Upgrading the fixed-time artificial insemination (FTAI) protocol in Romanian buffaloes
- Department of Clinical Sciences, Faculty of Veterinary Medicine, Iasi University of Life Sciences, Iasi, Romania
The present study describes the challenges of assisted reproduction in Romanian buffaloes while increasing the efficacy of artificial insemination by choosing the most suitable method. The modified fixed-time artificial insemination (FTAI) protocol with sexed semen was used to increase the conception rate. This study included a total of 80 buffalo heifers that received ovarian stimulation using the OvSynch protocol. Two groups (n = 40), namely, a control group, in which the classic FTAI method was performed, and an experimental group, in which deep intrauterine AI was performed in cows that had developed a dominant follicle (US+UcFTAI), were randomly selected. The conception rate (CR) was 63.6% in the experimental group, which was statistically higher (P < 0.05) than the control group (30%). The ultrasound examination indicated that, using the OvSynch protocol, 82.5% (33 out of 40) of buffaloes developed a dominant follicle (DF) while the distribution between the warm and cold seasons was 75 and 90%, respectively. The CR was 60% during the hot season and 66.6% during the cold season. At calving, 92.5% female fetuses were born. The improved FTAI method in this study enhanced the results by reducing the waste of sexed semen and maximizing the response to OvSynch, making it a recommendation for practitioners. This study presents preliminary results and highlights that genetic progress is difficult to achieve. A systematic approach is needed in order to choose the most suitable biotechnological method for each farm.
1. Introduction
Various animal husbandry systems are currently used in most buffalo breeding countries, such as India (1), Brazil (2), Italy (3), and China (4, 5), within smaller or larger farms. As the number of local buffaloes in Romania is constantly decreasing, the application of current reproductive biotechnologies in these breeds remains limited (6, 7). Several European countries with a strong tradition in milk buffalo production heavily rely on AI. Nevertheless, many farms continue to use bulls and combine AI with natural breeding methods (3).
The Romanian buffalo is characterized by seasonal reproductive activity, with over 50.8% spontaneous estrus occurring during the spring season. They typically reach sexual maturity at a later stage, after 22 months, and have a calving interval between 443 and 547 days. Estrus lasts only for a short duration of time, i.e., 8–12 h, and is weakly expressed clinically, with small dominant and ovulatory follicles measuring 9–1.2 cm and poorly developed CL of 1 cm. Although the ovaries are small in size, (measuring 24.3 mm in length, 18.25 mm in width, 13.15 mm in thickness, and weighing 4.53 g), they have a very high reactive activity. The conception rate achieved through AI varies between 26 and 30% (6, 8).
The current research study indicates promising prospects for combining assisted reproduction and genomic evaluation, which can be used successfully for populations with limited sizes (9). Deep uterine AI offers the advantage of depositing semen closer to the utero-tubal junction (10) while allowing for the use of a lower dose and sexed semen (11). Utilizing ipsilateral ultrasound guidance toward the ovary with DF (8) leads to an increase in the conception rate.
In the past decade, assisted reproduction in Romanian buffaloes has yielded unsatisfactory outcomes; however, research development and optimization of reproductive biotechniques have begun to produce promising results (6–8, 12, 13).
Anestrus in buffaloes poses a costly challenge and a reproductive problem, resulting in a low conception rate. Researchers worldwide have employed various estrus induction protocols utilizing different hormones to address anestrus, with varying success rates (14–18).
Poor endocrine status of buffaloes (19), lower number of follicles (20), high incidence of silent estrus, long calving interval (12), weak palpable ovarian structures on transrectal examination (18, 21), seasonality of reproduction and poor fertility (3), and high incidence of follicular atresia (22) are some of the limiting factors that probably resulted in moderate responses of buffalo cows to reproductive biotechnologies (18). Currently, there are several limiting factors affecting the implementation of AI in buffaloes. Therefore, the use of hormonal protocols associated with FTAI makes reproduction in buffaloes more advantageous and practical (18, 21, 23). Furthermore, the small number of genomically tested buffalo bulls, compared to cattle, and the low conception rates obtained in AI programs discouraged buffalo farmers from adopting this technique (6). Although reproduction management protocols are similar to those of cows, they have a limited application due to low estrus expression and poor detection rates, a variable duration of estrus, and difficulty in predicting the time of ovulation (12).
Since its development, ovulation synchronization (OvSynch) and timed artificial insemination (TAI) programs (OvSynch+TAI) have significantly reduced the time and costs associated with reproduction (24).
During the breeding season, using the OvSynch protocol together with FTAI for 16–20 h, after the second GnRH injection, yielded an acceptable CR (35%-56%) in buffaloes although lower conception rates (CR) have also been reported (25, 26). Subsequently, the model was adopted and tested on river buffaloes in different seasons (19, 21) and implemented to a limited extent in swamp buffaloes as well (22, 27).
These data could be beneficial in improving and establishing the most suitable reproductive model for future studies and for the efficiency of reproductive biotechnologies in the buffalo industry.
This study aims to describe the challenges of assisted reproduction in buffaloes, including low conception rates after implementing AI, susceptibility to seasonality, and difficulties in estrus detection. These challenges are primarily attributed to unique characteristics of the estrous cycle in Romanian buffaloes, where signs of estrus are weakly expressed clinically, accompanied by a short estrus duration, small dominant and pre-ovulatory follicles, and underdeveloped CL. Moreover, an improved FTAI method using sexed semen was tested to increase the conception rate.
2. Materials and methods
2.1. Buffalo cows
The experiment was performed on a total of 80 primiparous buffalo cows, specifically of the Romanian Indigenous Buffalo (RIB) variety, belonging to the Mediterranean breed. Our goal was to form a nucleus of females with increased genetic potential.
Young buffalo heifers were selected based on their age (over 22 months), weight (over 250 kg), and a body condition score (BCS) of around 3. In terms of sexual cyclicity, only cattle that had passed the onset of puberty and had active physiological structures on the ovaries were selected. The ovaries and uterus showed no signs of pathology, and the shape and size of the ovaries were within the normal range, measuring at least 2 cm in width and displaying physiological structures.
The group containing 80 female buffaloes was subjected to a thorough gynecological and general examination (see Figure 1). They were then divided into two groups: a control group and an experimental group, containing equal number of buffaloes in each group. Each female buffalo was randomly selected from either the control or experimental group (n = 40 FTAI/US+UcFTAI). Then, AI was performed using sexed semen from two fertile bulls during both the warm and cold seasons. It is worth noting that, locally, the breeding season aligns with the cold months (cold season).
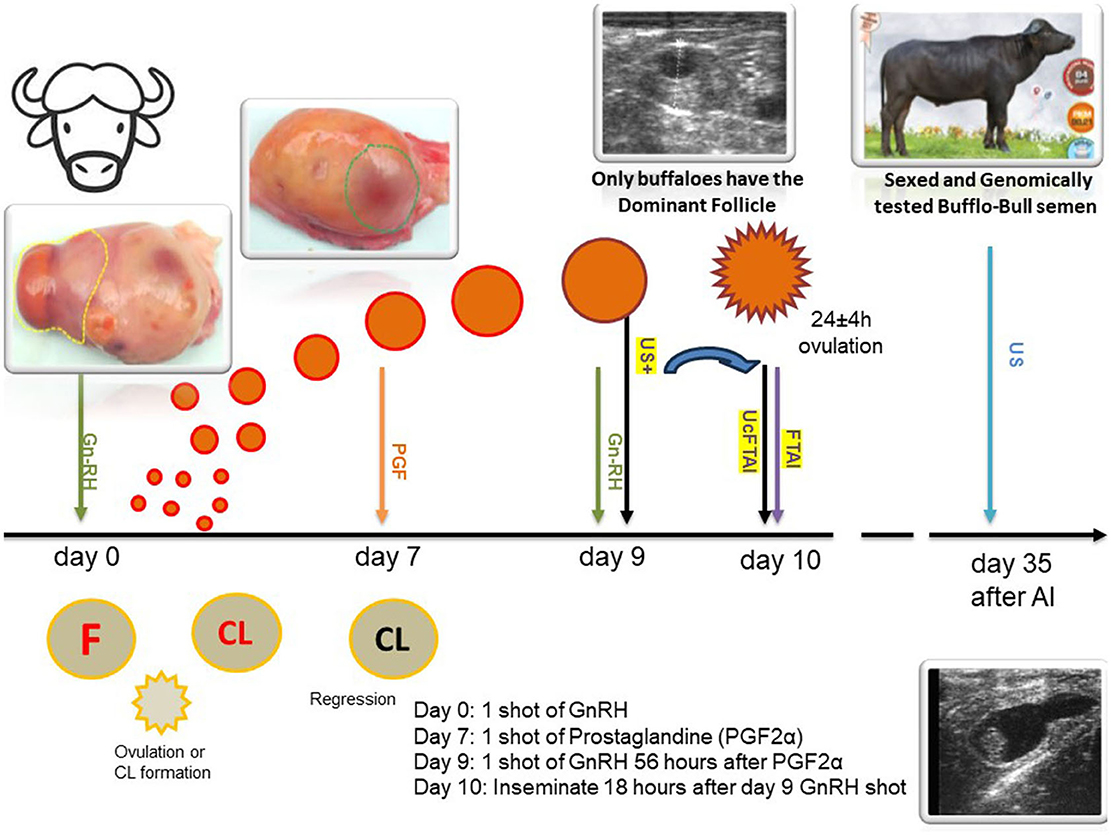
Figure 1. Scheme of the US+UcFTAI and FTAI method with semen sexed according to the OvSynch protocol in Romanian buffalo (CIB).
2.2. Buffalo bull semen
Two different bulls, tested genomically and using sexed semen, from Associatzione Nazionale Allevatori Specie Bufalina (Italy) Oro Mediterraneo and Aton del Parco, were used. The semen was purchased, transported, and stored in optimal conditions by a local importer, in straws containing 2 million X-chromosome-bearing sperm.
2.3. Care management
All biosecurity, nutrition, welfare, maintenance, vaccination, and deworming conditions were ensured. For ensuring that their living conditions were comfortable, the animals had thermic comfort, by preventing extreme heat or cold. This allowed for continuous reproductive activity, without the usual seasonal changes.
2.4. Ultrasound monitoring
Ovarian dynamics and follicular evolution of buffalo cows during the estrous synchronization treatment were monitored using transrectal ultrasonography with a Honda HS-1600V® ultrasound scanner from Japan equipped with a 5–7.5 MHz and Doppler transducer (8). Transrectal genital ultrasound examination was performed at three crucial time points: before assembling the experimental and control groups to exclude those with uterine/ovarian pathology, ensuring that only normally cycling cows were included; 18 h before the scheduled time for AI to select heifers that had a DF on the ovary (US+UcFTAI), and 35 days after AI for pregnancy diagnosis and calculation of the conception rate (see Figure 1).
2.5. Hormone treatment management
The groups, control and experimental groups, were formed after a thorough gynecological and general examination. Subsequently, the OvSynch (GnRH-PGF-GnRH) protocol was initiated. According to the treatment scheme, buffaloes received IM injections on day 0 and 9 with a dosage of 0.01 mg buserelin acetate (Receptal®, MDS, The Netherlands). On the seventh day, they received a PGF2alpha synthetic analog, that is, cloprostenol 500 μg (Estrumate®, MDS, The Netherlands).
2.6. Artificial insemination and time management (FTAI/US+UcFTAI)
The control group was artificially inseminated on day 10 without the need to detect clinical signs of estrus, following the classic FTAI method. In the experimental group (US+UcFTAI), to prevent waste, only buffaloes with a dominant follicle (DF) measuring at least 0.9 mm on the ovary were inseminated on day 9 (27). The AI method employed was recto-vaginal, with the female buffaloes inseminated once, 18 h after the second GnRH injection (FT-fixed times). Sexed semen was deposited only in the uterine horn ipsilateral to the ovary carrying the dominant follicle (deep Uc-unicornual). The insemination technique followed the classical Anglo-Saxon method, by transrectally locating the cervix and transcervical passage of the Cassou insemination gun. A plastic protective sheet was also used.
2.7. Statistical analyses
Data of conception rates, estrus, and ovulation across the groups were compared through the chi-squared test, and the value of p of < 0.05 was considered statistically significant. Statistical analysis was performed using Prism version 8 (GraphPad software 5.0. La Jolla, CA, USA, www.graphpad.com, accessed on 28 August 2023).
3. Results
The experimental group, using the modified OvSynch protocol, US+UcFTAI, achieved a CR of 63.6% (21 out of 33) with a p-value of 0.00405. In the control group, all females were inseminated using FTAI protocol, resulting in a conception rate of 30% (12 out of 40) with differences between the warm and cold seasons (25 vs. 35%) (P > 0.05) and between Oro and Aton bulls (35 vs. 25%) (p > 0.05). At parturition, 91.6% females were obtained (Table 1).
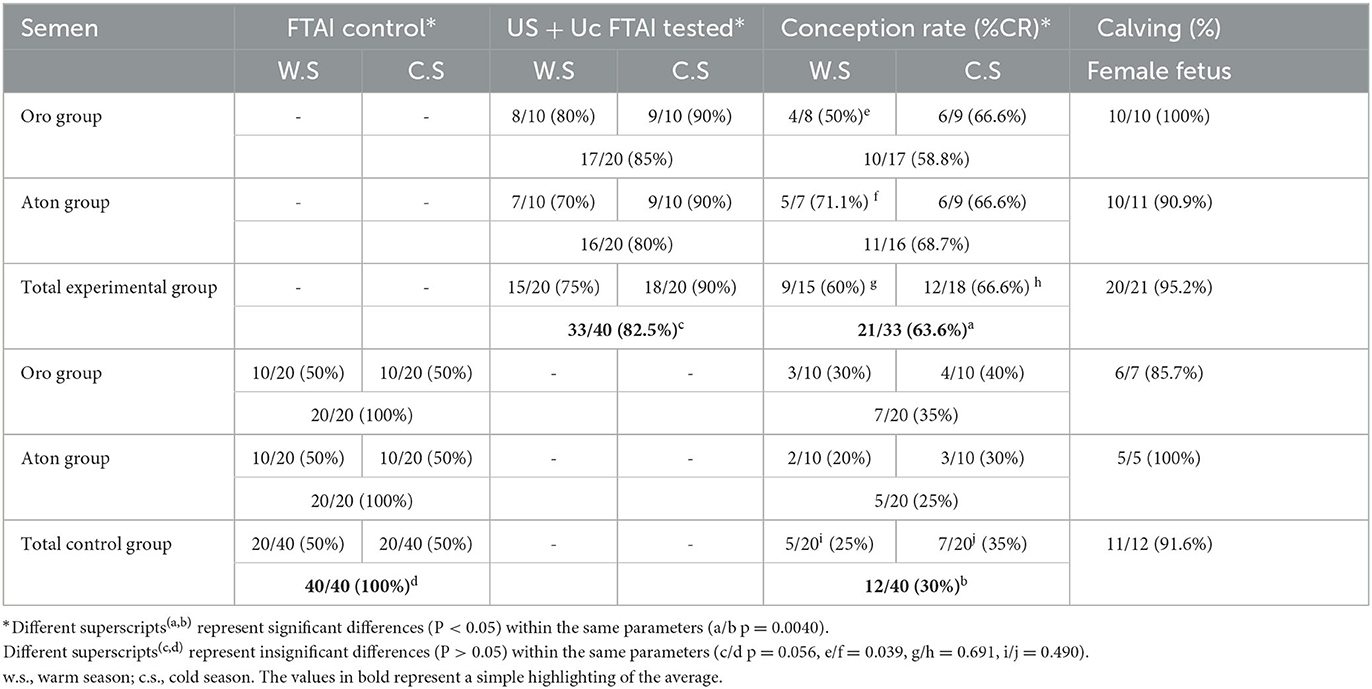
Table 1. Conception rate in Romanian Indigenous Buffalo (RIB) according to the FTAI and ES+UcFTAI protocol.
The statistical difference between the females inseminated in the control group and the experimental group that developed a DF was inconclusive with a p-value of 0.056 (>0.05). Before insemination, an ultrasound examination was performed. In the experimental group, 33 out of 40 buffaloes (82.5%) had a new dominant follicle of at least 0.9 cm in diameter in one of the ovaries. It is noteworthy to mention that 82.5% of females had a complete response and were considered to show a positive reaction to hormonal estrus induction, with or without behavioral clinical signs. These females were inseminated with semen from two buffalo bulls, with 85% from Oro's group and 80% from Aton's (see Table 1). Regarding seasonal distribution, the dominant follicle was detected in 75% (15 out of 20) of the buffaloes in the warm season and 90% (18 out of 20) during the cold season. The total conception rate was 63.6% (21 out of 33), which is statistically higher (p < 0.05) than the control group. The distribution between the two seasons (warm/cold) was 60% (9 out of 15) compared to 66.6% (12 out of 18), which was statistically insignificant (p > 0.05). By categories of bulls, the percentages were also similar, with 60% in Oro's group (9 out of 15) and 66.6% in Aton's (12 out of 18), (p < 0.05). A difference in fertility between bulls was only observed during the summer season, with 71.7% (5/7) in Aton's group and 50% (4/8) in Oro's group, but it was statistically insignificant (p > 0.05).
Regarding the sex ratio, after calving, 92.5% (20 out of 21) of female fetuses were obtained, and only in Aton's group, one male was born.
4. Discussion
As the number of buffaloes in Romania has been decreasing during the last few decades, it has become challenging to form homogeneous buffalo groups from the local variety (7). The seasonal buffalo anestrous in countries north of the equator has been attributed to factors such as thermic stress (India, Pakistan, and Egypt), low environmental humidity (Venezuela), or photoperiodicity (Italy and European Countries) (18). In Romania, the breeding season for the Romanian Indigenous Buffalo is considered to occur during the cold season (autumn–winter) (6, 12).
Some veterinary drugs, such as progestogens, gonadotropin-releasing hormone (GnRH), prostaglandin F2alpha (PGF2α), and eCG, have been used with different schemes, such as OvSynch, to control estrus (13) in buffaloes. However, the results have been variable and inconclusive (26, 27). Therefore, there is a need to continue the research efforts aimed at innovation in this field.
According to the literature, ovulation induced in heifers following the GnRH–PGF2α protocol can be anticipated within approximately 28 h (6, 21). The authors recommend the administration of GnRH at the end of the growth phase or at the beginning of the static phase of the dominant follicle, with GnRH administered within 72, 48, or 24 h after PGF2α (28).
Pursley et al. (29) were among the first to use a protocol for estrus synchronization and ovulation in cows with fixed-time insemination (19). Since then, various protocols based on OvSynch have emerged to improve CR (13, 21, 30). These protocols have also been applied to buffalo cows but yielded lower results compared to cows (26, 27). Recently, it was stated that CR varies between 30 and 60% (3, 4), depending on the season, treatment, management, or age (19), with rates ranging from 26 to 80%. The double OvSynch protocol, combined with ultrasound confirmation of pregnancy, was shown to improve the CR, in Italian farms, for constant milk production (3).
Genetic enhancement and the acquisition of individuals with superior production quality (18) can be achieved over several generations by implementing the classic breeding protocols and selecting only animals with validated productivity for breeding. Given that the buffalo population is small and the production value is low, it is necessary to use modern and current reproductive biotechnologies, such as artificial insemination (AI), as a measure of improvement and genetic progress in the buffalo herd. The methods and techniques are, and must be, in continuous development, with only those suitable for each farmer, species, and breed being employed (8, 24). It is noteworthy that AI is practiced very infrequently in Europe, with only 5% of buffaloes in Italy, and merely 0.1% in Romania (31).
Considering the physiological characteristics described above, regarding follicular development, the occurrence of a DF and ovulation following OvSynch and recognizing that not all females develop and ovulate a follicle, there arose a need to control the development of DFs through ultrasound examination before FTAI. The conception rate obtained was higher (63.6%) in this experimental group (US+UcFTAI). This increase was possible for two reasons: On the one hand, due to the fact that sexed semen was placed closer to the fertilization site, and on the other hand, due to the ultrasound selection of cows with highest likelihood to ovulate (present DF).
The results of this study (with CR of 63.6%) surpass other publications that only used the OvSynch protocol. These other studies reported conception rates of 48.8% (27), 56.5% (29) during the mating season, 56.7% (32) in multiparous females, and 6.9% (27) during the non-mating season. Other studies on CR after stimulation with OvSynch has shown variation between 36.0 and 42.55% when used during the breeding season with conventional semen (18, 30).
Studies published by Paul and Prakash, using the OvSynch protocol in non-lactating Murah buffaloes, reported an ovulation of 90% in 23.3 h, but the CR was 33.3% at FTAI (12 and 24 h after OvSynch) and 30.7% in spontaneous estrus (after 12 h of OvSynch-induced estrus) (33).
Moreover, by implementing the US+UcFTAI modified protocol in both the cold and warm seasons, a favorable ovarian reaction was observed in 82.5% of buffaloes. This study demonstrated consistent CR results between the warm and cold seasons, and because the maintenance and comfort conditions were optimal, the adverse effects of heat and frost, as well as ovarian inhibition, which are specific to buffaloes, were avoided.
5. Conclusion
Improvement of the FTAI protocol by incorporating ultrasound scanning and intracornual AI was performed to reduce waste and maximize the effectiveness of the OvSynch hormone therapy. The efficiency of the ovarian response was achieved through a careful gynecological selection of female heifers and bio-stimulation with bulls. It is recommended to conduct an ultrasound examination 18 h before FTAI, with the latter being ipsilateral and deeply intracornual. This approach is applied exclusively to buffaloes with DF larger than 0.9 cm. Thus, by implementing the upgraded protocol of UcFTAI with sexed semen, the objective of increasing the conception rate in buffalo populations with limited numbers becomes achievable and can potentially be expanded to a larger scale.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Iasi University of Life Sciences (IULS), Faculty of Veterinary Medicine, Bioethics Committee following the EU 2010/63 and National Directives Ord. 28/31–08–2011 and National Law 206/2004. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SC: Conceptualization, Data curation, Formal analysis, Investigation, Software, Writing—original draft, Writing—review and editing. PR: Resources, Supervision, Validation, Writing—review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
I would like to thank the pig farmer for his cooperation. I would also like to thank Alexandra Neamţu, Ph.D. in Obstetrics and Veterinary Andrology at IULS, for the English language corrections.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kumar L, Phogat JB, Pandey AK, Phulia SK, Kumar S, Dalal J. Estrus induction and fertility response following different treatment protocols in Murrah buffaloes under field conditions. Veterinary World. (2016) 9:1466. doi: 10.14202/vetworld.2016.1466-1470
2. Frares LF, Weiss RR, Kozicki LE, Santangelo RP, de Abreu RA, do Santos IW, et al. Estrus synchronization and Fixed Time Artificial Insemination (FTAI) in dairy buffaloes during seasonal anestrus. Braz Arch Biol Technol. (2013) 56:575–80. doi: 10.1590/S1516-89132013000400007
3. Otava G, Squicciarini S, Marc S, Suici T, Onan GW, Hutu I, et al. Effects of age and season on conception rate of Mediterranean Italian Dairy Buffalo (Bubalus bubalis) following oestrus synchronization and fixed-time artificial insemination. Reprod Domest Anim. (2021) 56:1511–8. doi: 10.1111/rda.14013
4. Abulaiti A, El-Qaliouby SH, El Bahgy HEK, Naseer S, Ahmed Z, Hua G, et al. GPGMH, a new fixed timed-AI synchronization regimen for swamp and river crossbred buffaloes (Bubalus bubalis). Front Vet Sci. (2021) 8:646247. doi: 10.3389/fvets.2021.646247
5. Abulaiti A, Riaz U, Naseer Z, Ahmed Z, Hua G, Yang L. Follicular dynamics during estrous cycle of pubertal, mature and postpartum crossbred (Nili Ravi × Jianghan) buffaloes. Animals. (2022) 12:1208. doi: 10.3390/ani12091208
6. Liliana C. Research on the implementation of biotechnical methods to stimulate the reproductive function of buffaloes (Carpathian Indigenous Buffalo). Ph Doctoral Thesis. USAMV, Iasi (2021).
7. Toba GF, Ciornei SG, Paraschivescu M, Toba GL, Liliana C, Banateanu F. Identification, monitoring and conservation of the biodiversity of the national heritage ofthe Romanian buffalo breed, using breeding biotechnologies. In: Harnessing Tangible and Intangible Assets in the Context of European Integration and Globalization: Challenges Ahead. (2021) I-ii:1127–1139.
8. Ciornei S. Reproductive Biotehnologies, Practical Guide. Iasi: Editura Ion Ionescu de la Brad (2021).
9. Shao B, Sun H, Ahmad MJ, Ghanem N, Abdel-Shafy H, Du C, et al. Genetic features of reproductive traits in bovine and buffalo: Lessons from bovine to buffalo. Front Genet. (2021) 12:617128. doi: 10.3389/fgene.2021.617128
10. Van Soom A, Verberckmoes S. Deep intrauterine insemination in cattle. Gynecol Obstet Fertil. (2004) 32:911–5. doi: 10.1016/j.gyobfe.2004.08.016
11. Meirelles C, Kozicki LE, Weiss RR, Segui MS, Souza A, dos Santos IW, et al. Comparison between deep intracornual artificial insemination (dIAI) and conventional artificial insemination (AI) using low concentration of spermatozoa in beef cattle. Braz Arch Biol Technol. (2012) 55:371–4. doi: 10.1590/S1516-89132012000300006
12. Ghineţ L, Drugociu D, Roşca P, Nechifor F, Agape G, Ciornei Ş. Seasonality of clinical estrus in buffalos. Lucrări Ştiin,tifice-Medicină Veterinară, Universitatea deştiinţe Agricole şi Medicină Veterinară Ion Ionescu de la Brad Iaşi. (2016) 59:348–52.
13. Ghineţ L, Ciornei LM, Drugociu DG, Roşca P, Ciornei ŞG. Induction of estrus in buffalo by luteolysis management (single PGF and dual administration). Lucrari Stiintifice-seria Medicina Veterinara. (2019) 62:442–4.
14. Barile VL, Galasso A, Marchiori E, Pacelli C, Montemurro N, Borghese A. Effect of PRID treatment on conception rate in Mediterranean buffalo heifers. Livestock Production Science. (2001) 68:283–7. doi: 10.1016/S0301-6226(00)00228-1
15. Neglia G, Gasparrini B, Di Palo R, De Rosa C, Zicarelli L, Campanile G. Comparison of pregnancy rates with two estrus synchronization protocols in Italian Mediterranean Buffalo cows. Theriogenology. (2003) 60:125–133. doi: 10.1016/S0093-691X(02)01328-6
16. Warriach HM, Channa AA, Ahmad H. Effect of oestrus synchronization methods on oestrus behavior, timing of ovulation and pregnancy rate during the breeding and low breeding seasons in Nili-Ravi buffaloes. Anim Reprod Sci. (2008) 107:62–7. doi: 10.1016/j.anireprosci.2007.06.007
17. Murugavel K, Antoine D, Raju MS, Lopez-Gatius F. The effect of addition of equine chorionic gonadotropin to a progesterone based estrous synchronization protocol in buffaloes (Bubalis bubalis) under tropical conditions. Theriogenology. (2009) 71:1120–6. doi: 10.1016/j.theriogenology.2008.12.012
18. Purohit GN, Duggal GP, Dadarwal D, Kumar D. Reproductive biotechnologies for improvement of buffalo: the current status Asian-Aust. J Anim Sci. (2003) 16:1071–86. doi: 10.5713/ajas.2003.1071
19. De Rensis F, Ronci G, Guarneri P, Nguyen BX, Presicce GA, Huszenicza G, et al. Conception rate after fixed time insemination following ovsynch protocol with and without progesterone supplementation in cyclic and non-cyclic Mediterranean Italian buffaloes (Bubalus bubalis). Theriogenology. (2005) 63:1824–31. doi: 10.1016/j.theriogenology.2004.07.024
20. Hufana-Duran D, Duran PG. Advanced reproductive technologies in water buffalo. In:Purohit GN, , editor. Bubaline Theriogenology. Berlin: Peter Lang Gmbhintl Verlag Wissensch (2015).
21. de Araujo Berber RC, Madureira EH, Baruselli PS. Comparison of two Ovsynch protocols (GnRH versus LH) for fixed timed insemination in buffalo (Bubalus bubalis). Theriogenology. (2002) 57:1421–30. doi: 10.1016/S0093-691X(02)00639-8
22. Liang XW, Qin GS, Chen MT, Liao CH, Zhang XF, Wei SJ. Technical study on estrus synchronization of buffalo. Anim Husb Vet Med. (2007) 39:6–9.
23. Ciornei SG, Drugociu D, Ciornei L, Roşca P. Ovarian response to P4-PGF-FSH treatment in Suffolk sheep and P4-PGF-PMSG synchronization in cross-bred ewes, for IVD and ET protocol. Vet Med Sci. (2022) 8:726–34. doi: 10.1002/vms3.705
24. Wang B, Xiao J, Ma Y, Gao C, Li H, Jia Y, et al. Comparison of the evaluation of combination of ultrasonography of the reproductive tract with hormone administration on dairy cow fertility. Front Vet Sci. (2022) 9:840724. doi: 10.3389/fvets.2022.840724
25. Barile VL. Fixed Time Artificial Insemination in Buffaloes. Bubaline Theriogenology. La Jolla, CA: International Veterinary Information Service (2019).
26. Brito LFC, Satrapa R, Marson EP, Kastelic JP. Efficacy of PGF2α to synchronize estrus in water buffalo cows (Bubalus bubalis) is dependent upon plasma progesterone concentration, corpus luteum size and ovarian follicular status before treatment. Anim Reprod Sci. (2002) 73:23–35. doi: 10.1016/S0378-4320(02)00124-0
27. Baruselli PS, Madureira EH, Barnabe VH, Barnabe RC, de Araújo Berber RC. Evaluation of synchronization of ovulation for fixed timed insemination in buffalo (Bubalus bubalis). Braz J Vet Res Anim Sci. (2003) 40:431–42. doi: 10.1590/S1413-95962003000600007
28. Doležel R, Cech S, Zajic J, Havliček V. Oestrus synchronization by PGF 2α and GnRH in intervals according to stage of follicular development at time of initial treatment in cows. Acta Veterinaria Brno. (2002) 71:101–8. doi: 10.2754/avb200271010101
29. Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology. (1995) 44:915–23. doi: 10.1016/0093-691X(95)00279-H
30. Monteiro BM, de Souza DC, Machado Vasconcellos GSF, Corrêa TB, Vecchio DV, de Sá Filho MF, et al. Ovarian responses of dairy buffalo cows to timed artificial insemination protocol, using new or used progesterone devices, during the breeding season (autumn–winter). Anim Sci J. (2016) 87:13–20. doi: 10.1111/asj.12400
31. Singh I, Balhara AK. New approaches in buffalo artificial insemination programs with special reference to India. Theriogenology. (2016) 86:194–9. doi: 10.1016/j.theriogenology.2016.04.031
32. Rabidas S, Gofur M. Synchronization of estrus using ovsynch protocol and fixed timed artificial insemination (FTAI) in indigenous dairy buffaloes: an effective buffalo breeding program in Bangladesh. Asian J Biol. (2017) 2:1–8. doi: 10.9734/AJOB/2017/31950
Keywords: estrus inductions, Romanian buffalo, FTAI, OvSynch, sperm
Citation: Ciornei SG and Roşca P (2023) Upgrading the fixed-time artificial insemination (FTAI) protocol in Romanian buffaloes. Front. Vet. Sci. 10:1265060. doi: 10.3389/fvets.2023.1265060
Received: 21 July 2023; Accepted: 22 September 2023;
Published: 27 October 2023.
Edited by:
Mustafa Numan Bucak, Selçuk University, TürkiyeReviewed by:
Gaffari Türk, Firat University, TürkiyeTohid Rezaei Topraggaleh, Urmia University of Medical Sciences, Iran
Mustafa Bodu, Selçuk University, Türkiye
Copyright © 2023 Ciornei and Roşca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petru Roşca, petru1065@yahoo.com
 Stefan Gregore Ciornei
Stefan Gregore Ciornei Petru Roşca
Petru Roşca