptability and Results. Transplantation (2015) 99(1):145–50. doi:10.1097/TP.0000000000000245
44. Kenn, K, Gloeckl, R, Soennichsen, A, Sczepanski, B, Winterkamp, S, Boensch, M, et al. Predictors of Success for Pulmonary Rehabilitation in Patients Awaiting Lung Transplantation. Transplantation (2015) 99(5):1072–7. doi:10.1097/TP.0000000000000472
45. Pehlivan, E, Balci, A, Kilic, L, and Kadakal, F. Preoperative Pulmonary Rehabilitation for Lung Transplant: Effects on Pulmonary Function, Exercise Capacity, and Quality of Life; First Results in Turkey. Exp Clin Transplant (2018) 16(4):455–60. doi:10.6002/ect.2017.0042
46. da Fontoura, FF, Berton, DC, Watte, G, Florian, J, Schio, SM, Camargo, JDP, et al. Pulmonary Rehabilitation in Patients With Advanced Idiopathic Pulmonary Fibrosis Referred for Lung Transplantation. J Cardiopulmonary Rehabil Prev (2018) 38(2):131–4. doi:10.1097/HCR.0000000000000315
47. Ochman, M, Maruszewski, M, Latos, M, Jastrzebski, D, Wojarski, J, Karolak, W, et al. Nordic Walking in Pulmonary Rehabilitation of Patients Referred for Lung Transplantation. Transpl Proc (2018) 50(7):2059–63. doi:10.1016/j.transproceed.2018.02.106
48. Byrd, R, Smith, P, Mohamedaly, O, Snyder, LD, and Pastva, AM. A 1-Month Physical Therapy-Based Outpatient Program for Adults Awaiting Lung Transplantation: A Retrospective Analysis of Exercise Capacity, Symptoms, and Quality of Life. Cardiopulmonary Phys Ther J (2019) 30(2):61–9. doi:10.1097/CPT.0000000000000087
49. Florian, J, Watte, G, Teixeira, PJZ, Altmayer, S, Schio, SM, Sanchez, LB, et al. Pulmonary Rehabilitation Improves Survival in Patients With Idiopathic Pulmonary Fibrosis Undergoing Lung Transplantation. Sci Rep (2019) 9:9347. doi:10.1038/s41598-019-45828-2
50. McAdams-DeMarco, MA, Ying, H, Van Pilsum Rasmussen, S, Schrack, J, Haugen, CE, Chu, NM, et al. Prehabilitation Prior to Kidney Transplantation: Results From a Pilot Study. Clin Transplant (2019) 33(1):e13450. doi:10.1111/ctr.13450
51. Kilic, L, Pehlivan, E, Balci, A, and Bakan, ND. Effect of 8-week Pulmonary Rehabilitation Program on Dyspnea and Functional Capacity of Patients on Waiting List for Lung Transplantation. Turkish Thorac J (2020) 21(2):110–5. doi:10.5152/TurkThoracJ.2019.18202
52. Pehlivan, E, Balci, A, and Kilic, L. The Effect of Pulmonary Rehabilitation on Dyspnea and Factors Related to Dyspnea in Lung Transplantation Candidates. Eur Res J (2020) 6(5):395–400. doi:10.18621/eurj.531507
53. Lorenz, EC, Hickson, LJ, Weatherly, RM, Thompson, KL, Walker, HA, Rasmussen, JM, et al. Protocolized Exercise Improves Frailty Parameters and Lower Extremity Impairment: A Promising Prehabilitation Strategy for Kidney Transplant Candidates. Clin Transpl (2020) 34(9):e14017. doi:10.1111/ctr.14017
54. Massierer, D, Bourgeois, N, Rakel, A, Prevost, K, Lands, LC, Poirier, C, et al. Changes in 6-minute Walking Distance in Lung Transplant Candidates While Participating in a Home-Based Pre-Habilitation Program-A Retrospective Chart Review. Clin Transplant (2020) 34(10):e14045. doi:10.1111/ctr.14045
55. Wickerson, L, Rozenberg, D, Helm, D, Gottesman, C, Mathur, S, and Singer, LG. Short Physical Performance Battery Scores at Lung Transplant Assessment: Relationship to Early Transplant Outcomes and Response to Pre-habilitation. J Heart Lung Transplant (2020) 39:S208–9. doi:10.1016/j.healun.2020.01.828
56. Lin, FP, Visina, JM, Bloomer, PM, Dunn, MA, Josbeno, DA, Zhang, X, et al. Prehabilitation-Driven Changes in Frailty Metrics Predict Mortality in Patients With Advanced Liver Disease. Am J Gastroenterol (2021) 116(10):2105–17. doi:10.14309/ajg.0000000000001376
57. Kerti, M, Bohacs, A, Madurka, I, Kovats, Z, Gieszer, B, Elek, J, et al. The Effectiveness of Pulmonary Rehabilitation in Connection With Lung Transplantation in Hungary. Ann (2021) 10(4):3906–15. doi:10.21037/apm-20-1783
58. Layton, AM, Irwin, AM, Mihalik, EC, Fleisch, E, Keating, CL, Dimango, EA, et al. Telerehabilitation Using Fitness Application in Patients With Severe Cystic Fibrosis Awaiting Lung Transplant: A Pilot Study. Int J Telemed Appl (2021) 2021:6641853. doi:10.1155/2021/6641853
59. Wickerson, L, Helm, D, Gottesman, C, Rozenberg, D, Singer, LG, Keshavjee, S, et al. Telerehabilitation for Lung Transplant Candidates and Recipients During the COVID-19 Pandemic: Program Evaluation. JMIR MHealth and UHealth (2021) 9(6):e28708. doi:10.2196/28708
60. Duarte-Rojo, A, Bloomer, PM, Rogers, RJ, Hassan, MA, Dunn, MA, Tevar, AD, et al. Introducing EL-FIT (Exercise and Liver FITness): A Smartphone App to Prehabilitate and Monitor Liver Transplant Candidates. Liver Transplant (2021) 27(4):502–12. doi:10.1002/lt.25950
61. Byrd, R, Vallabhajosula, S, Bailey, S, and Champion, T. Effects of Rehabilitation Before Lung Transplantation on Balance. Cardiopulmonary Phys Ther J (2022) 33(2):50–9. doi:10.1097/cpt.0000000000000187
62. Singer, JP, Soong, A, Bruun, A, Bracha, A, Chin, G, Hays, SR, et al. A Mobile Health Technology Enabled Home-Based Intervention to Treat Frailty in Adult Lung Transplant Candidates: A Pilot Study. Clin Transplant (2018) 32(6):e13274. doi:10.1111/ctr.13274
63. Anderson, MR, Easthausen, I, Gallagher, G, Udupa, J, Tong, Y, Torigian, D, et al. Skeletal Muscle Adiposity and Outcomes in Candidates for Lung Transplantation: A Lung Transplant Body Composition Cohort Study. Am J Respir Crit Care Med Conf Am Thorac Soc Int Conf ATS. (2020) 201(1):A2827. doi:10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2827
64. Morkane, CM, Kearney, O, Bruce, DA, Melikian, CN, and Martin, DS. An Outpatient Hospital-Based Exercise Training Program for Patients With Cirrhotic Liver Disease Awaiting Transplantation: A Feasibility Trial. Transplantation (2020) 104(1):97–103. doi:10.1097/TP.0000000000002803
65. Craig, JA, Miner, D, Remtulla, T, Miller, J, and Zanussi, LW. Piloting a Coping Skills Group Intervention to Reduce Depression and Anxiety Symptoms in Patients Awaiting Kidney or Liver Transplant. Health Soc Work (2017) 42(1):e44–e52. doi:10.1093/hsw/hlw064
66. Febrero, B, Ramirez, P, Martinez-Alarcon, L, Abete, C, Galera, M, Rios, A, et al. Group Psychotherapy Could Improve Depression in Cirrhotic Patients on the Liver Transplant Waiting List. Transpl Proc (2019) 51(1):28–32. doi:10.1016/j.transproceed.2018.02.206
67. Jutagir, DR, Saracino, RM, Cunningham, A, Foran-Tuller, KA, Driscoll, MA, Sledge, WH, et al. The Feasibility of a Group Stress Management Liver SMART Intervention for Patients With End-Stage Liver Disease: A Pilot Study. Palliat Support Care (2019) 17(1):35–41. doi:10.1017/S147895151800024X
68. Zhao, Q, Zhang, S, and Yu, R. Impact of Pre-Transplantation Psychological Counseling in Improving the Mental Well-Being of Patients on Hemodialysis. Front Psychiatr (2021) 12:594670. doi:10.3389/fpsyt.2021.594670
69. Zamora-Valdes, D, Watt, KD, Kellogg, TA, Poterucha, JJ, Di Cecco, SR, Francisco-Ziller, NM, et al. Long-Term Outcomes of Patients Undergoing Simultaneous Liver Transplantation and Sleeve Gastrectomy. Hepatology (2018) 68(2):485–95. doi:10.1002/hep.29848
70. Hollander, FM, van Pierre, DD, de Roos, NM, van de Graaf, EA, and Iestra, JA. Effects of Nutritional Status and Dietetic Interventions on Survival in Cystic Fibrosis Patients Before and After Lung Transplantation. J Cyst Fibros (2014) 13(2):212–8. doi:10.1016/j.jcf.2013.08.009
71. Ju, A, Cazzolli, R, Howell, M, Scholes-Robertson, N, Wong, G, and Jaure, A. Novel Endpoints in Solid Organ Transplantation: Targeting Patient-Reported Outcome Measures. Transplantation (2023). Publish Ahead of Print. doi:10.1097/TP.0000000000004537
72. Pearson, N, Naylor, PJ, Ashe, MC, Fernandez, M, Yoong, SL, and Wolfenden, L. Guidance for Conducting Feasibility and Pilot Studies for Implementation Trials. Pilot Feasibility Stud (2020) 6(1):167. doi:10.1186/s40814-020-00634-w
73. Perez-Saez, MJ, Morgado-Perez, A, Faura, A, Munoz-Redondo, E, Garriz, M, Muns, MD, et al. The FRAILMar Study Protocol: Frailty in Patients With Advanced Chronic Kidney Disease Awaiting Kidney Transplantation. A Randomized Clinical Trial of Multimodal Prehabilitation. Front Med (2021) 8:675049. doi:10.3389/fmed.2021.675049
Keywords: prehabilitation, solid organ transplant candidates, exercise, nutrition, psychosocial interventions
Citation: Annema C, De Smet S, Castle EM, Overloop Y, Klaase JM, Janaudis-Ferreira T, Mathur S, Kouidi E, Perez Saez MJ, Matthys C, Dobbels F, Ferrari P, Gołębiewska J, Mrzljak A, Girman P, Perch M, Lopez-Lopez V, White C, Koval D, Greenwood S and Monbaliu D (2023) European Society of Organ Transplantation (ESOT) Consensus Statement on Prehabilitation for Solid Organ Transplantation Candidates. Transpl Int 36:11564. doi: 10.3389/ti.2023.11564
Received: 11 May 2023; Accepted: 15 June 2023;
Published: 21 July 2023.
Copyright © 2023 Annema, De Smet, Castle, Overloop, Klaase, Janaudis-Ferreira, Mathur, Kouidi, Perez Saez, Matthys, Dobbels, Ferrari, Gołębiewska, Mrzljak, Girman, Perch, Lopez-Lopez, White, Koval, Greenwood and Monbaliu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Coby Annema, j.h.annema@umcg.nl
†These authors have contributed equally to this work
 Coby Annema
Coby Annema Stefan De Smet
Stefan De Smet Ellen M. Castle
Ellen M. Castle Yasna Overloop6,
Yasna Overloop6,  Fabienne Dobbels
Fabienne Dobbels Diethard Monbaliu
Diethard Monbaliu