Prevalence and Patient-Level Correlates of Intentional Non-Adherence to Immunosuppressive Medication After Heart-Transplantation—Findings From the International BRIGHT Study
- 1Nursing Science, Department of Public Health, University of Basel, Basel, Switzerland
- 2Pediatric Intensive Care Unit, University Children’s Hospital Basel, Basel, Switzerland
- 3Academic Centre for Nursing and Midwifery, Department of Public Health and Primary Care, KU Leuven, Leuven, Belgium
- 4School of Nursing and Health Studies, University of Missouri-Kansas City, Kansas City, MO, United States
After heart transplantation (HTx), non-adherence to immunosuppressants (IS) is associated with poor outcomes; however, intentional non-adherence (INA) is poorly understood regarding its international variability in prevalence, contributing factors and impact on outcomes. We investigated (1) the prevalence and international variability of INA, (2) patient-level correlates of INA, and (3) relation of INA with clinical outcomes. Secondary analysis of data from the BRIGHT study—an international multi-center, cross-sectional survey examining multi-level factors of adherence in 1,397 adult HTx recipients. INA during the implementation phase, i.e., drug holiday and dose alteration, was measured using the Basel Assessment of Adherence to Immunosuppressive Medications Scale© (BAASIS©). Descriptive and inferential analysis was performed with data retrieved through patient interview, patient self-report and in clinical records. INA prevalence was 3.3% (n = 46/1,397)—drug holidays: 1.7% (n = 24); dose alteration: 1.4% (n = 20); both: 0.1% (n = 2). University-level education (OR = 2.46, CI = 1.04–5.83), insurance not covering IS costs (OR = 2.21, CI = 1.01–4.87) and barriers (OR = 4.90, CI = 2.73–8.80) were significantly associated with INA; however, clinical outcomes were not. Compared to other single-center studies, this sample’s INA prevalence was low. More than accessibility or financial concerns, our analyses identified patient-level barriers as INA drivers. Addressing patients’ IS-related barriers, should decrease INA.
Introduction
After heart transplantation (HTx), patients need to adhere to a life-long immunosuppressive medication (IS) regimen [1]. Poor adherence to IS has been linked to poor clinical and economic outcomes [2].
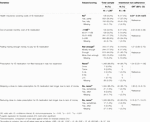
Following the Ascertaining Barriers to Compliance (ABC) taxonomy definition, medication adherence is the process by which a patient follows a medication regimen as prescribed. It has 3 phases: initiation, implementation, and persistence (Figure 1) [3]. While non-adherence can occur during any of these phases, after HTx, initiation of IS takes place under clinical supervision and therefore medication non-adherence (NA) is most common during the implementation and persistence phases [3]. Medication NA can be discerned as either intentional or unintentional [4, 5]. Intentional non-adherence (INA) refers to a rational decision-making process and the ability of a person to act on a behavior [6, 7]. This is opposed to unintentional non-adherence, a passive and intermittent process that results from forgetfulness, a lack of capacity, skills, and/or resources [6–11].

FIGURE 1. Process of medication adherence illustrating phases in which intentional non-adherence and intentional implementation non-adherence (i.e., drug holiday and dose alteration) may appear [3].
Rational decision-making is related to the ability to formulate and carry out a behavior. Within the context of INA, patients decide to reduce their dosing frequency or number of medications, or even to prematurely and unilaterally discontinue treatment (i.e., non-persistence) [9, 12]. This also includes consciously deciding to skip several consecutive doses (i.e., a drug holiday) or to alter the dose of medication (i.e., dose alteration) [13, 14]. The objective is often to avoid disturbing side-effects, to circumvent a restrictive schedule or taking constraints (e.g., having to take food simultaneously), or to generate a feeling of control [9]. Doses may also be omitted or reduced to make a prescription last longer [15].
To date, though, INA to IS (which we will refer to simply as INA) has received only limited attention in the HTx populations and has not been well-substantiated due to inconsistent definition and measurement and large international variability. INA has not been directly studied, and estimated prevalence of drug holidays or non-persistence to IS vary widely, respectively 0%–7.1% and 0.6%–3.1% [16–18].
Deviations from prescribed medication regimen may adversely influence its effect and put the patient at risk of negative clinical outcomes—acute rejection episodes, graft loss, and death [19, 20]. It is unclear how INA influences this risk and how prevalent it is [2, 21, 22].
The limited evidence on correlates of INA focuses on patient-level barriers: beliefs [11, 23], disruption of daily routine [23, 24], and knowledge gaps [5, 25, 26]. System-level correlates: financial barriers related to a lack of health insurance coverage or other sources of increased out-of-pocket monthly expenses [27–29], vary between healthcare systems and show high international variability in relation to INA.
The aims of this study were to 1) assess the prevalence and variability of INA in adult HTx internationally, 2) investigate patient-level correlates of INA, and 3) assess INA’s associations with clinical outcomes in adult HTx recipients.
Patients and Methods
Design and Sample
This is a secondary data analysis of the “Building research initiative group: chronic illness management and adherence in transplantation” (BRIGHT) study [30], an international, multi-center, cross-sectional survey examining multi-level factors related to IS adherence in HTx recipients. Detailed information on the BRIGHT study has been reported elsewhere [27, 30]. In a multi-stage sampling approach, a convenience sample including 11 countries, 36 HTx centers, and a random sample of HTx recipients was selected. Transplant recipients were included using seven criteria [30]: 1) ≥18 years old at time of inclusion; 2) transplanted and followed-up for routine care in participating centers; 3) first transplant; 4) single-organ transplant; 5) 1–5 years post-transplant; 6) could read in the languages spoken in the country of the participating center; and 7) could provide written informed consent. Exclusion criteria were: 1) had participated in an adherence intervention study within the past 6 months; or 2) were receiving professional support in taking medication at the time of this study.
Variables and Measurement
We based our analyses on data collected using the BRIGHT questionnaires (i.e., BRIGHT patient interview, BRIGHT patient self-report questionnaire) and on the BRIGHT data—including those relating to patient outcomes—collected from clinical files [27, 30]. Intentional NA—drug holidays and dose alterations—patient-level correlates and center location were assessed through patient interview transcripts and patients’ written self-reports [30].
Socio-Demographic Data
The following demographic data were assessed (see Table 1 for answer options) [30]: age (in years), gender, marital status, living situation, employment status, educational level (using a standardized categorization across countries), ethnicity and center location/country.
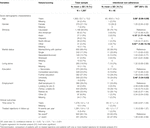
TABLE 1. Socio-demographic characteristics and clinical outcomes for total group and patients showing intentional non-adherence.
Intentional Non-Adherence
Intentional NA was assessed using 2 items from the 5-item Basel Assessment of Adherence to immunoSuppressive medIcation Scale (BAASIS© https://baasis.nursing.unibas.ch/) [32]. The first item, drug holiday, was operationalized for patients indicating they had skipped two or more consecutive doses of medication. The second, dose alteration, was operationalized for patients indicating that they had altered their prescribed IS dosage (i.e., they had taken more or fewer pills per dose than prescribed) over the last 4 weeks [27]. Intentional NA was operationalized as a positive answer to either of these two items.
IMBP Correlates of Intentional Non-Adherence
Fishbein’s Integrative Model of Behavioral Prediction (IMBP; Figure 2) [33] posits that Intention to perform is the most proximal determinant of health behavior. Intention to perform has three determinants: attitudes, norms and self-efficacy. An attitude is defined as a positive or negative feeling towards performing the behavior [34]. Subjective norms are defined as the beliefs an individual or a group has regarding whether or not to perform a given behavior [34]. Self-efficacy refers to the person’s beliefs regarding performing a recommended behavior, despite circumstances or barriers making it difficult [34]. Fishbein’s model acknowledged that the presence of personal or environmental barriers may hinder patients from acting upon their intentions and keep them from executing the recommended behavior (Figure 2) [34]. The next paragraphs describe the instruments to measure these five concepts. Information on the instruments’ psychometric properties can be found elsewhere [27].
Intention
Intention was operationalized as the cognitive representation of a person’s readiness to perform a given behavior [27]. As an indicator of the capacity of a person to take actions necessary to attain a target [36], it was assessed using 3 investigator-developed items (e.g., “I always intend to take my IS on time”) rated on a unidimensional 5-point Likert-type scale ranging from 1 (strongly disagree) to 5 (strongly agree) [27]. Intention was scored by calculating a mean across the 3 items. This subscale’s Cronbach’s alpha was 0.81 [27].
Attitudes
Attitudes were operationalized to reflect how favorably—such as important to avoid organ rejection—or unfavorably—such as poison—each patient considered IS. Attitudes are related to a patient’s degree of belief that a given behavior will lead to a favorable or unfavorable outcome [36]. Attitudes were assessed using a 21-item investigator-developed instrument asking patients’ to rate their concerns/worries (12 items, e.g., “Immunosuppressive medications are addictive”) as well as how necessary they considered their IS (9 items, e.g., “Immunosuppressive medications protect my heart”) [27, 30, 35]. Items were rated on a 5-point Likert-type scale ranging from 1 (strongly disagree) to 5 (strongly agree). Total scores for the positive attitudes—favorable—and worries—unfavorable—dimensions were calculated as the mean score over each item’s rating. The dimensions’ Cronbach’s alphas were, respectively 0.77 and 0.66 [27].
Norms
Regarding norms, the operational definition used here relates to patients’ perceptions of social pressure or relevant others’ beliefs that may influence their decision-making about medication taking [27]. Important influences may include others’ approval or disapproval of a behavior or the knowledge that some behaviors cannot be performed without assistance [36]. An 11-item investigator-developed instrument based on previous work [24, 37–41] was used to measure normative beliefs about IS (e.g., “Some of my family members disapprove that I have to take immunosuppressive medications”) [24, 27, 30]. Patients were asked to rate items on a 5-point Likert-type scale ranging from 1 (strongly disagree) to 5 (strongly agree). As psychometric analysis confirmed the instrument’s unidimensionality, a mean score was calculated across all items. This instrument’s Cronbach’s alpha was 0.94 [27].
Self-Efficacy
Self-efficacy was defined as the patients’ confidence in their ability to take their IS in a given situation [27]. This confidence depends on perceived skills and possibly the expected cooperation of others [36]. Regarding IS, self-efficacy behavior was assessed using the 23-item Long-Term Medication Behavior Self-Efficacy Scale [42]. Items were rated on a 5-point Likert-type scale ranging from 1 (not at all confident) to 5 (totally confident). As psychometric analysis showed that this scale is unidimensional, an overall mean score was calculated for self-efficacy. Cronbach’s alpha was 0.98 [42].
Barriers
Barriers were operationalized as personal circumstances or environmental constraints that might either prevent a patient from enacting an intended behavior or limit their capacity to perform desired actions [12]. The 19-item IS Medication Adherence Barriers instrument represents barriers identified by patients attempting to follow IS regimens [30]. Items (e.g., “I find it hard to swallow my IS medication”, “I find it hard to take my IS medication because I experience side-effects,” or “I find it hard to go away from home and plan the day because I have to take my IS medication”) are rated on a unidimensional 5-point Likert scale ranging from 1 (never) to 5 (always). A mean score across the 19 items is then calculated. This instrument was developed by the Transplant360 Task Force [43]. Its Cronbach’s alpha was 0.89.
Financial Barriers
Financial barriers—healthcare system-level factors—, are cost-related difficulties that hinder a patient from enacting a behavior [36]. Those affecting IS taking are often related to health insurance not or only partially covering the medication costs, necessitating high monthly expenditures [15]. Financial barriers were assessed using six investigator-developed items, which were dichotomized for the purpose of this study: Health insurance covering costs of IS (no versus yes partly, yes fully); Out-of-pocket monthly cost of IS (0–$20, $20.01–$60, $60.01–$110 versus >110$); Feeling that one has enough money to pay for IS (not enough versus mostly enough, enough, more than enough); Prescription for IS not filled because it was too expensive (never versus once, twice, 3–4x, 5–6x, ≥7x); Skipping a dose to make prescription for IS last longer due to lack of money (no never versus yes sometimes, yes often); and Reducing dose to make prescription for IS last longer due to lack of money (no never versus yes sometimes, yes often).
Clinical Outcomes
Two clinical outcomes were assessed (see Table 1): time since transplantation (in years); and number of treated rejections experienced per year in follow-up.
Data Collection
The BRIGHT study’s data collection has been described previously [27, 30]. Data were collected from early 2012–early 2017 [27].
Data Analysis
We used descriptive statistics as appropriate based on measurement levels and data distributions. Hierarchical inferential statistics, i.e., multilevel logistic regression analysis, was used to assess associations between INA (i.e., drug holiday and dose alteration), IMBP correlates (Figure 2) and clinical outcomes, while controlling for international variability. Socio-demographic characteristics, financial barriers and clinical outcomes that initial analyses suggested were significantly associated with INA were included in the model. Financial barrier-related data were dichotomized before inclusion. Generalized linear regression with random effects was used in the multilevel analysis of international variability. However, the small INA sample size did not allow for moderator analysis with significant or otherwise meaningful results.
Missing data analysis was performed, including a visual analysis with Amelia II [44] (multiple imputation software). Analysis of distribution did not reveal any substantial differences between the 20 patients (1.4%) who provided insufficient information relative to BAASIS© to assess adherence [32]. For further analysis, the authors proceeded with list-wise deletion.
The software package used for statistical analysis was R, version 4.0.2, 2020-06-22. [45] Statistical significance was set at p<.05.
Results
Sample Characteristics
This analysis included 1,397 patients (details provided elsewhere) [27]. Participants’ mean age was 53.7 (±13.2) years; 27.1% were female; 84.9% were of Caucasian origin. At time of interview, most (68.4%) were married or living with partners; 19.0% were living alone. The majority (72.8%) had completed secondary school or higher, with 22.0% holding University degrees; 26.2% were employed or self-employed; 28.9% were temporarily or fully unable to work; and 33.4% were retired. Financial barriers such as health insurance not covering IS costs and high monthly out-of-pocket IS expenses were reported respectively by 9.2% and 9.5% of patients. A more detailed overview of patient-level characteristics can be found in the Tables 1, 2.
Intentional Non-Adherence
Prevalence
Intentional NA was observed in 46 of 1,397 patients (3.3%). Drug holidays were reported by 24 (1.7%), dose alteration by 20 (1.4%). Two (0.1%) reported a combination of drug holiday and dose alteration.
International Variability
International variability was high, with INA prevalence spanning from 0% in Germany to 9.8% in Australia (Figure 3). Drug holidays ranged from 0% in Germany to 4.3% in Switzerland, and dose alteration from 0% in Germany to 7.8% in Australia.
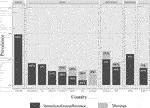
FIGURE 3. Prevalence of intentional non-adherence internationally. Sample, N (%): Australia, 51 (3.7); Switzerland, 47 (3.4); UK, 99 (7.1); Belgium, 74 (5.3); France, 158 (11.5); Spain, 223 (16.2); Italy, 109 (7.9); Germany, 65 (4.8); Canada, 119 (8.7); USA, 337 (24.3); Brazil, 100 (7.2). Missings: France, 1.3%; Spain, 1.8%; Italy, 1.8%; Germany, 3.0%; Canada, 1.7%; USA, 0.9%; Mean, 1.0%.
Correlates of Intentional Non-Adherence
In univariable analyses, lower age (OR = 0.98, CI = 0.96–0.99), being of Asian or other origin (OR = 4.10, CI = 1.17–14.19 and OR = 5.02, CI = 1.66–15.16), and university education were associated with higher INA (OR = 2.46, CI = 1.04–5.83). Lack of insurance coverage for IS was the only financial barrier significantly related to a higher risk of INA (OR = 2.21, CI = 1.01–4.87). Low intention was strongly related to INA (OR = 0.54, CI = 0.38–0.77). High worries (OR = 1.81, CI = 1.15–2.85), low self-efficacy (OR = 0.59, CI = 0.44–0.80) and high barriers (OR = 4.90, CI = 2.73–8.80) also significantly increased the odds for INA (Table 3).
The multivariate analysis of demographic correlates showed that having a university degree was significantly related to INA (OR = 2.95, CI = 1.05–8.29). Intentional NA was strongly associated with the IMBP correlate barriers (OR = 4.81, CI = 2.17–10.65) and insurance not covering IS costs (OR = 2.32, CI = 1.02–5.25).
When controlling for differences between countries (as a random effect), being of Asian origin (b = 0.076, p = 0.036), being a widow (b = 0.077, p = 0.012), not living alone (b = 0.032, p = 0.035) and having a university degree (b = 0.035, p = 0.035) correlated with a higher risk of INA. Barriers remained the only IMBP that is associated with a higher risk of INA (b = 0.11, p < 0.001).
Clinical Outcomes
On average, patients had been transplanted 3.4 years (±1.4) and had experienced 0.9 (±1.5) treated rejections per year in follow-up. The proportion of patients who had experienced at least one rejection episode during follow-up was not significantly higher in those reporting INA (n = 22/46, 47.8%) than in the overall sample (n = 551/1,397, 39.4%; OR = 1.48, CI = 0.82–2.68).
Discussion
To our knowledge, this is the first study to investigate the prevalence and correlates of INA to immunosuppressive medication after HTx internationally. Its major strengths are its international multisite sample and the use of a theoretical model to guide the exploration of correlates of intentional non-adherence [3, 19, 46, 47].
Intentional Non-Adherence
Our sample’s overall INA rate, 3.3% (n = 46/1,397), was lower than those reported in comparable clinical populations [32]. Few studies have been published distinguishing drug holiday and dose alterations of IS after HTx using the BAASIS©; [32, 48–51]. BAASIS©, as a self-report method, is embedded in the ABC taxonomy, assessing phases of medication adherence and providing bases for operationalization and assessment of INA (i.e., drug holidays or dose alterations) [46, 52, 53]. Respectively, three and four studies have reported higher prevalence either of drug holidays (8.3%–11.0%) [49–51] or of dose alterations (5.6%–12.1%) [48–51]. Skipping multiple doses—drug holiday—represents a higher risk for negative clinical consequences and is especially concerning [17, 40, 54]. Despite similar medication regimens described, i.e., drug type and twice-daily dosing, patients included in these studies had longer times—4.8 and 7.5 years—since transplantation [48, 49]; and non-adherence has been shown, although inconsistently, to increase over time [55–57]. Compared to non-adherence rates for other types of medication (e.g., adjuvant endocrine therapy in breast cancer: 7%–14%; anti-retroviral therapy in HIV: 17.8%; tyrosine kinase inhibitors in chronic myeloid leukemia: 27%), the rates reported for post-HTx INA to immunosuppressants are among the lowest in literature [52, 58–63]. This may be explained by immunosuppressants’ low forgiveness—the need for extremely close adherence to maintain their effects [64–66]—which focusses patients’ attention very closely on their regimens [67–69]. Compared to recipients of other solid organs—such as lung, liver, and kidney—[17, 25, 54, 70] heart recipients’ low INA rates may also reflect the limited therapeutic options available in case of graft rejection, dysfunction or loss [20, 71]. While kidney recipients have the option, for example, of dialysis or renal transplantation from living donors, a heart transplant is usually a one in a life-time gift [51, 72–75].
International Variations and Financial Barriers
Our findings show that INA prevalence varies internationally, the highest rates being observed in Australia (9.8%), Brazil (6.0%) and Canada (5.0%). A range of country-level correlates (e.g., insurance coverage, financial barriers, access to medication) have been offered as explanations [76–78]. Measurable moderating variables, such as low insurance coverage for IS in Australia, the USA and Canada [76], or the perceived financial burden of high monthly out-of-pocket expenses in Switzerland [29] may help explain some disparities. Low accessibility, such as greater distance to the transplant center, does not seem to favor INA. [29, 77] When referring to delayed access to a specialist or higher waiting times for appointments, e.g., Canada and oppositely Germany, low accessibility appears to match higher INA rates. [29] This implies that better organized services help compensate low accessibility and prevent INA. [77].
Correlates of Intentional Non-Adherence
Belonging to an ethnic minority—more specifically, being of Asian or of other origin—increased the odds of INA. This may result from lower levels of support within these populations [79–81] or variations in social desirability across ethnic groups regarding organ transplantation [80]. Social norms may also increase the tendency to underreport INA in favor of other forms of NA, such as forgetfulness [82]. In line with previous research, having a university degree was significantly related to higher rates of INA [71, 83]. It may be assumed that higher-educated persons feel they have the skills to recognize and weigh IS-related benefits and risks [72]. It also strongly suggests that INA does not arise from a lack of understanding [84] or health literacy [58, 70, 85–87]. Instead, it suggests that INA is more closely related to the decision-making process outlined by the theory of planned behavior [88] and how the patient balances the benefits of following the IS regimen against the risks and barriers, e.g., side-effects, taking constraints or disruption of their normal routines [5, 17, 81, 89].
IMBP Correlates of Intentional Non-Adherence
Worries (i.e., negative feelings) towards following the IS regimen as prescribed were particularly strongly related to INA. This supports the idea that intentional behavior, even regarding the weighing-out of necessities and concerns, is tipped more by patients’ fears and worries (e.g., “IS medication is toxic for my body” or “doctors place too much trust in IS medication”) than by clinicians’ assurances that IS is necessary and beneficial [25, 75, 88]. Therefore, a slightly heightened sense of worry could greatly increase a patient’s risk of attempting to modulate the IS’ side-effects (e.g., “When I suffer from uncomfortable side effects, it is best if I reduce the dosage of my IS medication a little”) [90] or to increase their compatibility with daily routines (e.g., “Taking IS medication disrupts my daily life”) [5, 88].
Self-efficacy correlated strongly with lower rates of INA. Our results show lower levels of self-efficacy in patients indicating INA than in the overall sample (3.95 ± 0.89 vs. 4.36 ± 0.81, p < .01). Self-efficacy relates to patients’ beliefs in their ability to affect a situation. It is demonstrated by patients being confident about taking IS in a given situation [27, 91]. Patients experiencing IS constraints may be tempted to cut back on or briefly halt their IS to limit their side-effects, test their effectiveness or increase their sense of control over their disease and its treatment [92]. When such INA behaviors occur, they reflect low self-efficacy, but foster a false sense of control [5]. This, in turn, leads to intentional and fully conscious non-adherence [91, 93].
Despite the intention to adhere to IS regimen, multiple barriers may hinder a patient from performing the necessary behaviors, such as taking multiple pills at once, taking IS whilst busy with other matters, taking them despite side-effects or having to follow an inconvenient schedule. Consequently, barriers were the strongest predictor of INA. Indeed, even when behaviors are intended, certain barriers can prevent patients from enacting them. This tendency supports the hypothesis that regimen-related constraints, especially difficulties taking IS, are more critical than the suspicion that IS is harmful [58].
Recent findings focusing on cost-related medication non-adherence also show that some financial barriers may relate to patient-level factors rather than healthcare system-level factors, i.e., whether “health insurance covers the cost of IS” or “monthly out-of-pocket expenses for IS [are manageable]” [51, 76, 94]. Examples of patient-level factors include attempts to “make prescriptions last longer” or “delay IS medication refills,” and relate closely to how patients prefer to allocate funds [15, 76]. Regarding INA, these results emphasize the importance of addressing financial barriers at the patient level [76].
Limitations
The reliability of patient self-report is strongly dependent on the data collection techniques used, e.g., patient interview, and on how the patient understands collected information will be used. Both the wording of questions and the interviewer’s attitude may influence the accuracy of the responses, as patients may believe it is more acceptable to have forgotten a dose than to have intentionally/purposely not taken it, i.e., social desirability bias. And if non-adherent patients refuse to participate because they consider their behaviors unacceptable, this will skew prevalence estimates for those behaviors downwards [52, 95–97]. At the same time, self-report helps gain a deeper insight into how IS is taken (i.e., number of pills taken per dose, doses taken) and why (i.e., open question on adherence) [96, 98]. Because our analyses of patients’ behaviors rely quite heavily on those patients’ underlying intentions, we assume our findings offer a firm basis for future research on targeted interventions [46, 96].
Although our operational definition implied a link between non-persistence and rational decision making, we did not approach non-persistence as INA. This sample’s IS non-persistence rate (i.e., discontinuation of the regimen) was very low (N = 7, 0.5%). This finding echoed those of other studies, all of which reported very small prevalence (0.6%–3.1%) of medication non-persistence [17, 18, 49]. In all cases, including cases with a high relative rate of missing information on INA—e.g., Spain, Italy, Germany—, the number of cases involved were too low to allow in-depth analyses. Still, considering the clinical impact of non-persistence; [20, 65, 99, 100], further insight is needed to determine, for example, whether this measurement arises from a misunderstanding of the question. For example, there needs to be a clear distinction between interruptions in IS use that arise from regimen changes versus those where, contrary to their clinicians’ advice, patients simply abandon their IS regimens for prolonged periods; [101–103]. The former represents a therapeutic adjustment, the latter a potentially life-threatening behavior based on a conscious but misguided (and hopefully preventable) decision [67, 83].
Also, as this was a cross-sectional study, no longitudinal data were collected. Therefore, it is not possible to draw inferences regarding INA’s development or evolution. Patients were asked about their non-adherence over the last month. This cannot cover possible life-cycles of INA behaviors (i.e., it is not possible to say whether patients go through phases during which the type and level of non-adherence behaviors change) [92, 104]. While current findings suggest that non-adherence increases over time, [52, 57, 66, 70], applying these findings to INA will require data on intentionality and negative perceptions (worries) collected across multiple time points. In short, capturing INA’s dynamic underlying nature will require further longitudinal research [105].
Conclusion
Based on a validated measurement (i.e., the BAASIS©) of intentional non-adherence to immunosuppressive medication (INA) [32], and referring to Fishbein’s Integrative Model of Behavioral Prediction to further understand INA-relevant behavior, this large multi-center study assessed the prevalence of INA on an international level. INA occurs when patients intentionally alter their medication regimens against medical advice, i.e., via drug holidays and/or dose alteration. Our analyses indicated that the correlates most strongly associated with INA were having a university-level education, belonging to an ethnic minority, or lacking health insurance that covered IS costs. As reasons, patients commonly cite worries (e.g., burdensome side-effects) or barriers (e.g., constraints related to their medication regimens), or a desire to regain a sense of control over their lives. In addition to highlighting the importance of patient-level factors associated specifically with INA, these findings support the development and use of individually-tailored interventions to decrease INA.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical approval was obtained from the participating centers’ ethical boards or commissions prior to data collection. Informed written consent was obtained from all included patients, in line with guidelines of the Declaration of Helsinki [106]. Anonymity and confidentiality of data and patient information were assured during the study and the secondary analysis [27, 30].
Author Contributions
SG, FD, and CR are BRIGHT study’s principal and co-investigators. For the current study, MM, LB, and SG analyzed the data and wrote and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that the BRIGHT study received funding from the International Transplant Nurses Society (ITNS), the International Society for Heart and Lung Transplantation (ISHLT), the Swiss Academy of Medical Sciences (SAMW), as well as an unrestricted research grant from Astellas Pharma. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to all of the BRIGHT study’s patients and clinicians for their contributions of data, expertise and time, to Chris Shultis for his editing, to Remon Helmy for his assistance in preparing this manuscript, and to Kris Denhaerynck for his support with the statistical analyses. Also, we cordially thank our fellow Masters students at the University of Basel’s Institute of Nursing Science for their critical revisions.
Abbreviations
HTx, Heart transplantation; INA, Intentional nonadherence; IS, Immunosuppressive medication.
References
1. Wilhelm, MJ. Long-term Outcome Following Heart Transplantation: Current Perspective. J Thorac Dis (2015) 7:549–51. doi:10.3978/j.issn.2072-1439.2015.01.46
2. De Geest, S, Denhaerynck, K, and Dobbels, F. Clinical and Economical Consequences of Nonadherence to Immunosuppressive Drugs in Adult Solid Organ Transplantation (Invited Editor: Dr. Federico Oppenheime). In: DJ Grinyò, editor. International Transplantation Updates 2011. Barcelona, Spain: Permanyer Publications (2011). p. 63–81.
3. Vrijens, B, De Geest, S, Hughes, DA, Przemyslaw, K, Demonceau, J, Ruppar, T, et al. A New Taxonomy for Describing and Defining Adherence to Medications. Br J Clin Pharmacol (2012) 73:691–705. doi:10.1111/j.1365-2125.2012.04167.x
4. van Dulmen, S, Sluijs, E, van Dijk, L, de Ridder, D, Heerdink, R, Bensing, J, et al. Furthering Patient Adherence: a Position Paper of the International Expert Forum on Patient Adherence Based on an Internet Forum Discussion. BMC Health Serv Res (2008) 8:47. doi:10.1186/1472-6963-8-47
5. Horne, R, Chapman, SC, Parham, R, Freemantle, N, Forbes, A, and Cooper, V. Understanding Patients' Adherence-Related Beliefs about Medicines Prescribed for Long-Term Conditions: a Meta-Analytic Review of the Necessity-Concerns Framework. PLoS One (2013) 8:e80633. doi:10.1371/journal.pone.0080633
6. Johnson, MJ. The Medication Adherence Model: a Guide for Assessing Medication Taking. Res Theor Nurs Pract (2002) 16:179–92. doi:10.1891/rtnp.16.3.179.53008
7. Wroe, AL. Intentional and Unintentional Nonadherence: a Study of Decision Making. J Behav Med (2002) 25:355–72. doi:10.1023/a:1015866415552
8. Gadkari, AS, and McHorney, CA. Unintentional Non-adherence to Chronic Prescription Medications: How Unintentional Is it Really? BMC Health Serv Res (2012) 12:98. doi:10.1186/1472-6963-12-98
9. Horne, R. Compliance, Adherence, and Concordance: Implications for Asthma Treatment. Chest (2006) 130(1):65S–72S. doi:10.1378/chest.130.1_suppl.65S
10. Campbell, TS, Johnson, JA, and Zernicke, KA. Unintentional Nonadherence. In: MD Gellman, and JR Turner, editors. Encyclopedia of Behavioral Medicine. New York: Springer (2013).
11. Barber, N, Safdar, A, and Franklin, BD. Can Human Error Theory Explain Non-adherence? Pharm World Sci (2005) 27:300–4. doi:10.1007/s11096-005-0355-7
12. Fishbein, M, Hennessy, M, Yzer, M, and Douglas, J. Can We Explain Why Some People Do and Some People Do Not Act on Their Intentions? Psychol Health Med (2003) 8:3–18. doi:10.1080/1354850021000059223
13. Iihara, N, Nishio, T, Okura, M, Anzai, H, Kagawa, M, Houchi, H, et al. Comparing Patient Dissatisfaction and Rational Judgment in Intentional Medication Non-adherence versus Unintentional Non-adherence. J Clin Pharm Ther (2014) 39:45–52. doi:10.1111/jcpt.12100
14. Griva, K, Davenport, A, Harrison, M, and Newman, SP. Non-adherence to Immunosuppressive Medications in Kidney Transplantation: Intent vs. Forgetfulness and Clinical Markers of Medication Intake. Ann Behav Med (2012) 44:85–93. doi:10.1007/s12160-012-9359-4
15. Evans, RW, Applegate, WH, Briscoe, DM, Cohen, DJ, Rorick, CC, Murphy, BT, et al. Cost-related Immunosuppressive Medication Nonadherence Among Kidney Transplant Recipients. Clin J Am Soc Nephrol (2010) 5:2323–8. doi:10.2215/CJN.04220510
16. De Geest, S, Burkhalter, H, Bogert, L, Berben, L, Glass, TR, Denhaerynck, K, et al. Describing the Evolution of Medication Nonadherence from Pretransplant until 3 Years post-transplant and Determining Pretransplant Medication Nonadherence as Risk Factor for post-transplant Nonadherence to Immunosuppressives: The Swiss Transplant Cohort Study. Transpl Int (2014) 27:657–66. doi:10.1111/tri.12312
17. Cossart, AR, Staatz, CE, Campbell, SB, Isbel, NM, and Cottrell, WN. Investigating Barriers to Immunosuppressant Medication Adherence in Renal Transplant Patients. Nephrology (Carlton) (2019) 24:102–10. doi:10.1111/nep.13214
18. Michaud, L, Ludwig, G, Berney, S, Rodrigues, S, Niquille, A, Santschi, V, et al. Immunosuppressive Therapy after Solid-Organ Transplantation: Does the INTERMED Identify Patients at Risk of Poor Adherence? Pharm Pract (2016) 14:822. doi:10.18549/PharmPract.2016.04.822
19. Fine, RN, Becker, Y, De Geest, S, Eisen, H, Ettenger, R, Evans, R, et al. Nonadherence Consensus Conference Summary Report. Am J Transpl (2009) 9:35–41. doi:10.1111/j.1600-6143.2008.02495.x
20. De Geest, S, Dobbels, F, Fluri, C, Paris, W, and Troosters, T. Adherence to the Therapeutic Regimen in Heart, Lung, and Heart-Lung Transplant Recipients. J Cardiovasc Nurs (2005) 20(5):S88–S98. doi:10.1097/00005082-200509001-00010
21. Denhaerynck, K, Burkhalter, F, Schafer-Keller, P, Steiger, J, Bock, A, and De Geest, S. Clinical Consequences of Non Adherence to Immunosuppressive Medication in Kidney Transplant Patients. Transpl Int (2009) 22:441–6. doi:10.1111/j.1432-2277.2008.00820.x
22. Takemoto, SK, Pinsky, BW, Schnitzler, MA, Lentine, KL, Willoughby, LM, Burroughs, TE, et al. A Retrospective Analysis of Immunosuppression Compliance, Dose Reduction and Discontinuation in Kidney Transplant Recipients. Am J Transpl (2007) 7:2704–11. doi:10.1111/j.1600-6143.2007.01966.x
23. Hansen, R, Seifeldin, R, and Noe, L. Medication Adherence in Chronic Disease: Issues in Posttransplant Immunosuppression. Transpl Proc (2007) 39:1287–300. doi:10.1016/j.transproceed.2007.02.074
24. Chisholm, MA, Williamson, GM, Lance, CE, and Mulloy, LL. Predicting Adherence to Immunosuppressant Therapy: a Prospective Analysis of the Theory of Planned Behaviour. Nephrol Dial Transpl (2007) 22:2339–48. doi:10.1093/ndt/gfm149
25. Griva, K, Neo, HLM, and Vathsala, A. Unintentional and Intentional Non-adherence to Immunosuppressive Medications in Renal Transplant Recipients. Int J Clin Pharm (2018) 40:1234–41. doi:10.1007/s11096-018-0652-6
26. Iihara, N, Tsukamoto, T, Morita, S, Miyoshi, C, Takabatake, K, and Kurosaki, Y. Beliefs of Chronically Ill Japanese Patients that lead to Intentional Non-adherence to Medication. J Clin Pharm Ther (2004) 29:417–24. doi:10.1111/j.1365-2710.2004.00580.x
27. Denhaerynck, K, Berben, L, Dobbels, F, Russell, CL, Crespo-Leiro, MG, Poncelet, AJ, et al. Multilevel Factors Are Associated with Immunosuppressant Nonadherence in Heart Transplant Recipients: The International BRIGHT Study. Am J Transpl (2018) 18:1447–60. doi:10.1111/ajt.14611
28. Berben, L, Dobbels, F, Engberg, S, Hill, MN, and De Geest, S. An Ecological Perspective on Medication Adherence. West J Nurs Res (2012) 34:635–53. doi:10.1177/0193945911434518
29. Schoen, C, Osborn, R, Squires, D, and Doty, MM. Access, Affordability, and Insurance Complexity Are Often Worse in the United States Compared to Ten Other Countries. Health Aff (Millwood) (2013) 32:2205–15. doi:10.1377/hlthaff.2013.0879
30. Berben, L, Denhaerynck, K, Dobbels, F, Engberg, S, Vanhaecke, J, Crespo-Leiro, MG, et al. Building Research Initiative Group: Chronic Illness Management and Adherence in Transplantation (BRIGHT) Study: Study Protocol. J Adv Nurs (2015) 71:642–54. doi:10.1111/jan.12519
31. Cohen, J, Cohen, P, West, SG, and Aiken, LS. Applied Multiples Regression/correlation Analysis for the Behavioral Sciences. 3ed. New York: Routledge (2003).
32. Denhaerynck, K, Dobbels, F, Koštálová, B, and De Geest, SBAASIS consortium. Psychometric Properties of the BAASIS: A Meta-Analysis of Individual Participant Data. Transplantation (2023). [Epub Ahead of Print]. doi:10.1097/TP.0000000000004574
33. Fishbein, M. A Reasoned Action Approach to Health Promotion. Med Decis Making (2008) 28:834–44. doi:10.1177/0272989X08326092
34. Fishbein, M, and Yzer, CM. Using Theory to Design Effective Health Behavior Interventions. Theor Health Interventions (2003) 13:164–83. doi:10.1111/j.1468-2885.2003.tb00287.x
35. Schmid-Mohler, G, Thut, MP, Wuthrich, RP, Denhaerynck, K, and De Geest, S. Non-adherence to Immunosuppressive Medication in Renal Transplant Recipients within the Scope of the Integrative Model of Behavioral Prediction: a Cross-Sectional Study. Clin Transpl (2010) 24:213–22. doi:10.1111/j.1399-0012.2009.01056.x
36. Ajzen, I. The Theory of Planned Behavior: Frequently Asked Questions. Hum Behav Emerg Tech (2020) 2:314–24. doi:10.1002/hbe2.195
37. Butler, JA, Peveler, RC, Roderick, P, Smith, PW, Horne, R, and Mason, JC. Modifiable Risk Factors for Non-adherence to Immunosuppressants in Renal Transplant Recipients: a Cross-Sectional Study. Nephrol Dial Transpl (2004) 19:3144–9. doi:10.1093/ndt/gfh505
38. Denhaerynck, K, Steiger, J, Bock, A, Schafer-Keller, P, Kofer, S, Thannberger, N, et al. Prevalence and Risk Factors of Non-adherence with Immunosuppressive Medication in Kidney Transplant Patients. Am J Transpl (2007) 7:108–16. doi:10.1111/j.1600-6143.2006.01611.x
39. Gifford, AL, Bormann, JE, Shively, MJ, Wright, BC, Richman, DD, and Bozzette, SA. Predictors of Self-Reported Adherence and Plasma HIV Concentrations in Patients on Multidrug Antiretroviral Regimens. J Acquir Immune Defic Syndr (2000) 23:386–95. doi:10.1097/00126334-200004150-00005
40. Greenstein, S, and Siegal, B. Compliance and Noncompliance in Patients with a Functioning Renal Transplant: a Multicenter Study. Transplantation (1998) 66:1718–26. doi:10.1097/00007890-199812270-00026
41. Russell, CL, Kilburn, E, Conn, VS, Libbus, MK, and Ashbaugh, C. Medication-taking Beliefs of Adult Renal Transplant Recipients. Clin Nurse Spec (2003) 17:200-8–209-30. doi:10.1097/00002800-200307000-00018
42. Denhaerynck, K, Abraham, I, Gourley, G, Drent, G, De Vleeschouwer, P, Papajcik, D, et al. Validity Testing of the Long-Term Medication Behavior Self-Efficacy Scale. J Nurs Meas (2003) 11:267–82. doi:10.1891/jnum.11.3.267.61271
43.The Transplant360 Task Force. TRANPLANT360 (2017). Available from: https://www.transplant360.com (Accessed June 29, 2017).
44. Honaker, J, King, G, and Blackwell, M. Amelia II: A Program for Missing Data. J Stat Softw (2011) 45(7):1–47. doi:10.18637/jss.v045.i07
45.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2022). Available at: https://www.R-project.org/.
46. Dima, AL, Allemann, SS, Dunbar-Jacob, J, Hughes, DA, Vrijens, B, and Wilson, IB. TEOS: A Framework for Constructing Operational Definitions of Medication Adherence Based on Timelines-Events-Objectives-Sources. Br J Clin Pharmacol (2021) 87:2521–33. doi:10.1111/bcp.14659
47. De Geest, S, Zullig, LL, Dunbar-Jacob, J, Helmy, R, Hughes, DA, Wilson, IB, et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med (2018) 169:30–5. doi:10.7326/M18-0543
48. Doesch, AO, Mueller, S, Akyol, C, Erbel, C, Frankenstein, L, Ruhparwar, A, et al. Increased Adherence Eight Months after Switch from Twice Daily Calcineurin Inhibitor Based Treatment to once Daily Modified Released Tacrolimus in Heart Transplantation. Drug Des Devel Ther (2013) 7:1253–8. doi:10.2147/DDDT.S52820
49. Shemesh, Y, Peles-Bortz, A, Peled, Y, HarZahav, Y, Lavee, J, Freimark, D, et al. Feelings of Indebtedness and Guilt toward Donor and Immunosuppressive Medication Adherence Among Heart Transplant (HTx) Patients, as Assessed in a Cross-Sectional Study with the Basel Assessment of Adherence to Immunosuppressive Medications Scale (BAASIS). Clin Transpl (2017) 31:10. doi:10.1111/ctr.13053
50. Pereira, GLA, Almeida, GPL, Rocha, EMM, Braga, CJM, and Aragao, GF. Pharmaceutical Care in Immunosuppressive Therapy for Heart Transplant Patients in Ceará. MOJ Biol Med (2022) 7:2. doi:10.15406/mojbm.2022.07.00166
51. Poltronieri, NVG, Moreira, RSL, Schirmer, J, and Roza, BA. Medication Non-adherence in Heart Transplant Patients. Rev Esc Enferm USP (2020) 54:e03644. doi:10.1590/S1980-220X2019009203644
52. Moon, Z, Moss-Morris, R, Hunter, MS, Norton, S, and Hughes, LD. Nonadherence to Tamoxifen in Breast Cancer Survivors: A 12 Month Longitudinal Analysis. Health Psychol (2019) 38:888–99. doi:10.1037/hea0000785
53. Low, JK, Manias, E, Crawford, K, Walker, R, Mulley, WR, Toussaint, ND, et al. Improving Medication Adherence in Adult Kidney Transplantation (IMAKT): A Pilot Randomised Controlled Trial. Sci Rep (2019) 9:7734. doi:10.1038/s41598-019-44002-y
54. Ganjali, R, Ghorban Sabbagh, M, Nazemiyan, F, Mamdouhi, F, Badiee Aval, S, Taherzadeh, Z, et al. Factors Associated with Adherence to Immunosuppressive Therapy and Barriers in Asian Kidney Transplant Recipients. Immunotargets Ther (2019) 8:53–62. doi:10.2147/ITT.S212760
55. Foley, L, Larkin, J, Lombard-Vance, R, Murphy, AW, Hynes, L, Galvin, E, et al. Prevalence and Predictors of Medication Non-adherence Among People Living with Multimorbidity: a Systematic Review and Meta-Analysis. BMJ Open (2021) 11:e044987. doi:10.1136/bmjopen-2020-044987
56. Gokoel, SRM, Gombert-Handoko, KB, Zwart, TC, van der Boog, PJM, Moes, D, and de Fijter, JW. Medication Non-adherence after Kidney Transplantation: A Critical Appraisal and Systematic Review. Transpl Rev (Orlando) (2020) 34:100511. doi:10.1016/j.trre.2019.100511
57. Tsapepas, D, Langone, A, Chan, L, Wiland, A, McCague, K, and Chisholm-Burns, M. A Longitudinal Assessment of Adherence with Immunosuppressive Therapy Following Kidney Transplantation from the Mycophenolic Acid Observational REnal Transplant (MORE) Study. Ann Transpl (2014) 19:174–81. doi:10.12659/AOT.890216
58. Cea-Calvo, L, Marin-Jimenez, I, de Toro, J, Fuster-RuizdeApodaca, MJ, Fernandez, G, Sanchez-Vega, N, et al. Different Associations of Intentional and Non-intentional Non-adherence Behaviors with Patient Experience with Healthcare and Patient Beliefs in Medications: A Survey of Patients with Chronic Conditions. Patient Prefer Adherence (2020) 14:2439–50. doi:10.2147/PPA.S281985
59. Lowry, KP, Dudley, TK, Oddone, EZ, and Bosworth, HB. Intentional and Unintentional Nonadherence to Antihypertensive Medication. Ann Pharmacother (2005) 39:1198–203. doi:10.1345/aph.1E594
60. Brett, J, Fenlon, D, Boulton, M, Hulbert-Williams, NJ, Walter, FM, Donnelly, P, et al. Factors Associated with Intentional and Unintentional Non-adherence to Adjuvant Endocrine Therapy Following Breast Cancer. Eur J Cancer Care (Engl) (2016) 27:e12601. doi:10.1111/ecc.12601
61. Mentz, RJ, Greiner, MA, Muntner, P, Shimbo, D, Sims, M, Spruill, TM, et al. Intentional and Unintentional Medication Non-adherence in African Americans: Insights from the Jackson Heart Study. Am Heart J (2018) 200:51–9. doi:10.1016/j.ahj.2018.03.007
62. Efficace, F, Rosti, G, Cottone, F, Breccia, M, Castagnetti, F, Iurlo, A, et al. Profiling Chronic Myeloid Leukemia Patients Reporting Intentional and Unintentional Non-adherence to Lifelong Therapy with Tyrosine Kinase Inhibitors. Leuk Res (2014) 38:294–8. doi:10.1016/j.leukres.2013.07.003
63. Helmy, R, Scalso de Almeida, S, Denhaerynck, K, Berben, L, Dobbels, F, Russell, CL, et al. Prevalence of Medication Nonadherence to Co-medication Compared to Immunosuppressants in Heart Transplant Recipients: Findings from the International Cross-Sectional BRIGHT Study. Clin Ther (2019) 41:130–6. doi:10.1016/j.clinthera.2018.11.007
64. Blaschke, TF, Osterberg, L, Vrijens, B, and Urquhart, J. Adherence to Medications: Insights Arising from Studies on the Unreliable Link between Prescribed and Actual Drug Dosing Histories. Annu Rev Pharmacol Toxicol (2012) 52:275–301. doi:10.1146/annurev-pharmtox-011711-113247
65. Dobbels, F, De Geest, S, van Cleemput, J, Droogne, W, and Vanhaecke, J. Effect of Late Medication Non-compliance on Outcome after Heart Transplantation: a 5-year Follow-Up. J Heart Lung Transpl (2004) 23:1245–51. doi:10.1016/j.healun.2003.09.016
66. Kostalova, B, Mala-Ladova, K, Kubena, AA, Horne, R, Dusilova Sulkova, S, and Maly, J. Changes in Beliefs about Post-Transplant Immunosuppressants over Time and its Relation to Medication Adherence and Kidney Graft Dysfunction: A Follow-Up Study. Patient Prefer Adherence (2021) 15:2877–87. doi:10.2147/PPA.S344878
67. Laederach-Hofmann, K, and Bunzel, B. Noncompliance in Organ Transplant Recipients: a Literature Review. Gen Hosp Psychiatry (2000) 22:412–24. doi:10.1016/s0163-8343(00)00098-0
68. Denhaerynck, K, Dobbels, F, Cleemput, I, Desmyttere, A, Schafer-Keller, P, Schaub, S, et al. Prevalence, Consequences, and Determinants of Nonadherence in Adult Renal Transplant Patients: a Literature Review. Transpl Int (2005) 18:1121–33. doi:10.1111/j.1432-2277.2005.00176.x
69. De Geest, S, Abraham, I, Moons, P, Vandeputte, M, Van Cleemput, J, Evers, G, et al. Late Acute Rejection and Subclinical Noncompliance with Cyclosporine Therapy in Heart Transplant Recipients. J Heart Lung Transpl (1998) 17:854–63.
70. Lennerling, A, Kisch, A, and Anna, F. Non-adherence to Immunosuppressant after Lung Transplantation – A Common Risk Behavior. Open Nurs J (2019) 13:108–15. doi:10.2174/1874434601913010108
71. Dew, MA, DiMartini, AF, De Vito Dabbs, A, Myaskovsky, L, Steel, J, Unruh, M, et al. Rates and Risk Factors for Nonadherence to the Medical Regimen after Adult Solid Organ Transplantation. Transplantation (2007) 83:858–73. doi:10.1097/01.tp.0000258599.65257.a6
72. Doyle, IC, Maldonado, AQ, Heldenbrand, S, Tichy, EM, and Trofe-Clark, J. Nonadherence to Therapy after Adult Solid Organ Transplantation: A Focus on Risks and Mitigation Strategies. Am J Health Syst Pharm (2016) 73:909–20. doi:10.2146/ajhp150650
73. Butler, JA, Roderick, P, Mullee, M, Mason, JC, and Peveler, RC. Frequency and Impact of Nonadherence to Immunosuppressants after Renal Transplantation: a Systematic Review. Transplantation (2004) 77:769–76. doi:10.1097/01.tp.0000110408.83054.88
74. Reese, PP, Bloom, RD, Trofe-Clark, J, Mussell, A, Leidy, D, Levsky, S, et al. Automated Reminders and Physician Notification to Promote Immunosuppression Adherence Among Kidney Transplant Recipients: A Randomized Trial. Am J Kidney Dis (2017) 69:400–9. doi:10.1053/j.ajkd.2016.10.017
75. Lennerling, A, and Forsberg, A. Self-reported Non-adherence and Beliefs about Medication in a Swedish Kidney Transplant Population. Open Nurs J (2012) 6:41–6. doi:10.2174/1874434601206010041
76. Schonfeld, S, Denhaerynck, K, Berben, L, Dobbels, F, Russell, CL, Crespo-Leiro, MG, et al. Prevalence and Correlates of Cost-Related Medication Nonadherence to Immunosuppressive Drugs after Heart Transplantation: The International Multicenter Cross-Sectional Bright Study. J Cardiovasc Nurs (2020) 35:519–29. doi:10.1097/JCN.0000000000000683
77. Marsicano-Souza, EO, Colugnati, F, Geest, S, and Sanders-Pinheiro, H. Nonadherence to Immunosuppressives and Treatment in Kidney Transplant: ADHERE BRAZIL Study. Rev Saude Publica (2021) 55:33. doi:10.11606/s1518-8787.2021055002894
78. Abshire Saylor, M, Denhaerynck, K, Mielke, J, Davidson, PM, Dobbels, F, Russell, CL, et al. Multi-level Correlates of Received Social Support Among Heart Transplant Recipients in the International BRIGHT Study: a Secondary Analysis. Eur J Cardiovasc Nurs (2022) 21:857–67. doi:10.1093/eurjcn/zvac041
79. Bae, SG, Kam, S, Park, KS, Kim, KY, Hong, NS, Kim, KS, et al. Factors Related to Intentional and Unintentional Medication Nonadherence in Elderly Patients with Hypertension in Rural Community. Patient Prefer Adherence (2016) 10:1979–89. doi:10.2147/ppa.s114529
80. Brod, M, Rana, A, and Barnett, AH. Adherence Patterns in Patients with Type 2 Diabetes on Basal Insulin Analogues: Missed, Mistimed and Reduced Doses. Curr Med Res Opin (2012) 28:1933–46. doi:10.1185/03007995.2012.743458
81. Scheel, JF, Schieber, K, Reber, S, Stoessel, L, Waldmann, E, Jank, S, et al. Psychosocial Variables Associated with Immunosuppressive Medication Non-adherence after Renal Transplantation. Front Psychiatry (2018) 9:23. doi:10.3389/fpsyt.2018.00023
82. Atkins, L, and Fallowfield, L. Intentional and Non-intentional Non-adherence to Medication Amongst Breast Cancer Patients. Eur J Cancer (2006) 42:2271–6. doi:10.1016/j.ejca.2006.03.004
83. Kung, M, Koschwanez, HE, Painter, L, Honeyman, V, and Broadbent, E. Immunosuppressant Nonadherence in Heart, Liver, and Lung Transplant Patients: Associations with Medication Beliefs and Illness Perceptions. Transplantation (2012) 93:958–63. doi:10.1097/TP.0b013e31824b822d
84. Daleboudt, GM, Broadbent, E, McQueen, F, and Kaptein, AA. Intentional and Unintentional Treatment Nonadherence in Patients with Systemic Lupus Erythematosus. Arthritis Care Res (Hoboken) (2011) 63:342–50. doi:10.1002/acr.20411
85. Lindquist, LA, Go, L, Fleisher, J, Jain, N, Friesema, E, and Baker, DW. Relationship of Health Literacy to Intentional and Unintentional Non-adherence of Hospital Discharge Medications. J Gen Intern Med (2012) 27:173–8. doi:10.1007/s11606-011-1886-3
86. Nafradi, L, Galimberti, E, Nakamoto, K, and Schulz, PJ. Intentional and Unintentional Medication Non-adherence in Hypertension: The Role of Health Literacy, Empowerment and Medication Beliefs. J Public Health Res (2016) 5:762. doi:10.4081/jphr.2016.762
87. Fan, JH, Lyons, SA, Goodman, MS, Blanchard, MS, and Kaphingst, KA. Relationship between Health Literacy and Unintentional and Intentional Medication Nonadherence in Medically Underserved Patients with Type 2 Diabetes. Diabetes Educator (2016) 42:199–208. doi:10.1177/0145721715624969
88. Hugon, A, Roustit, M, Lehmann, A, Saint-Raymond, C, Borrel, E, Hilleret, MN, et al. Influence of Intention to Adhere, Beliefs and Satisfaction about Medicines on Adherence in Solid Organ Transplant Recipients. Transplantation (2014) 98:222–8. doi:10.1097/tp.0000000000000221
89. Moon, Z, Moss-Morris, R, Hunter, MS, and Hughes, LD. More Than Just Side-Effects: The Role of Clinical and Psychosocial Factors in Non-adherence to Tamoxifen. Br J Health Psychol (2017) 22:998–1018. doi:10.1111/bjhp.12274
90. Geissler, J, Efficace, F, Bombaci, F, De Jong, J, Gavin, AM, Dziwinski, EJ, et al. Factors Predicting Intentional Non-adherence in Chronic Myeloid Leukemia: A Multivariate Analysis on 2546 Patients by the CML Advocates Network. Blood (2013) 122:4023. doi:10.1182/blood.v122.21.4023.4023
91. Silva, AN, Moratelli, L, Tavares, PL, Marsicano, EO, Pinhati, RR, Colugnati, FA, et al. Self-efficacy Beliefs, Locus of Control, Religiosity and Non-adherence to Immunosuppressive Medications in Kidney Transplant Patients. Nephrology (Carlton) (2016) 21:938–43. doi:10.1111/nep.12695
92. Huyard, C, Derijks, L, Haak, H, and Lieverse, L. Intentional Nonadherence as a Mean to Exert Control. Qual Health Res (2017) 27:1215–24. doi:10.1177/1049732316688882
93. Ostini, R, and Kairuz, T. Investigating the Association between Health Literacy and Non-adherence. Int J Clin Pharm (2014) 36:36–44. doi:10.1007/s11096-013-9895-4
94. Wayda, B, Clemons, A, Givens, RC, Takeda, K, Takayama, H, Latif, F, et al. Socioeconomic Disparities in Adherence and Outcomes after Heart Transplant: A UNOS (United Network for Organ Sharing) Registry Analysis. Circ Heart Fail (2018) 11:e004173. doi:10.1161/CIRCHEARTFAILURE.117.004173
95. Schafer-Keller, P, Steiger, J, Bock, A, Denhaerynck, K, and De Geest, S. Diagnostic Accuracy of Measurement Methods to Assess Non-adherence to Immunosuppressive Drugs in Kidney Transplant Recipients. Am J Transpl (2008) 8:616–26. doi:10.1111/j.1600-6143.2007.02127.x
96. Williams, AB, Amico, KR, Bova, C, and Womack, JA. A Proposal for Quality Standards for Measuring Medication Adherence in Research. AIDS Behav (2013) 17:284–97. doi:10.1007/s10461-012-0172-7
97. Helmy, R, Duerinckx, N, De Geest, S, Denhaerynck, K, Berben, L, Russell, CL, et al. The International Prevalence and Variability of Nonadherence to the Nonpharmacologic Treatment Regimen after Heart Transplantation: Findings from the Cross-Sectional BRIGHT Study. Clin Transpl (2018) 32:e13280. doi:10.1111/ctr.13280
98. Deschamps, AE, De Geest, S, Vandamme, AM, Bobbaers, H, Peetermans, WE, and Van Wijngaerden, E. Diagnostic Value of Different Adherence Measures Using Electronic Monitoring and Virologic Failure as Reference Standards. AIDS Patient Care STDS (2008) 22:735–43. doi:10.1089/apc.2007.0229
99. Ruygrok, PN, Agnew, TM, Coverdale, HA, Whitfield, C, and Lambie, NK. Survival after Heart Transplantation without Regular Immunosuppression. J Heart Lung Transpl (1994) 13:208–11.
100. Ortalli, G. Suicide by Interruption of Immunosuppressive Therapy. J Cardiothorac Vasc Anesth (1992) 6:644. doi:10.1016/1053-0770(92)90134-s
101. Chang, DH, Youn, J-C, Dilibero, D, Patel J, K, and Kobashigawa J, A. Heart Transplant Immunosuppression Strategies at Cedars-Sinai Medical Center. Int J Heart Fail (2021) 3:15–30. doi:10.36628/ijhf.2020.0034
102. Kasiske, BL, Chakkera, HA, Louis, TA, and Ma, JZ. A Meta-Analysis of Immunosuppression Withdrawal Trials in Renal Transplantation. J Am Soc Nephrol (2000) 11:1910–7. doi:10.1681/ASN.V11101910
103. Londono, MC, Rimola, A, O'Grady, J, and Sanchez-Fueyo, A. Immunosuppression Minimization vs. Complete Drug Withdrawal in Liver Transplantation. J Hepatol (2013) 59:4. doi:10.1016/j.jhep.2013.04.003
104. Hewison, A, Atkin, K, McCaughan, D, Roman, E, Smith, A, Smith, G, et al. Experiences of Living with Chronic Myeloid Leukaemia and Adhering to Tyrosine Kinase Inhibitors: A Thematic Synthesis of Qualitative Studies. Eur J Oncol Nurs (2020) 45:101730. doi:10.1016/j.ejon.2020.101730
105. Nguyen, TM, La Caze, A, and Cottrell, N. What Are Validated Self-Report Adherence Scales Really Measuring?: a Systematic Review. Br J Clin Pharmacol (2014) 77:427–45. doi:10.1111/bcp.12194
Keywords: immunosuppression, heart transplantation, medication non-adherence, intentional non-adherence, correlates
Citation: Marston MT, Berben L, Dobbels F, Russell CL and de Geest S (2023) Prevalence and Patient-Level Correlates of Intentional Non-Adherence to Immunosuppressive Medication After Heart-Transplantation—Findings From the International BRIGHT Study. Transpl Int 36:11308. doi: 10.3389/ti.2023.11308
Received: 25 February 2023; Accepted: 15 June 2023;
Published: 10 July 2023.
Copyright © 2023 Marston, Berben, Dobbels, Russell and de Geest. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sabina de Geest, sabina.degeest@unibas.ch
 Mark T. Marston
Mark T. Marston Lut Berben
Lut Berben Fabienne Dobbels
Fabienne Dobbels Cynthia L. Russell4 and
Cynthia L. Russell4 and  Sabina de Geest
Sabina de Geest