Is transanal irrigation the best treatment possibility for low anterior resection syndrome? A multicenter, randomized clinical trial: study protocol
- 1Department of Colorectal Surgery, East Sussex Healthcare Trust, Hastings, United Kingdom
- 2Faculty of Medicine, Vilnius University, Vilnius, Lithuania
- 3Department of Surgical Oncology, National Cancer Institute, Vilnius, Lithuania
- 4Department of Rehabilitation Physical and Sports Medicine, Faculty of Medicine, Institute of Health Sciences, Vilnius University, Vilnius, Lithuania
- 5Department of Surgery, Aarhus University Hospital, Aarhus, Denmark
- 6Danish Cancer Society Centre for Research on Survivorship and Late Adverse Effects After Cancer in the Pelvic Organs, Aarhus University Hospital, Aarhus, Denmark
Background: Up to 50% of patients who undergo rectal resection suffer from various and partly severe functional problems, despite the preservation of the anal sphincter. These complaints are defined as low anterior resection syndrome (LARS). So far, there are no randomized clinical trials regarding the most effective treatment for LARS. Our aim is to evaluate whether transanal irrigation improves bowel function and quality of life in patients following low anterior resection compared to best supportive care.
Methods: Patients who have undergone low anterior resection will be approached for this study. On patient's visit, complaints regarding the defecation as well as any deterioration in their overall quality of life will be assessed using questionnaires such as the Low Anterior Resection Syndromes score, Wexner score, European Organization for Research and Treatment of Cancer (EORTC) Quality of Life (QOL) CR-29, and Measure Yourself Medical Outcome Profile tool. Few additional target questions will be also asked, such as “Would you recommend the treatment to anybody; did you expect the improvement following the treatment; etc.” Questionnaires and scales will be filled on follow-up visits every 3 months for 1 year.
Discussion: This multicenter, randomized controlled trial will lead to a better understanding of LARS treatment. Moreover, it will be a hypothesis-generating study and will inform areas needing future prospective studies.
Clinical Trial Registration: ClinicalTrials.gov, identifier (NCT05920681).
Introduction
Neoadjuvant treatment with low anterior resection and the formation of anastomosis provides excellent oncological results and is currently the gold standard of rectal cancer treatment (1). Preoperative chemoradiotherapy, vascular dissection, and surgical removal of the rectum and mesorectum cause significant colorectal motility impairment. This results in a variety of symptoms (multiple bowel movements, recurrent urge episodes, hoarding, urinary, fecal incontinence, etc.) that are associated with severe impairment of quality of life. These complaints are summarized as low anterior resection syndrome (LARS) (2). Moreover, surgery may lead to increased morbidity, prolonged hospital stay, readmission, sepsis, and death (3, 4).
Our previous studies showed the prevalence of LARS following rectal surgery in Lithuania reaches up to 75% (5). More importantly, it is a long-term effect—50% of patients have these symptoms 5 or more years after the surgery (6).
Transanal irrigation (enema) (TAI) is a promising treatment modality for LARS patients with increasing prospective data from published studies (7). Only a few clinical trials have been published in the literature where the benefit of transanal irrigations in the treatment of LARS has been investigated (8–10). In 2018, a study conducted in Italy (8), with 27 patients, evaluated transanal irrigation as a potentially beneficial treatment modality in the treatment of LARS. It was shown that the use of TAI demonstrates notable efficacy in treating LARS and resulted in improved continence and quality of life. However, this study included patients with chronic LARS as well as patients with early symptoms following the surgery. Some patients’ symptoms may improve over the time following the operation, while others can suffer from colonic dysfunction and nerve damage, which are strongly related to the main symptoms of LARS, but do not seem to be influenced by time (8). Similar studies were conducted in Germany (2018 and 2023) (9, 10), which have also shown the benefit of transanal irrigation in controlling LARS, but due to time constraints, some patients refused this method. While patients treated by TAI showed significant improvements in bowel movements, a notable portion decided to stop the treatment and relied on supportive therapy only (9).
Despite these data, the use of TAI remains a matter of debate. In addition, there are only few randomized clinical trials so far comparing TAI with best supportive care (8, 9, 11, 12). Main limitations of this study are that a significant amount of patients (six patients, reduction of 27.27%) dropped out of the intervention (TAI) group (9), the small numbers, and the short follow-up period (8, 11, 12).
Thus far, there are no randomized clinical trials confirming or denying the hypothesis regarding the most effective treatment for LARS. Treatment recommendations for LARS have been published in 2021 (13). Here, authors propose to initiate the treatment with best supportive treatment. If it fails, transanal irrigations are started. Moreover, if this fails, invasive procedures are recommended (such as sacral nerve modulation or stoma). Some authors recommend the perineal stoma as an alternative stoma formation site in patients were sphincter preservation is not possible (14). However, data on the risk of LARS in this subgroup of patients is still lacking.
Objectives
The primary objective of this study was to evaluate if transanal irrigation improves bowel function and quality of life in patients following low anterior resection compared with best supportive care.
Specific objectives were as follows:
1. To assess the proportion of patients with transanal irrigation that reduces the symptoms of LARS (decrease in absolute score).
2. To assess the proportion of patients with best supportive care that reduces the symptoms of LARS (decrease in absolute score).
3. Compare results between groups.
Methods and analysis
Study design
This is a multicenter randomized clinical trial. Centers from Lithuania (four centers), UK (at least one center), Denmark (at least one center), and other countries will be invited to participate through the European Society of Coloproctology’s (ESCP) trial map.
The main objective of this clinical trial is to evaluate whether transanal irrigation improves bowel function and quality of life in patients following low anterior resection best supportive care. This will be accomplished by recording the patient's complaints (defecation, urination problems, deterioration of quality of life) after the operation, filling the LARS score (3, 15), Wexner score (16), and quality of life questionnaires [European Organization for Research and Treatment of Cancer (EORTC) CR29 (17) and Measure Yourself Medical Outcomes Profile (MYMOP)] (18) with additional questions: Would you advice this treatment to anybody else? Did your quality of live improve? Did the bowel function improve? Are you satisfied with the treatment? Did you expect the treatment would help? These questions will be rated from 0 to 5. All will be filled in again during the visit every 3 months for 1 year.
Study population
All patients who developed LARS and met the inclusion criteria will be offered participation in this clinical trial.
Eligibility criteria
Inclusion criteria are as follows:
• Subject is an adult (≥18 years).
• Agrees to participate in a study.
• A low anterior resection (robotic, laparoscopic, or open) was performed [anastomosis up to 5–7 cm from the anocutaneous line when assessed with a finger or endo(recto)scope] following long-course (chemo)radiotherapy [the patients treated with 5-fluorouracil (FU) or capecitabine and radiation for 5 weeks; radiation therapy is given once a day at 1.8 Gy/day for a total of 50.0 Gy (1.8 Gy/fraction to the gross tumor and 45 Gy to pelvic lymph nodes)].
• >3 months have passed since the operation or the closure of the ileostomy (if formed).
• No anastomotic leak or stenosis (assessed clinically, during examination, and/or via a proctogram).
• LARS >30 points (major LARS).
Exclusion criteria are as follows:
• Tumor recurrence/progression.
• Pregnancy.
• Diagnosed with inflammatory bowel disease (ICD codes K50–59).
• Side-to-end anastomosis.
• Palliative care.
• Will not be able to perform irrigation.
Recruitment
Patients with LARS and meeting the inclusion criteria will be offered participation in this clinical trial upon consultation with an abdominal surgeon or surgeon at the National Cancer Institute clinic (or any other participating center). The patient will be given time to think as much as necessary. All questions related to the clinical trial will be answered. The patient's decision to participate in the study or not will not have any effect on their further treatment and/or surveillance. Patients who have consented and signed the personal information form and the consent form will be included in the study. Only centers with a high volume of documented colorectal surgery can be included (centers performing at least 50 low anterior resections following neoadjuvant chemoradiotherapy per year may be assessed as candidates for participation).
Informed consent
After patients are invited to participate in the study, they will be provided with information about this study. The research doctor or the person conducting the research authorized by the researcher provides information about the study to the subject. The doctor-researcher or the person conducting the research authorized by the researcher explains the information related to the research and the objectives of the research. If patients agree to participate in the study, they sign the informed consent form, indicating their name, surname, date, and time. The investigator will sign and date the consent form. The informed consent form is signed in two copies, one is given to the patient participating in the study and the other is kept at the study center (National Cancer Institute), in a secure place, with limited access to only the personnel of this biomedical study. The principal investigator is responsible for the storage of research documents.
Randomization
Individuals participating in this study will be randomly divided into groups A and B by randomization (computer-generated random numbers and sealed envelopes will be used). The probability of falling into one or the other group is equal.
• Group A. This is a group of patients who will be subjected to transanal irrigation (experimental).
• Group B. This is a group of patients who will receive only the best currently in use maintenance treatment (control).
During the research, the name and surname of the subject will be replaced by a special code, restricting the identity of the subject. This code will be used in all study documents except the consent form. Only the main researcher and his representatives will have access to these data, and the staff performing the statistical analysis will not know which group the subject belongs to. Only the data analysts will be blinded to the patients groups (will get only data with names of Groups A and B, without any notification, of which group is which).
Interventions
During the study, transanal irrigations will be used, which are considered safe procedures that do not pose additional risks to the patient.
Transanal irrigation
Transanal irrigation will be applied to patients who will enter the experimental group. The patient lies on the left or right side depending on the main hand with the knees bent. With the main hand, the TAI tip (cone catheter) lubricated with a lubricant is carefully introduced. The TAI bag (Coloplast Peristeen Transanal Irrigation System) is filled with warm water—it can be boiled or just from the tap. The contents of the TAI bag are slowly administered through the anus—the cone catheter is inserted [the starting volume is 500 ml of warm (around body temperature) water and it can be increased up to 1 L eventually over a 3- to 4-week period]. The duration of the TAI is about 15–20 min. Afterward, the subject goes to defecate until the bowel is empty. This action should be repeated daily.
The patients will be instructed by the treating physician and will be contacted within 3–4 weeks on the course of the procedure.
In case of bleeding or abdominal pain, patients were instructed to contact the team member at any time. For all other questions regarding TAI, the instructor could be contacted during office hours. All the adverse events and complications will be assessed weekly. In case of the high number of complications, the study will be ended.
Best supportive care
The control group will receive best supportive care: diet modification (low-fiber diet and personal recommendations were given), medications (bulk-forming agents and loperamide), and, if needed, diapers. All patients were instructed regarding the pelvic floor muscle training (Kegel exercise).
No patients received biofeedback therapy or any other interventions such as sacral nerve stimulation or percutaneous tibial nerve stimulation.
All the team members will be provided with the video teaching material and instructions for the procedures to be performed for both groups.
Assessments
Data collection will take place during the patient visit. Demographic and clinical examination data will be collected from the medical documentation at the research center. During the visit, the patient's complaints after the operation (defecation, deterioration of quality of life) will be recorded, and LARS, Wexner scale, and quality of life questionnaires will be filled during the visit. Questionnaires and scales will be filled again during the visit every 3 months for 1 year. Other tests that will be performed during the visit will be long-term follow-up tests, an integral part of the treatment, not related to the clinical trial.
At the second stage, a longer follow-up will be planned—for more than 2 years after the end of treatment.
Sample size
A sample size of 40 is planned (an improvement of 5 points on the LARS scale):
• 20 transanal irrigation group (experimental) and
• 20 best supportive care group (control).
To demonstrate a 5-point difference (with 80% certainty) between the intervention group and the control group, 34 patients were required to participate in the study (17 in each study arm). Taking into account a drop off of 20%, at least 20 patients per group will be needed. The primary endpoint was LARS score analyzed by unpaired t test.
Outcome measures
The objectives of the trial are to evaluate in what proportion of patients (percentage) transanal irrigation and in what proportion of patients (percentage) the best supportive treatment reduces the symptoms of LARS score (change in absolute score), compare the results, and evaluate the statistical reliability. The secondary outcomes would be assessing the change in single LARS score's items.
Data analysis
Statistical methods will be used for data analysis using the SPSS program.
Statistical analysis
For statistical analysis, we will use the intention to treat principle.
Data collection and management
Data collection
Data obtained from demographic and clinical examination during the initial assessment and from the medical documentation at the study center will be collected. List of data to be obtained are subject's gender, age, height, weight, concomitant diseases, and medications used (their names and doses). The patient's complaints (defecation, urination problems, deterioration of quality of life) after surgery are recorded; LARS is evaluated according to questionnaires at each visit. The data collected via paper will later be uploaded into Excel, where it will be depersonalized. All questionnaires used have been validated (Wexner, LARS), or permission to use them has been obtained from the authors.
Data management
All information will be recorded in electronic and paper documents created specifically for the clinical trial. No patient identifiable data (name, date of birth, address, etc.) will be recorded. Registered local investigators will have individual password-protected access to their unit's data entered into an electronic database. During the running of the audit, only local data will be visible to investigators; other sites’ data will not be accessible.
The main researcher, the research investigators, persons authorized by the ethics committees, or persons authorized by other controlling institutions will be able to get acquainted with the data collected for the purpose of the study, which allow the direct identification of the subject.
The data will be processed in a computerized manner, via electronic research documents and password-protected data (Appendix 1). Only the researchers know the access codes.
Management and safety
During the study, transanal irrigation (enema) will be used—a safe procedure that may cause only minor inconveniences: longer delay in the morning toilet, nausea, abdominal bloating, increased bowel movements after the procedure, anus pain, or other rare side effects [e.g., intestinal perforation, which according to the literature occurs in 1 in 166,000 procedures (19, 20)].
These inconveniences will be recorded in the electronic journal. Moreover, some patients will receive the best supportive treatment, which is a safe treatment because it is non-interventional and therefore does not pose any additional risk to the patient.
Discussion
In this study, we aim to evaluate if transanal irrigation improves bowel function and quality of life in patients following low anterior resection compared with best supportive care.
We have designed this study for a couple of reasons. Just recently, public guidelines were issued (13). The authors recommend conservative management as a first step. Even though there is little evidence that dietary modifications are effective for LARS patients, good results with a reduction of non-soluble fiber intake seems reasonable. The use of anti-diarrheal agents such as loperamide, if necessary, can also apply to LARS. The authors also recommend patient consultation before any treatment initiation and risk of LARS assessment. Moreover, all the dietary instructions or medications were prescribed just after the surgery. Together with best supportive treatment, pelvic floor muscle training with biofeedback may be advised. If “conservative” treatments are not helpful, patients may be advised to use TAI, sacral neuromodulation (SNM), or percutaneous tibial nerve stimulation. TAI seems to be a promising treatment modality. It has at least two benefits—first, as the bowel following irrigation is empty, the patient will have pseudocontinence; second, the bowel is “taught” to do the defecation movements at same time. Only a few randomized clinical trials are present comparing TAI vs. best supportive treatment (8–13). Main limitations of these trials are the small sample size and relatively short follow-up.
Therefore, our study will be the first international multicenter study including patients with LARS and using transanal irrigation. Moreover, a patient representative was included in the protocol recommending to choose the correct questions and questionnaires.
Trial status
The first patient was included in August 2023. At the time of protocol revision (January 2024), two centers in Lithuania, one in Portugal, and one in UK are actively recruiting patients for the study, and 24 patients have already been included.
Data availability statement
All the data will be accessible from the corresponding author upon reasonable request.
Ethics statement
The studies involving humans were approved by Vilnius Bioethics Committee nr 2013. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MK: Data curation, Formal Analysis, Methodology, Writing – original draft. IC: Data curation, Investigation, Software, Writing – original draft. AA: Formal Analysis, Investigation, Resources, Writing – review & editing. TA: Funding acquisition, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. PC: Conceptualization, Methodology, Writing – review & editing. AD: Conceptualization, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to thank our patient (wished to stay anonymous) who underwent the low anterior resection and helped with study protocol preparation and questionnaire formation for the participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1Jei sutikimą dalyvauti tyrime duoda pats asmuo.
2Jei sutikimą dalyvauti tyrime duoda kitas asmuo.
Abbreviations
LARS, low anterior resection syndrome; TAI, transanal irrigation; ESCP, European Society of Coloproctology; EORTC, European Organization for Research and Treatment of Cancer; UK, United Kingdom; MYMOP, Measure Yourself Medical Outcomes Profile.
References
1. National Comprehensive Cancer Network. Available online at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (Accessed May 30, 2023).
2. Keane C, Wells C, O’Grady G, Bissett IP. Defining low anterior resection syndrome: a systematic review of the literature. Colorectal Dis. (2017) 19(8):713–22. doi: 10.1111/codi.13767
3. Tevis SE, Kennedy GD. Postoperative complications: looking forward to a safer future. Clin Colon Rectal Surg. (2016) 29(3):246–52. doi: 10.1055/s-0036-1584501
4. Mulita F, Liolis E, Akinosoglou K, Tchabashvili L, Maroulis I, Kaplanis C, et al. Postoperative sepsis after colorectal surgery: a prospective single-center observational study and review of the literature. Prz Gastroenterol. (2022) 17(1):47–51. doi: 10.1007/s11377-021-00584-6
5. Samalavicius NE, Dulskas A, Lasinskas M, Smailyte G. Validity and reliability of a Lithuanian version of low anterior resection syndrome score. Tech Coloproctol. (2016) 20(4):215–20. doi: 10.1007/s10151-015-1424-0
6. Dulskas A, Kavaliauskas P, Pilipavicius L, Jodinskas M, Mikalonis M, Samalavicius NE. Long-term bowel dysfunction following low anterior resection. Sci Rep. (2020) 10(1):11882. doi: 10.1038/s41598-020-68900-8
7. Embleton R, Henderson M. Using transanal irrigation in the management of low anterior resection syndrome: a service audit. Br J Nurs. (2021) 30(21):1226–30. doi: 10.12968/bjon.2021.30.21.1226
8. Martellucci J, Sturiale A, Bergamini C, Boni L, Cianchi F, Coratti A, et al. Role of transanal irrigation in the treatment of anterior resection syndrome. Tech Coloproctol. (2018) 22(7):519–27. doi: 10.1007/s10151-018-1829-7
9. Rosen HR, Boedecker C, Fürst A, Krämer G, Hebenstreit J, Kneist W. Prophylactic transanal irrigation (TAI) to prevent symptoms of low anterior resection syndrome (LARS) after rectal resection: results at 12-month follow-up of a controlled randomized multicenter trial. Tech Coloproctol. (2020) 24(12):1247–53. doi: 10.1007/s10151-020-02261-2
10. Rosen H, Sebesta CG, Sebesta C. Management of low anterior resection syndrome (LARS) following resection for rectal cancer. Cancers (Basel). (2023) 15(3):778. doi: 10.3390/cancers15030778
11. Pieniowski EHA, Bergström CM, Nordenvall CAM, Westberg KS, Johar AM, Tumlin Ekelund SF, et al. A randomized controlled clinical trial of transanal irrigation versus conservative treatment in patients with low anterior resection syndrome after rectal cancer surgery. Ann Surg. (2023) 277(1):30–7. doi: 10.1097/SLA.0000000000005482
12. Meurette G, Faucheron JL, Cotte E, Denost Q, Portier G, Loriau J, et al. Low anterior resection syndrome after rectal resection management: multicentre randomized clinical trial of transanal irrigation with a dedicated device (cone catheter) versus conservative bowel management. Br J Surg. (2023) 110(9):1092–5. doi: 10.1093/bjs/znad078
13. Christensen P, Im Baeten C, Espín-Basany E, Martellucci J, Nugent KP, Zerbib F, et al. Management guidelines for low anterior resection syndrome—the MANUEL project. Colorectal Dis. (2021) 23(2):461–75. doi: 10.1111/codi.15517
14. Verras GI, Filis D, Panagiotopoulos I, Liolis E, Kehagias D, Bousis D, et al. Perineal pseudocontinent colostomy: an alternative method to promote patients’ satisfaction and safety? Prz Gastroenterol. (2023) 18(2):216–8. doi: 10.5114/pg.2022.116998
15. Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. (2012) 255(5):922–8. doi: 10.1097/SLA.0b013e31824f1c21
16. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. (1993) 36(1):77–97. doi: 10.1007/BF02050307
17. EORTC Quality of Life Group. EORTC QLQ—CR29, Version 2.1. Available online at: https://www.eortc.org/app/uploads/sites/2/2018/08/Specimen-CR29-English-2.1.pdf (Accessed January 14, 2024).
18. Paterson C. Measuring outcomes in primary care: a patient generated measure, MYMOP, compared with the SF-36 health survey. Br Med J. (1996) 312:1016–20. doi: 10.1136/bmj.312.7037.1016
19. Christensen P, Krogh K, Buntzen S, Payandeh F, Laurberg S. Long-term outcome and safety of transanal irrigation for constipation and fecal incontinence. Dis Colon Rectum. (2009) 52(2):286–92. doi: 10.1007/DCR.0b013e3181979341
20. Christensen P, Krogh K, Perrouin-Verbe B, Leder D, Bazzocchi G, Petersen Jakobsen B, et al. Global audit on bowel perforations related to transanal irrigation. Tech Coloproctol. (2016) 20(2):109–15. doi: 10.1007/s10151-015-1400-8
Appendix 1

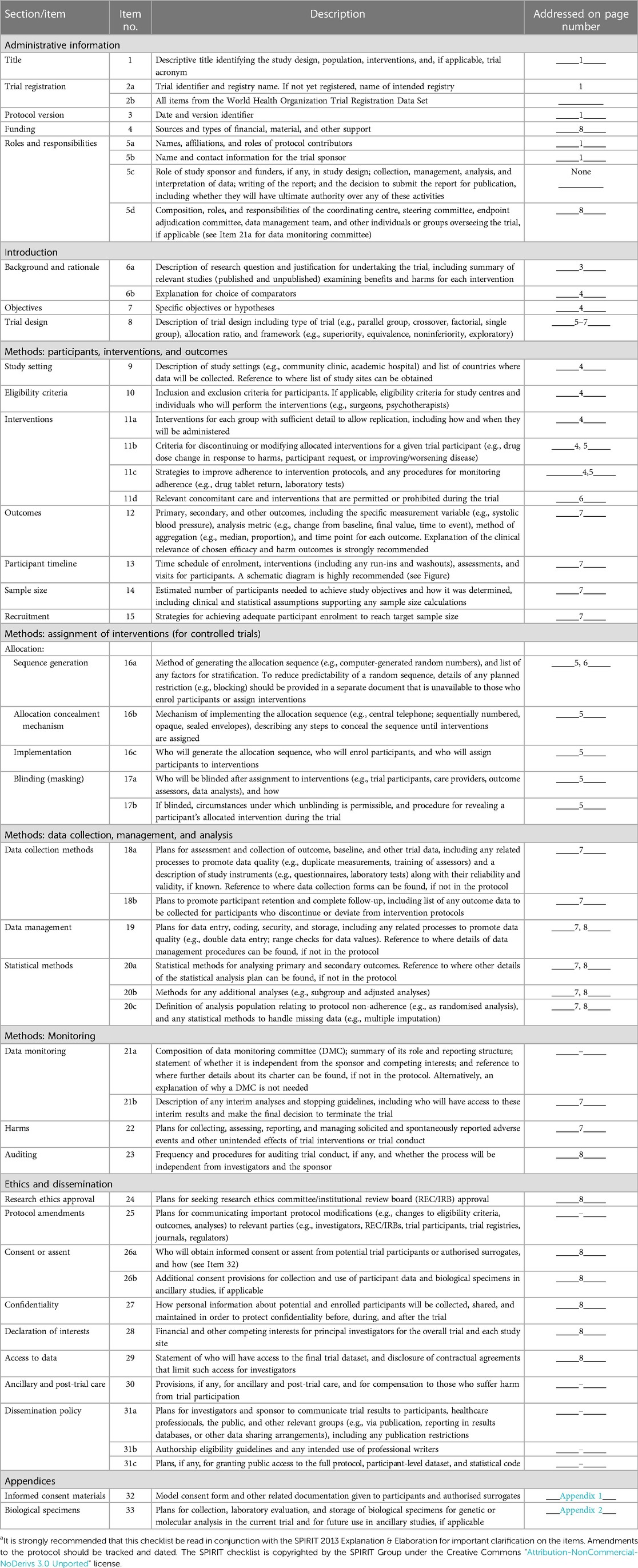
SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documentsa.
Appendix 2
SUTIKIMAS DALYVAUTIBIOMEDICININIAME TYRIME(pavyzdinė forma, palikti tik tyrimui aktualią informaciją, žr. išnašas)
1. Aš perskaičiau šią Informuoto asmens sutikimo formą ir supratau man pateiktą informaciją.
2. Man buvo suteikta galimybė užduoti klausimus ir gavau mane tenkinančius atsakymus.
3. Supratau, kad galiu bet kada pasitraukti iš tyrimo, nenurodydama(s) priežasčių.1
4. Supratau, kad asmuo, dėl kurio dalyvavimo biomedicininiame tyrime aš duodu sutikimą, gali bet kada pasitraukti iš tyrimo, nenurodydamas priežasčių.2
5. Supratau, kad norėdama(s) atšaukti sutikimą dalyvauti biomedicininiame tyrime, raštu turiu apie tai informuoti tyrėją/kitą jo įgaliotą biomedicininį tyrimą atliekantį asmenį.
6. Patvirtinu, kad turėjau užtektinai laiko apsvarstyti man suteiktą informaciją apie biomedicininį tyrimą.
7. Supratau, kad dalyvavimas šiame tyrime yra savanoriškas.
8. Patvirtinu, kad sutikimą dalyvauti šiame biomedicininiame tyrime duodu laisva valia.
9. Leidžiu naudoti asmens duomenis ta apimtimi ir būdu, kaip nurodyta Informuoto asmens sutikimo formoje.
10. Patvirtinu, kad gavau Informuoto asmens sutikimo formos egzempliorių, pasirašytą tyrėjo/ kito jo įgalioto biomedicininį tyrimą atliekančio asmens.
Patvirtinu, kad suteikiau informaciją apie biomedicininį tyrimą aukščiau nurodytam asmeniui.
Patvirtinu, kad asmeniui (ar kitam sutikimą duoti turinčiam teisę asmeniui) buvo skirta pakankamai laiko apsispręsti dalyvauti biomedicininiame tyrime, atsižvelgiant į biomedicininio tyrimo pobūdį, taip pat įvertinus kitas aplinkybes, galinčias daryti įtaką priimamam sprendimui.
Aš skatinau asmenį (ar kitą sutikimą turintį teisę duoti asmenį) užduoti klausimus ir į juos atsakiau.
Keywords: fecal incontinence, treatment of bowel dysfunction, colorectal surgery, low anterior resection syndrome, quality of life, transanal irrigation
Citation: Klimovskij M, Civilka I, Aleinikov A, Aukstikalnis T, Christensen P and Dulskas A (2024) Is transanal irrigation the best treatment possibility for low anterior resection syndrome? A multicenter, randomized clinical trial: study protocol. Front. Surg. 11:1384815. doi: 10.3389/fsurg.2024.1384815
Received: 10 February 2024; Accepted: 22 April 2024;
Published: 13 May 2024.
Edited by:
Christopher Young, University of Kansas Medical Center, United StatesReviewed by:
Francesk Mulita, General University Hospital of Patras, GreeceAssad Zahid, The University of Sydney, Australia
Marie Shella De Robles, Wollongong University Hospital, Australia
© 2024 Klimovskij, Civilka, Aleinikov, Aukstikalnis, Christensen and Dulskas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Audrius Dulskas, audrius.dulskas@gmail.com;
audrius.dulskas@nvi.lt
 Michail Klimovskij
Michail Klimovskij Ignas Civilka2
Ignas Civilka2  Tomas Aukstikalnis
Tomas Aukstikalnis Peter Christensen
Peter Christensen Audrius Dulskas
Audrius Dulskas