Doenitin-1: A novel Kunitz family protein with versatile functions during feeding and reproduction of the tick Haemaphysalis doenitzi
- Hebei Key Laboratory of Animal Physiology, Biochemistry and Molecular Biology, College of Life Sciences, Hebei Normal University, Shijiazhuang, China
As obligate blood-feeding ectoparasites, ticks secrete a great diversity of antithrombin molecules during feeding. In this study, a novel antithrombin gene named Doenitin-1 was characterized from the tick Haemaphysalis doenitzi. It has an open reading frame size of 426 bp; it encodes 141 amino acids and has a predicted molecular weight of 15.8 kDa. The fibrinogen coagulation test showed that the time of coagulation was increased significantly with increase in rDoenitin-1 protein concentration, and the activated partial thromboplastin time (APTT) and prothrombin time (PT) assays showed that rDoenitin-1 significantly prolonged the coagulation time of APTT, indicating that rDoenitin-1 has an anticoagulant activity in vitro. In addition, rDoenitin-1 presents a significant inhibitory activity in thrombin and cathepsin G. The hemolysis rate of rDoenitin-1 in healthy human blood cells was 4.25%, and no obvious hemolysis activity was observed. The comparison with other life stages shows that the higher expression occurs in adults, and tissue comparison indicated a higher expression in the midgut. The RNAi results indicated that interference of Doenitin-1 significantly reduced the engorgement rate and egg hatchability of H. doenitzi, and that the engorged body weight was slightly reduced. In conclusion, the results suggested that the novel gene Doenitin-1 functions in blood-feeding of H. doenitzi and performs various functions during feeding and reproduction of H. doenitzi. Doenitin-1 may be a potential vaccine candidate for tick control and for developing new antithrombotic drugs in the future.
Introduction
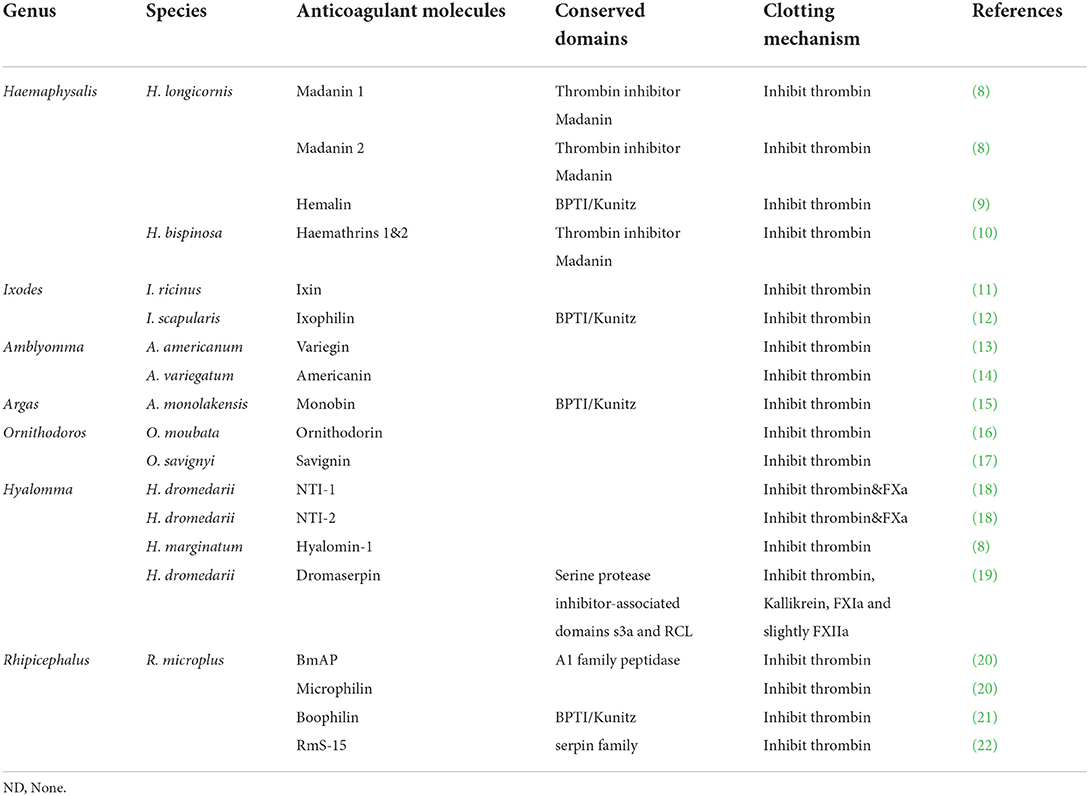
Ticks are obligate blood-feeding ectoparasites that can transmit a wide diversity of pathogens, including bacteria, viruses, and protozoans, to their hosts, causing great damages to wild animals, affecting livestock production, and threatening human health (1). Ixodid ticks usually feed for several days or weeks on their hosts and secrete many functional molecules during feeding (2). Among them, anticoagulation molecules play important roles in facilitating tick feeding (3). Anticoagulation is mainly attributed to inhibition of the clotting cascade, especially the key coagulation factors FXa and thrombin (4). Thrombin is usually composed of 36–259 amino acids; it can hydrolyze specific peptide bonds in fibrinogen to form insoluble fibrin and activate platelets and clotting factors to enhance clotting cascade (5). Therefore, a thrombin inhibitor is the most common anticoagulant molecule found in blood-sucking parasites to ensure successful blood-feeding and survival (6, 7). To date, several anticoagulants have been identified from the midgut, salivary glands, and hemolymph of ticks (Table 1). These molecules have a direct effect by keeping the blood in liquid state to ensure successful feeding of ticks (23).
Thrombin inhibitors are major members of the serine peptidase inhibitor family and have some biological activities like other family members. For example, the boophilin from Rhipicephalus (Boophilus) microplus was found to inhibit trypsin, neutrophil elastase, and bovine thrombin (21). Similarly, the anticoagulant molecules hemathrins 1 and 2 showed inhibitory effects on serine peptidases, such as thrombin, trypsin, and plasmin (10). However, the peptidases with Kunitz domains found in ticks may not only have an antithrombin function, but they also regulate host immunity (24, 25).
Haemaphysalis doenitzi is a three-host tick and widely distributed in South China, including in Fujian, Hainan, Yunnan, and Taiwan (26). In this study, a novel antithrombin gene was characterized from the tick H. doenitzi, and its inhibition of serine peptidase activity and hemolytic activity was assayed. Subsequently, potential functions were explored during blood-feeding and reproduction of H. doenitzi in the hope of identifying potential vaccine candidates for tick control and future development of new antithrombotic drugs.
Materials and methods
Collection and feeding of ticks
Wild adult H. doenitzi ticks were collected from vegetation by flag-dragging in Cangxi county (31°37′-32°10′N, 105°43′-106°28′E), Sichuan province. They were placed on the ears of New Zealand white rabbits glued with cloth bags for feeding. Those in non-parasitic stages are kept in an incubator at 26 ± 1°C and 75 ± 3% relative humidity under a 16/8-ligh/dark photoperiod. All experiments in this study were administrated with the permission of the Animal Ethics Committee of the Hebei Normal University (Protocol Number: IACUC-156027).
RNA extraction, cDNA synthesis, cloning, and sequence analysis
A number of unfed adult ticks with total body weights ranging from 50 to 80 mg were randomly selected and extracted for RNA according to the instructions of the TransZol™ Up Plus RNA Kit (TransGen Biotech, China). A cDNA library was synthesized according to Easy Script® first-strand cDNA Synthesis SuperMix (TransGen Biotech, China) and stored at −80°C.
Based on the transcriptome library of H. doenitzi deposited on GenBank (accession number: MZ981733), specific primers (forward primer 5′-CAGCGAAATGGCTTCTG-3′ and reverse primer 5′-TCAGTATTTGCAGGCGAAC-3′) were designed using Primer Premier 5.0 (Premier Biosoft International, United States). The PCR program was as follows: 94°C for 10 min, followed by 28 cycles each at 94°C for 30 s, 56°C for 30 s, 72°C for 1 min, and then a final elongation at 72°C for 10 min. The target PCR product was isolated by agarose gel electrophoresis (1%) and purified with gel extraction kits. Subsequently, the purified PCR product was inserted into the pMD®19-T Simple Vector (BGI, China) for sequencing. The confirmed sequence was analyzed by BLAST on NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Multiple sequence alignments were performed using the DNAMAN software (http://www.lynnon.com). The analysis selected H. longicornis (BAH02683.1), H. flava (ANA67892.1), R. microplus (XP_037274758.1), D. variabilis (ACF35510.1), and I. scapularis (XP_029835135.2). Then, the molecular weight (MW) and theoretical isoelectric point (pI) were predicted using Compute PI/Mw (http://web.expasy.org/compute_pi/). Signal peptides of the sequence were predicted based on SignalP (http://www.cbs.dtu.dk/services/SignalP/).
Spatiotemporal expression patterns of Doenitin-1
A total of 120 partially fed female adult ticks were used to collect hemolymph, salivary glands, ovaries and midguts, and eggs (incubated for 10 days); larvae (10 days after hatching), nymphs (10 days after molting), and adults (10 days after molting) were used to determine different life stages (unfed) and different tissue expression patterns of Doenitin-1. Total RNA was extracted as described above, and specific primers (qRT-PCR forward primer 5′-ATGGCTTCTGTCGGCTTCC-3′ and qRT-PCR reverse primer 5′-TTAGAGTCTCGAAGTTGTTCTCGTT-3′) were used. β-actin was used as a reference gene (β-actin forward primer 5′-CGTTCCTGGGTATGGAATCG-3′ and β-actin reverse primer 5′-TCCACGTCGCACTTCATG-3′) (27). Then, the SPSS 16.0 software for Windows (SPSS Inc, USA) was used to conduct a one-way ANOVA for qRT-PCR results (28).
Expression and purification of Doenitin-1 recombinant protein
The fragment was inserted into pET-32a with the Not I and BamH I restriction sites and then transformed into Escherichia coli (TransGen Biotech, China) cells and cultured overnight at 37°C. We added IPTG until the final concentration was 0.2 mM, and E. coli was induced for 6 h at 200 r/min at 37°C. The bacteria were centrifuged at 12,000 × g for 10 min, and the resultant supernatant was filtered with a 0.4-μm filter.
Recombinant proteins were purified using a ProteinIso™ Ni-NTA Resin kit (TransGen Biotech, China). The sample was added to the chromatography column filled with Ni-NTA for affinity for 30 min. Then, it was eluted with different imidazole concentrations (20, 50 100, 200, 250, and 500 mM) to get the recombinant protein in the gravity affinity chromatography. Finally, after elution with a 3-ml elution buffer (0.025 M NaCl, 0.05 M NaHPO4, and 0.02 M imidazole, pH = 7.4), the chromatography column was sealed with 3 ml anhydrous ethanol.
Preliminary purified samples were lyophilized and then diluted to a suitable concentration with ddH2O. Subsequently, dialysis membranes with a molecular weight cutoff of 8–14 kDa (Solarbio, China) and an ultrafiltration centrifugal tube (3 kDa) were used to further purify the eluted recombinant protein. Aliquots of a 20-μl sample (1 mg/ml) was boiled for 5 min and then separated by SDS-PAGE (12%). The concentration of the purified protein was determined using a BCA protein detection kit (Transgenic, China). The purified recombinant protein was identified by LC-MS/MS using a M-Class nanoACQUITY ultra performance liquid chromatography (UPLC) (Waters, USA) and a Q Exactive HF mass spectrometer (Thermo Fisher Scientific).
Activated partial thromboplastin time (APTT) and prothrombin time (PT) assays
Healthy adult human venous blood (2 ml) was collected in anticoagulant tubes containing sodium (KWS, China) and centrifuged at 4,000 r/min for 5 min to separate the plasma. The activated partial thromboplastin time (APTT) kit and the prothrombin time (PT) kit were purchased from Sunbio (China). The plasma and reagents were incubated at 37°C for 3 min. First, we used an ACLTOP 700 coagulation analyzer (Werfen, Spain) to test APTT and PT as the group of control. The results were recorded, and three normal samples were selected (PT normal value: 12–16 s; normal APTT: 24–36 s). Second, the recombinant protein was diluted at different concentrations of 0, 3.5, 7, 14, and 28 μM. Then, the groups were added separately to normal plasma samples, and the APTT and PT results were recorded after re-mixing. The experiment was repeated thrice.
Fibrinogen coagulation test
After mixing 80 μl fibrinogen solution and 80 μl rDoenitin-1 with different concentrations (0, 0.5, 1, 1.5, 2, and 2.5 μM), the mixtures were mixed and placed at a 37°C constant temperature water bath and incubated for 2 min. Then, 80 μl thrombin (Sigma, China) was added, and the absorbance of the mixture was immediately determined at 650 nm every 12 s with a UV spectrophotometer and monitored for 30 min. The experiment was repeated thrice.
Inhibitory effect of rDoenitin-1 on serine peptidase activity
rDoenitin-1 was mixed with three serine peptidases (thrombin, trypsin, and cathepsin G; Sigma, China). According to Assumpção et al. (29), corresponding chromogenic substrates were added to detect the residual activity of the peptidase. The buffers selected for the reaction of thrombin, trypsin, and cathepsin G with recombinant proteins were 50 mM Tris-HCl (pH 8), 150 mM NaCl, 20 mM CaCl2, and 0.01% Triton X-100. Boc-ASP-Pro-Arg-AMC (24.2 μM), Boc-GLN-Ala-Arg-AMC (250 μM), and SUC-Ala-Ala-Pro-Phe-AMC (250 μM) (Njpeptide, China) were selected as the chromogenic substrate for thrombin (27 nM), trypsin (10 nM) and cathepsin G (10 nM), respectively, and the concentration was 24.2 μM when the substrate was dissolved in a buffer under dark conditions. Dissolved thrombin at a concentration of 27 nM was used. The recombinant protein was diluted at a concentration gradient (32, 16, 8, 4, 2, 1, and 0.5 nM for thrombin; 200, 100, 50, 25, 12.5, and 6.25 nM for trypsin and cathepsin G) and no recombinant protein at 0 nM. Serine peptidase (20 μl) and recombinant protein (20 μl) with different concentration gradients were placed into an incubator and incubated at 37°C for 30 min (40-μl buffer was added into the control well). On a 96-well black ELISA plate (Corning, United States), a 60-μl buffer was added to each well, followed by the addition of a 100-μl color developing substrate under dark conditions. The microplate was then put into a microplate meter; the excitation wavelength was set at 355 nm and the emission wavelength at 460 nm to measure the OD value of each well. The experimental group OD value was Asample, the recombinant protein concentration at 0 nM was Ablack, and the control well OD value was Acontrol. Residual peptidase activity (%) = (Asample-Acontrol)/Ablack × 100% (30). The experiment was repeated thrice.
Hemolytic activity test
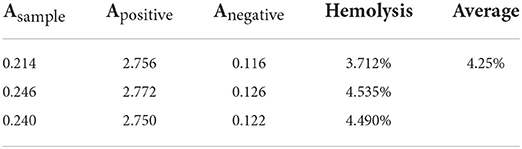
The healthy adult venous blood (6 ml) was mixed with 6 ml normal saline and centrifuged for 4 min at 3,000 × g, and the supernatant discarded. An appropriate amount of normal saline was added and centrifuged for 4 min at 3, 000 × g, and the supernatant was discarded. We repeated the previous step until the supernatant of the liquid changes from red to transparent after centrifugation. We added 9 ml normal saline to suspend the packed red blood cells and added the following solution into a 1.5-ml EP tube according to the following system: positive control group (absorbance value is A): 20 μl 1%Triton X-100 and hematocyte suspension 980 μl; negative control group (absorbance value is B): 20 μl normal saline and hematocyte suspension 980 μl; experimental group (absorbance value is C): 20 μl recombinant protein and hematocyte suspension 980 μl. The reagents were placed in a 37°C constant temperature water bath and incubated for 1 h; 300 μl of the supernatant was collected after centrifugation at 3,000 × g for 3 min. The absorbance value of each group was determined with a UV spectrophotometer at 540 nm. Three replicates were performed for each group.
Three repeated tests were performed to calculate the hemolysis rate of rDoenitin-1 according to the following formula: hemolysis rate =[(C–B)/(A–B)] × 100%.
Functions of Doenitin-1 during feeding and reproduction of H. doenitzi
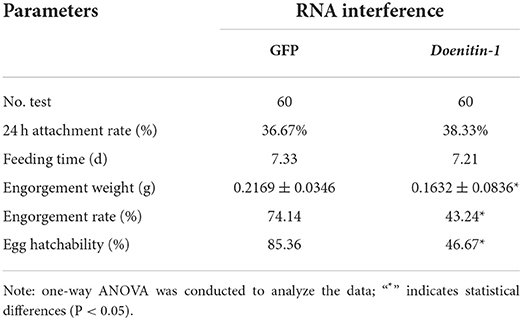
The versatile functions of Doenitin-1 during feeding and reproduction of H. doenitzi were explored using RNAi. Briefly, the primers including the T7 promoter sequence at the 5′-end of both primers (Doenitin-1 primer: 5′-TAATACGACTCACTATAGGAACTGGCTGCAAGCCAG-3′ and 5′- TCAGTATTTGCAGGCGAAC-3′; 5′-AACTGGCTGCAAGCCAG-3′ and 5′- TAATACGACTCACTATAGGTCAGTATTTGCAGGCGAAC-3′; GFP primer: 5′-TAATACGACTCACTATAGGGACGTAAACGGCCACAAGT-3′ and 5′- GCTTCTCGTTGGGGTCTTT-3′; 5′-GACGTAAACGGCCACAAGT-3′ and 5′- TAATACGACTCACTATAGGGCTTCTCGTTGGGGTCTTT-3′) were used to synthesize dsRNA. Unfed female and male ticks with uniform body size were selected, and the interference group was injected with Doenitin-1 dsRNA, while the control group was injected with GFP dsRNA. About 0.6 μl dsRNA (2,400 ng) was injected into the fourth basal segment of the abdomen of each female tick under a microscope using a beveled Hamilton's needle (10 μl). The injected ticks were placed in an incubator and observed for 24 h. After 24 h of observation, dead or weak ticks were removed, and ticks in the interference group and the control group were put into ear bags with a ratio of 20 female ticks to 15 male ticks per rabbit ear. Twenty-four hours later, the number of feeding ticks was checked to calculate the rate of attachment rate. After 4 days, 2–3 partially fed female ticks were removed from each group to determine the relative expression of the genes by qPCR. The experimental data were analyzed according to the 2−ΔΔCt algorithm, and the RQ value of each sample was calculated according to the formula and analyzed by biostatistics with the SPSS software. The time, body weight, and engorgement rate were recorded after the ticks were collected. Engorged female ticks were placed in an incubator, and the oviposition rate and hatching rate of eggs were recorded.
Statistical analysis
A statistical analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL). Data organization and analysis were performed with Graphpad Prism 8.0.1. The statistical method were one-way ANOVA and Student's t test. Whether there is a significant difference between different samples is indicated by different letters. All the data were expressed as mean ± standard deviation (SD) or percentage. P < 0.05 was considered statistically significant.
Results
Sequence analysis and spatiotemporal expression
The open reading frame (ORF) of Doenitin-1 (accession number: MZ981733) is 426 bp and encodes 141 amino acids (Figure 1). It is predicted that Doenitin-1 is 15.8 kDa, and that the theoretical isoelectric point (pI) is 4.76. It is presumed that Doenitin-1 contains a signal peptide of 15 amino acids and two Kunitz domains. Identity-based BLAST searches showed that 90% identity was found with H. longicornis (BAH02683.1), 83% identity with H. flava (ANA67892.1), 66% identity with R. microplus (Q8WPI2.1), 63% identity with Dermacentor variabilis (ACF35510.1), and 59% identity with Ixodes scapularis (XP_002434145.1). The multiple sequence alignment is shown in Figure 2.

Figure 1. Nucleotide sequence and deduced amino acid sequence of Doenitin-1. Row 1: Number position, which is counted every 10 bases. Row 2: Nucleotide sequence. Row 3: Amino acid sequence. The red area is the BPTI/Kunitz family of serine protease inhibitor domain; the yellow area is the Kunitz/Bovine pancreatic trypsin inhibitor domain; signal peptides are underlined; the solid red box is the BPTI/Kunitz binding presenting loop; the red dotted box is the Kunitz/Bovine pancreatic trypsin binding presenting loop. Both domains belong to the BPTI/Kunitz family of serine protease inhibitors.
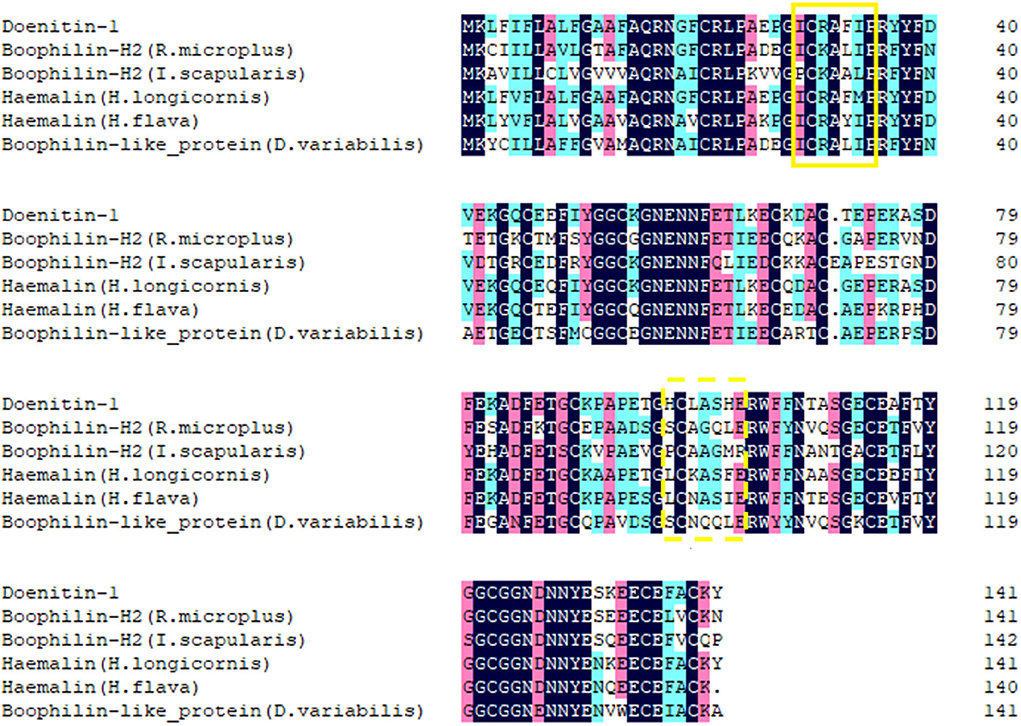
Figure 2. Alignment of Doenitin-1 with its homologs in other tick species. The identical amino acid sequence is in the last row. The solid yellow box is the BPTI/Kunitz substrate binding presenting loop; the yellow dotted box is the Kunitz/Bovine pancreatic trypsin binding presenting loop. The black highlight homology level equals 100%, the pink highlight homology level is greater than or equal to 75%, the blue is greater than or equal to 50%.
The qRT-PCR results showed that Doenitin-1 was expressed in all tissues (partially fed) and stages (unfed) (Figure 3). By one-way ANOVA, the expression level of Doenitin-1 in the midgut was significantly higher than in other tissues (P < 0.05) (Figure 3A), and the highest expression level was found in unfed adult ticks (Figure 3B).
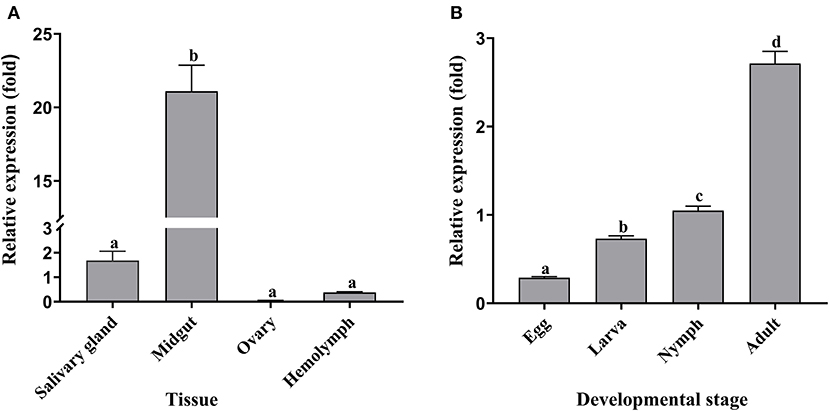
Figure 3. Spatiotemporal expression level of Doenitin-1 in H. doenitzi. (A) Different tissues of Doenitin-1. (B) Different stages of Doenitin-1. The SPSS 16.0 software for Windows (SPSS Inc, IL, United States) was used to conduct a one-way ANOVA for qRT-PCR results. Different letters indicate statistical differences (P < 0.05).
Test of APTT and PT of rDoenitin-1
The recombinant protein of Doenitin-1 was successfully expressed through a prokaryotic expression system, and the molecular weight of the fusion protein was about 35 kDa (immature protein 15.8 kDa and label protein 19 kDa). The optimum elution concentration of imidazole for preliminary purification was 250 mM. After further purification by dialysis and concentration by ultrafiltration, the SDS-PAGE showed that purified rDoentitin-1 was free of impurities (Figure 4). The mass spectrometric data were searched in the UniProt protein database contain rDoenitin protein sequence using Proteome Discoverer 2.2 (Thermo Fisher Scientific). After enzymatic digestion, the peptides of the recombinant protein identified by LC-MS/MS correctly matched with the rDoenitin-1 protein (Table 2), which implied the E. coli prokaryotic expression system we constructed correctly expressed the rDoenitin protein of of the tick H. doenitzi. The normal APTT coagulation was 28.37 ± 1.47 s without rDoenitin-1. APTT coagulation was significantly prolonged after the addition of different concentrations of rDoenitin-1 (P < 0.05). When the concentration was 28 μM, APTT coagulation was extended to 77.8 ± 5.66 s (Figure 5A). However, PT coagulation was not significantly prolonged (Figure 5B). These results indicated that rDoenitin-1 can inhibit the intrinsic coagulation pathway but has no obvious effect on the extrinsic coagulation pathway.
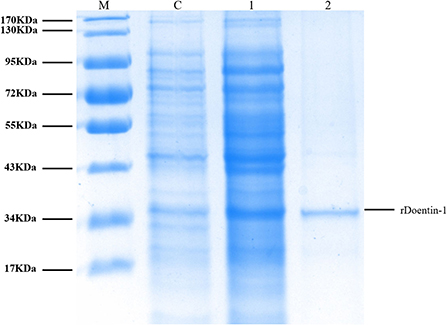
Figure 4. SDS-PAGE (12%) gel profile of rDoenitin-1 stained with Coomassie brilliant blue [M: molecular mass maker; C: before induction (control group); 1: after 0.2 mM IPTG induction for 5 h at 37°C; 2: recombinant rDoenitin-1 purified by column, semi-permeable membrane dialysis and an ultrafiltration membrane].
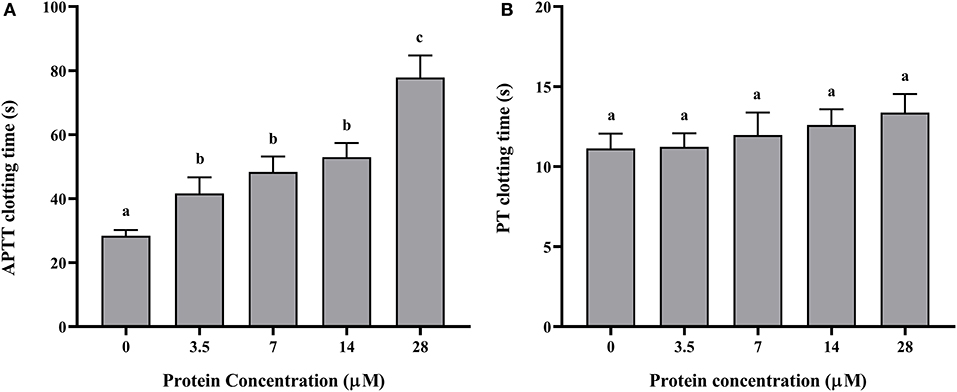
Figure 5. (A) APTT and (B) PT determination of rDoenitin-1. Coagulation time at different concentrations (0, 3.5, 7, 14, and 28 μM) of the recombinant protein. One-way ANOVA was conducted to analyze the data. Different letters indicate a significant difference between the two groups (P < 0.05).
Fibrinogen coagulation test
Fibrinogen coagulation time was 2.03 ± 0.68 min without rDoenitin-1, and fibrinogen coagulation occurred almost immediately. When the concentration of rDoenitin-1 was 2.5 μM, fibrinogen solidification time was extended to 29.67 ± 2.08 min (Figure 6A). The results showed that coagulation time was increased significantly with increase in rDoenitin-1 protein concentration, indicating that the inhibition of fibrinogen clot formation was gradually enhanced.
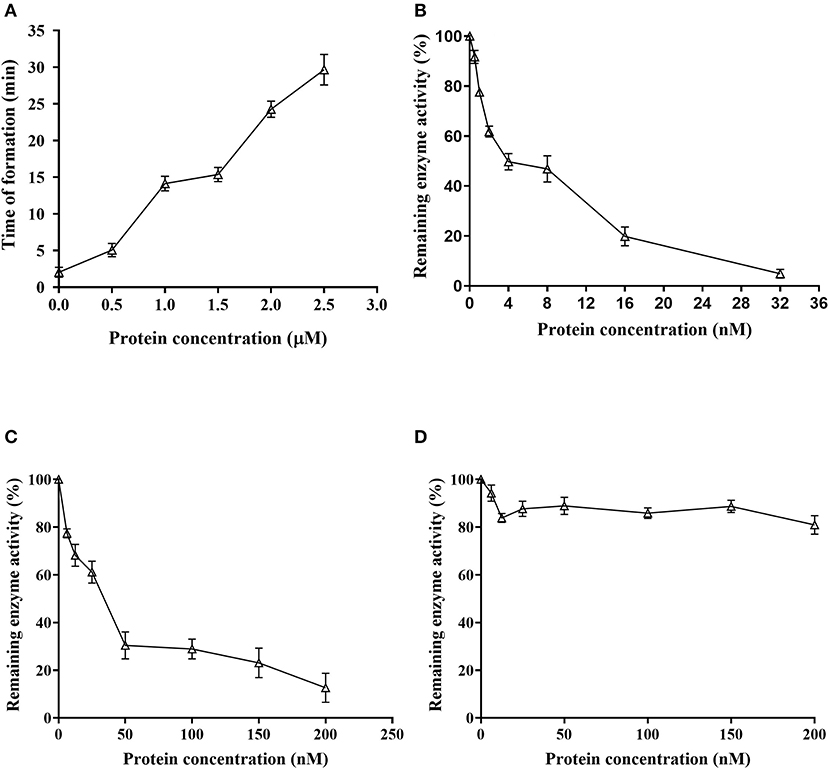
Figure 6. Function analysis of Doenitin-1. (A) rDoenitin-1 coagulation time at different concentrations (0, 0.5, 1, 1.5, 2, and 2.5 μM). Residual peptidase activity (%) of rDoenitin-1 on thrombin (B), cathepsin G activity (C) and trypsin (D) by rDoenitin-1.
Inhibitory effect of rDoenitin-1 on serine peptidase activity and its hemolytic activity
When rDoenitin-1 concentration was 0.5 nM, the residual peptidase activity of thrombin was 91.67%. When rDoenitin-1 concentration was 32 nM, the residual peptidase activity of thrombin was 4.85% (Figure 6B). The IC50 for thrombin was 6.546 nM (R2 = 0.9677). It can be seen that thrombin residual peptidase activity was gradually decreased with the increase in recombinant protein concentration, indicating inhibition of chromogenic substrate hydrolysis. When rDoenitin-1 concentration was 6.25 nM, the residual peptidase activity of cathepsin G was 77.39%. When rDoenitin-1 concentration was 200 nM, the residual peptidase activity of cathepsin G was 12.68 ± 5% (Figure 6C). The IC50 for cathepsin G was 30.01 nM (R2 = 0.9259). It showed that with increase in recombinant protein concentration, the residual peptidase activity of cathepsin G was gradually decreased, and no significant correlation was found between protein concentration and residual peptidase activity. There was no significant change in the residual peptidase activity of trypsin in the results (Figure 6D), indicating that rDoenitin-1 has no significant inhibitory effect on trypsin. The hemolytic rate of rDoenitin-1 was 4.25% (<5%), indicating that rDoenitin-1 had no significant hemolytic activity in healthy adult blood cells (Table 3).
Versatile functions of Doenitin-1 during feeding and reproduction of H. doenitzi
The qPCR results showed that the relative expression level of Doenitin-1 in the control group was 2.93 times higher than that of the interference group, indicating that the expression of Doenitin-1 was reduced by 65.7% (Figure 7). The engorgement weight and engorgement rate of the female ticks were significantly reduced after Doenitin-1 interference (P < 0.05). Compared with the GFP group, the egg hatchability was decreased significantly (P < 0.05), the egg hatchability of the GFP group was 85.36%, while it was only 46.67% in the experimental group. There were no significant changes in feeding time and 24-h attachment rate (P > 0.05) (Table 4). This result would suggest that Doenitin-1 is not directly involved in the feeding site but may play a role in keeping the blood meal in liquid form in the gut.

Figure 7. RNAi efficiency verification of H. doenitzi. Student's t test was conducted to analyze the data. Different letters indicate a significant difference between the two groups (P < 0.05).
Discussion
With the wide use of natural thrombin inhibitors, more and more thrombin inhibitors have been discovered from a great diversity of animals, including Anopheles albimanus, Najahaje, Vespa bicolor Fabricius, Bombina microdeladigitora, and Schistosomiasis mansoni (31–35). The wide use of thrombin inhibitors in different taxa suggests their importance in homeostasis regulation in most branches of life. To start blood feeding, ticks necessarily have to break their host's skin, which can cause a series of defense responses, including pain, hemostasis, complement activation, inflammation, and tissue repair of hosts (36, 37). In order to evade these defense mechanisms, ticks synthesize a range of anticoagulants, such as thrombin inhibitors (38). So far, a large number of anticoagulants have been identified that come from midguts, salivary glands, and hemolymph. These have a direct effect on blood-sucking to ensure a successful engorgement of ticks (23, 39). Besides, anticoagulants keep the blood liquid to enhance digestion by ticks. Currently, thrombin inhibitors have been found in some ticks, such as R. microplus (21), H. longicornis (9), and Ornithodoros moubata (16). Recent research shows that iripin-8 is a tick serpin with a conserved reactive center loop, which inhibits erythrocyte lysis to play a part in antihemostatic activity by complement. Moreover, it may mediate interference with host innate immunity (40).
In this study, a new anticoagulant was isolated from H. doenitzi and named Doenitin-1. It belongs to the Kunitz family and has two Kunitz domains, which are typical structures of thrombin inhibitors (41). The isoelectric point of Doenitin-1 is close to that reported for the thrombin inhibitors hemalin (9) and boophilin (42), which bind to the negative charged exosite 1 of thrombin with their C-terminal domain to inhibit thrombin (21, 43). Kunitz peptidase inhibitors are widely distributed in animals and plants, and are involved in a series of different functions with their target peptidase inhibitors (41, 44). In this study, the inhibitory effect of recombinant protein on three serine proteases was tested. The IC50 of rhemathrin-1/2 isolated by Brahma et al. for thrombin was 46.13 and 40.05 μM, and that of Variegin was 0.99 nM (10, 13). The isolated simukunin from salivary glands of black flies has an IC50 of 217.4 nM for cathepsin G and 379.3 nM for trypsin (45). During the onset of tick feeding, the host produces immunologic rejection reaction and secretes a water-like fluid to the wound site to prevent the blood-sucking process of the tick (46). Studies found that active substances, such as thrombin inhibitors, secreted by salivary glands could not only cope with host immune defense and weaken the immunological rejection of ticks but also assist in the process of pathogen infection of the host (47). The rIris isolated from I. ricinus could significantly inhibit thrombin and cytokines, evading the host immune system (48). Currently, there are many studies on tick-derived peptidase inhibitors containing the Kunitz domain and not only have an antithrombin function but also can regulate host immunity (24, 25).
Anticoagulants play an important role in the blood-sucking process of blood-feeding arthropods. It can help ticks parasitize on the body of the host, smoothly sucking blood and finishing blood meal digestion. In this study, the engorgement body weight of female ticks injected with Doenitin-1 dsRNA was significantly decreased, whereas the engorgement rate and egg hatchability were significantly decreased, which indicated that the decreased expression level of Doenitin-1 could inhibit the blood-sucking of female ticks. During feeding, the rabbit ears inoculated with ticks in the experimental group were ulcerated and suppurated where inflammation was obvious, leading the death of the ticks and suggesting that Doenitin-1 might inhibit host inflammation to some extent. It has been found that anticoagulant molecules in ticks could inhibit cytokines and affect the immune function of hosts (24, 48). After interference with hemalin, the blood-feeding period of ticks was prolonged and the engorgement rate was decreased, but the engorgement body weight, egg weight, and egg hatchability did not change significantly (9), while after interference with Doenitin-1, the engorgement body weight, engorgement rate, and egg hatchability were significantly reduced, indicating that the blood digestion and absorption processes were interfered. However, the 24-h attachment rate and feeding time showed no significant difference (P > 0.05) when compared with the control group. These results indicate that the thrombin inhibitor may regulate tick growth and development during blood-sucking. The hemolysis test showed that rDoenitin-1 had a hemolysis rate of 4.25%, which could be considered as having no hemolysis activity.
In this study, a novel anticoagulant from H. doenitzi named Doenitin-1 was characterized. The BLAST result showed that it belongs to the Kunitz family and that it has two Kunitz domains. They are disulfide rich alpha+beta folds, which are typical structures of thrombin inhibitors (41). The C-terminal of doentin-1 bind to the negatively charged Exosite I site to inhibit thrombin, which are similar to Hemalin and Boophilin (9, 21, 42), which the C-terminal of thrombin inhibitors bind to the negatively charged Exosite I site to inhibit thrombin (21). In conclusion, the recombinant protein rDoenitin-1 has a significant inhibitory effect on specific peptidases. Doenitin-1 plays an important role in oviposition, embryonic development, blood absorption, and digestion in adult ticks. The above results provide an important reference for development of new approaches for tick control and antithrombotic drugs. However, the limitation of this article is that the conformation of the natural protein and its enzymological characteristics have not been studied in-depth, so further study will be carried out using the natural protein of Doenitin-1 isolated from ticks, which will elucidate the molecular basis of the enzymes and their key roles in regulation of tick development and the blood-sucking process.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The animal study was reviewed and approved by Animal Ethics Committee of the Hebei Normal University.
Author contributions
JZL, KW, and XY contributed to conception and design of the study. ZG and SZ organized the database. HL and YS performed the statistical analysis. JLL wrote the first draft of the manuscript. XS and ZY wrote sections of the manuscript. All the authors contributed to manuscript revision, read and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (31472050), Natural Science Foundation of Hebei Province (C2022205026), and Foundation of Hebei Educational Committee (ZD2020168, ZD2022008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. de la Fuente J, Estrada-Peña A, Venzal JM, Kocan KM, Sonenshine DE. Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci. (2008) 13:6938–46. doi: 10.2741/3200
2. Sonenshine DE, Roe RM. Overwiew. In: Sonenshine DE, Roe RM, editors. Biology of Ticks, Vol. 1. Oxford: Oxford University Press (2014).
3. Šimo L, Kazimirova M, Richardson J, Bonnet SI. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Front Cell Infect Microbiol. (2017) 7:281. doi: 10.3389/fcimb.2017.00281
4. Yang XH, Meng H, Qi LL, Liu JZ. Tick salivary gland anti-hemostatic molecules and research progress. Chin J Vector Biol Control. (2017) 28:400–4.
5. Scheraga HA. The thrombin-fibrinogen interaction. Biophys Chem. (2004) 112:117–30. doi: 10.1016/j.bpc.2004.07.011
6. Schwienhorst A. Direct thrombin inhibitors - a survey of recent developments. Cell Mol Life Sci. (2006) 63:2773–91. doi: 10.1007/s00018-006-6219-z
8. Jablonka W, Kotsyfakis M, Mizurini DM, Monteiro RQ, Lukszo J, Drake SK, et al. Identification and mechanistic analysis of a novel tick-derived inhibitor of thrombin. PLoS ONE. (2015) 10:13–29. doi: 10.1371/journal.pone.0133991
9. Liao M, Zhou JL, Gong HY, Boldbaatar D, Shirafuji R, Battur B, et al. Hemalin, a thrombin inhibitor isolated from a midgut cDNA library from the hard tick Haemaphysalis longicornis. J Insect Physiol. (2009) 55:165–74. doi: 10.1016/j.jinsphys.2008.11.004
10. Brahma RK, Blanchet G, Kaur S, Kini RM, Doley R. Expression and characterization of haemathrins, madanin-like thrombin inhibitors, isolated from the salivary gland of tick Haemaphysalis bispinosa (Acari: Ixodidae). Thromb Res. (2017) 152:20–9. doi: 10.1016/j.thromres.2017.01.012
11. Hoffmann A, Walsmann P, Riesener G, Paintz M, Markwardt F. Isolation and characterization of a thrombin inhibitor from the tick Ixodes ricinus. Pharmazie. (1991) 46:209–12.
12. Narasimhan S, Perez O, Mootien S, DePonte K, Koski RA, Fikrig E, et al. Characterization of ixophilin, a thrombin inhibitor from the gut of Ixodes scapularis. PLoS ONE. (2013) 8:e68012. doi: 10.1371/journal.pone.0068012
13. Koh CY, Kazimirova M, Trimnell A, Takac P, Labuda M, Nuttall PA, et al. Variegin, A novel fast and tight binding thrombin inhibitor from the tropical bont tick. J Bio Chem. (2007) 282:29101–13. doi: 10.1074/jbc.M705600200
14. Zhu KC, Bowman AS, Brigham DL, Essenberg RC, Dillwith JW, Sauer JR. Isolation and characterization of Americanin, a specific inhibitor of thrombin, from the salivary glands of the lone star tick Amblyomma americanum. Exp Parasitol. (1997) 87:30–8. doi: 10.1006/expr.1997.4175
15. Mans BJ, Andersen JF, Schwan TG, Ribeiro JM. Characterization of anti-hemostatic factors in the argasid, Argas monolakensis: implications for the evolution of blood-feeding in the soft tick family. Insect Biochem Mol Biol. (2008) 38:22–41. doi: 10.1016/j.ibmb.2007.09.002
16. van de Locht A, Stubbs TM, Bode W, Friedrich T, Bollschweiler C, Hoffken W, et al. The ornithodorin-thrombin crystal structure, a key to the TAP enigma? EMBO J. (1996) 15:6011. doi: 10.1002/j.1460-2075.1996.tb00989.x
17. Nienaber J, Gaspar A, Neitz AWH. Savignin, a potent thrombin inhibitor isolated form the salivary glands of the tick Ornithodoros savignyi (Acari: Argasidae). Exp Parasitol. (1999) 93:82–91. doi: 10.1006/expr.1999.4448
18. Ibrahim MA, Ghazy AH, Maharem T, Khalil M. Isolation and properties of two forms of thrombin inhibitor from the nymphs of the camel tick Hyalomma dromedarii (Acari: Ixodidae). Exp Appl Acarol. (2001) 25:675–98. doi: 10.1023/A:1016136207308
19. Aounallah H, Fessel MR, Goldfeder MB, Carvalho E, Bensaoud C, Chudzinski-Tavassi AM, et al. rDromaserpin: a novel anti-hemostatic serpin, from the salivary glands of the hard tick Hyalomma dromedarii. Toxins. (2021) 13:913. doi: 10.3390/toxins13120913
20. Ciprandi A, de Oliveira SK, Masuda A, Horn F, Termignoni C. Boophilus microplus: its saliva contains microphilin, a small thrombin inhibitor. Exp Parasitol. (2006) 114:40–6. doi: 10.1016/j.exppara.2006.02.010
21. Macedo-Ribeiro S, Almeida C, Calisto BM, Friedrich T, Mentele R, Sturzebecher J, et al. Isolation, cloning and structural characterisation of Boophilin, a multifunctional Kunitz-Type proteinase inhibitor from the cattle tick. PLoS ONE. (2008) 3:e1624. doi: 10.1371/journal.pone.0001624
22. Xu T, Lew-Tabor A, Rodriguez VM. Effective inhibition of thrombin by Rhipicephalus microplus serpin-15 (RmS-15) obtained in the yeast Pichia pastoris. Ticks Tick Borne Dis. (2016) 7:180–7. doi: 10.1016/j.ttbdis.2015.09.007
23. Maritz-Olivier C, Stutzer C, Jongejan F, Neitz AWH, Gaspar ARM. Tick anti-hemostatics: targets for future vaccines and therapeutics. Trends Parasitol. (2007) 23:397–407. doi: 10.1016/j.pt.2007.07.005
24. Wood JP, Ellery PER, Maroney SA, Mast AE. Biology of tissue factor pathway inhibitor. Blood. (2014) 123:2934–43. doi: 10.1182/blood-2013-11-512764
25. Qiao RQ, Zhou JL. Advances in ticks containing Kunitz functional domain serine protease inhibitors. Prog Vet Med. (2013) 34:97–101.
26. Chen Z, Yang X, Bu F, Yang X, Yang X, Liu J. Ticks (Acari: Ixodoidea:Argasidae, Ixodidae) of China. Exp Appl Acarol. (2010) 51:393–404. doi: 10.1007/s10493-010-9335-2
27. Vaz IDS, Imamura S, Nakajima C, Cardoso FCD, Ferreira CAS, Renard G, et al. Molecular cloning and sequence analysis of cDNAs encoding for Boophilus microplus, Haemaphysalis longicornis and Rhipicephalus appendiculatus actions. Vet Parasitol. (2005) 127:147–55. doi: 10.1016/j.vetpar.2004.10.002
28. Hellemans J, Mortier G, Paepe AD, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Bio. (2007) 8:2934–943. doi: 10.1186/gb-2007-8-2-r19
29. Assumpção TC, Ma D, Mizurini DM, Kini RM, Ribeiro JM, Kotsyfakis M, et al. In vitro mode of action anti-thrombotic activity of Boophilin, a multifunctional kunitz protease inhibitor from the midgut of a tick vector of babesiosis, Rhipicephalus microplus. PLoS Neglect Trop D. (2016) 10:e0004298. doi: 10.1371/journal.pntd.0004298
30. Zhang H, Zhang W, Wang X, Zhou Y, Na W, Zhou J. Identification of a cysteine-rich antimicrobial peptide from salivary glands of the tick Rhipicephalus haemaphysaloides. Peptides. (2010) 32:441–6. doi: 10.1016/j.peptides.2010.12.004
31. Valenzuela JG, Francischetti IMB, Ribeiro JMC. Purification, cloning, synthesis of a novel salivary anti-thrombin from the mosquito Anopheles albimanus. Biochemistry US. (1999) 38:11209–15. doi: 10.1021/bi990761i
32. Osipov AV, Filkin SY, Makarova YV, Tsetlin VI, Utkin YN. A new type of thrombin inhibitor, noncytotoxic phospholipase A2, from the Najahaje cobra venom. Toxicon. (2010) 55:186–94. doi: 10.1016/j.toxicon.2009.07.011
33. Yang XB, Wang YK, Lu ZK, Zhai L, Jiang JG, Liu JZ, et al. A novel serine protease inhibitor from the venom of Vespa bicolor Fabricius. Comp Biochem Physiol B Biochem Mol Biol. (2009) 153:116–20. doi: 10.1016/j.cbpb.2009.02.010
34. Lu XY, Ma YF, Wu J, Lai R. Two serine protease inhibitors from the skin secretions of the toad, Bombina microdeladigitora. Comp Biochem Phys B. (2008) 149:608–12. doi: 10.1016/j.cbpb.2007.12.005
35. Lin YL, He SP. Sm22.6 antigen is an inhibitor to human thrombin. Mol Biochem Parasit. (2006) 147:95–100. doi: 10.1016/j.molbiopara.2006.01.012
36. Rodriguez-Valle M, Xu T, Kurscheid S, Lew-Tabor AE. Rhipicephalus microplus serine protease inhibitor family : annotation, expression and functional characterisation assessment. Parasit Vectors. (2015) 8:7. doi: 10.1186/s13071-014-0605-4
37. Francischetti IMB, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JMC. The role of saliva in tick feeding. Front Biosci. (2010) 14:2051–88. doi: 10.2741/3363
38. Zhang X, Lv SH, Wang JH. Progress in the study of anticoagulant substances from ticks. Chin J Cell Biol. (2016) 38:1572–8.
39. Steen NA, Barker SC, Alewood PF. Proteins in the saliva of the Ixodida (ticks): pharmacological features and biological significance. Toxicon. (2006) 47:1–20. doi: 10.1016/j.toxicon.2005.09.010
40. Kotal J, Polderdijk SGI, Langhansova H, Ederova M, Martins LA, Berankova Z, et al. Ixodes ricinus salivary Serpin Iripin-8 inhibits the intrinsic pathway of coagulation and complement. Int J Mol Sci. (2021) 22:9480. doi: 10.3390/ijms22179480
41. Ranasinghe S, McManus DP. Structure and function of invertebrate Kunitz serine protease inhibitors. Dev Comp Immunol. (2012) 39:219–27. doi: 10.1016/j.dci.2012.10.005
42. Soares TS, Watanabe RMO, Tanaka-Azevedo AM, Torquato RJS, Lu S, Figueiredo AC. Expression and functional characterization of boophilin, a thrombin inhibitor from Rhipicephalus (Boophilus) microplus midgut. Vet Parasitol. (2012) 187:521–8. doi: 10.1016/j.vetpar.2012.01.027
43. Jmel MA, Aounallah H, Bensaoud C, Mekki I, Kotsyfakis M. Insights into the role of tick salivary protease inhibitors during ectoparasite-host crosstalk. Int J Mol Sci. (2021) 22:892. doi: 10.3390/ijms22020892
44. Blisnick AA, Thierry F, Bonnet SI. Serine protease inhibitors in ticks: an overview of their role in tick biology and tick-borne pathogen transmission. Front Cell Infect Microbiol. (2017) 7:199. doi: 10.3389/fcimb.2017.00199
45. Tsujimoto H, Kotsyfakis M, Francischetti IM, Eum JH, Strand MR, Champagne DE. Simukunin from the salivary glands of the black fly Simulium vittatum inhibits enzymes that regulate clotting and inflammatory responses. PLoS ONE. (2012) 7:e29964. doi: 10.1371/journal.pone.0029964
47. Chmelar J, Calvo E, Pedra JHF, Francischetti IMB, Kotsyfakis M. Tick salivary secretion as a source of antihemostatics. J Proteomics. (2012) 75:3842–54. doi: 10.1016/j.jprot.2012.04.026
Keywords: Haemaphysalis doenitzi, thrombin inhibitor, anticoagulant activity, blood-sucking, hemolysis activity
Citation: Lu J, Wang K, Gao Z, Zhang S, Li H, Shi Y, Song X, Liu J, Yu Z and Yang X (2022) Doenitin-1: A novel Kunitz family protein with versatile functions during feeding and reproduction of the tick Haemaphysalis doenitzi. Front. Vet. Sci. 9:872244. doi: 10.3389/fvets.2022.872244
Received: 09 February 2022; Accepted: 13 July 2022;
Published: 10 August 2022.
Edited by:
Nicola Pugliese, University of Bari Aldo Moro, ItalyReviewed by:
Ben J. Mans, Agricultural Research Council, South AfricaWeiqing Zheng, Nanchang Centre for Diseases Control and Prevention, China
Copyright © 2022 Lu, Wang, Gao, Zhang, Li, Shi, Song, Liu, Yu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolong Yang, yangxl@hebtu.edu.cn; Zhijun Yu, yuzhijun@hebtu.edu.cn
†These authors have contributed equally to this work
 Jialin Lu
Jialin Lu Kuang Wang
Kuang Wang Zhihua Gao†
Zhihua Gao†  Jingze Liu
Jingze Liu Zhijun Yu
Zhijun Yu