Can an incomplete ERAS protocol reduce postoperative complications compared with conventional care in laparoscopic radical resection of colorectal cancer? A multicenter observational cohort and propensity score-matched analysis
- 1Department of Gastrointestinal Surgery and Department of Emergency Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Minimally Invasive Surgery, Affiliated Hospital of Putian University, Teaching Hospital of Fujian Medical University, Putian, China
- 3Department of Gastrointestinal Surgery, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 4Department of Gastrointestinal Surgical Oncology, Fujian Cancer Hospital and Fujian Medical University Cancer Hospital, Fuzhou, China
Background: The patients undergoing laparoscopic radical colorectomy in many Chinese hospitals do not achieve high compliance with the ERAS (enhanced recovery programs after surgery) protocol.
Methods: The clinical data from 1,258 patients were collected and divided into the non-ERAS and incomplete ERAS groups.
Results: A total of 1,169 patients were screened for inclusion. After propensity score-matched analysis (PSM), 464 pairs of well-matched patients were generated for comparative study. Incomplete ERAS reduced the incidence of postoperative complications (p = 0.002), both mild (6.7% vs. 10.8%, p = 0.008) and severe (3.2% vs. 6.0%, p = 0.008). Statistically, incomplete ERAS reduced indirect surgical complications (27,5.8% vs. 59, 12.7) but not local complications (19,4.1% vs. 19, 4.1%). The subgroup analysis of postoperative complications revealed that all patients benefited from the incomplete ERAS protocol regardless of sex (male, p = 0.037, 11.9% vs. 17.9%; female, p = 0.010, 5.9% vs. 14.8%) or whether neoadjuvant chemotherapy was administered (neoadjuvant chemotherapy, p = 0.015, 7.4% vs. 24.5%; no neoadjuvant chemotherapy, p = 0.018, 10.2% vs. 15.8%). Younger patients (<60 year, p = 0.002, 7.6% vs. 17.5%) with a low BMI (<22.84, 9.4% vs. 21.1%, p < 0.001), smaller tumor size (<4.0 cm, 8.1% vs. 18.1%, p = 0.004), no fundamental diseases (8.8% vs. 17.0%, p = 0.007), a low ASA score (1/2, 9.7% vs. 16.3%, p = 0.004), proximal colon tumors (ascending/transverse colon, 12.2% vs. 24.3%, p = 0.027), poor (6.1% vs. 23.7%, p = 0.012)/moderate (10.3% vs. 15.3%, p = 0.034) tumor differentiation and no preoperative neoadjuvant radiotherapy (10.3% vs. 16.9%, p = 0.004) received more benefit from the incomplete ERAS protocol.
Conclusion: The incomplete ERAS protocol decreased the incidence of postoperative complications, especially among younger patients (<60 year) with a low BMI (<22.84), smaller tumor size (<4.0 cm), no fundamental diseases, low ASA score (1/2), proximal colon tumors (ascending/transverse colon), poor/moderate differentiation and no preoperative neoadjuvant radiotherapy. ERAS should be recommended to as many patients as possible, although some will not exhibit high compliance. In the future, the core elements of ERAS need to be identified to improve the protocol.
Introduction
Since the ERAS (enhanced recovery programs after surgery) protocol was first recommended, it has gained widespread popularity as a common concept in different professional fields (1–11). During the past decade, many surgeons in China have also used ERAS. Some studies have reported perioperative benefits in patients who are highly compliant with the ERAS protocol (12–14). However, ERAS is not used for all patients in many Chinese hospitals, and some patients do not achieve high compliance with the protocol. This is common among patients not included in research studies, as most studies focus on highly compliant ERAS patients and seem to ignore the nonadherent subset of this population. Our previous study showed that ERAS failure occurred in 38 (17.9%) patients undergoing ERAS among 212 selected gastric cancer patients because of the following factors: advanced age, high ASA score, lack of preoperative education and combined surgery (15). The results of that study indicated that even some select ERAS patients failed to adhere to ERAS. In that study, ERAS failure was defined as more than 7 days of hospitalization due to postoperative complications, unplanned readmission or death within 30 days of surgery. If the program is extended to all patients, the failure rate will be even higher. Other researchers have also reported such failures after colorectal cancer surgery. Studies conducted by Korean researchers on ERAS in colorectal cancer patients have reached similar conclusions (16, 17). Therefore, a new question arises: Do such cases of failure still confer some advantage over no ERAS protocol? To answer this question, we collected more case data and conducted this retrospective cohort comparison study using PSM. To the best of our knowledge, few studies have involved large volumes of patients with low compliance to ERAS protocols versus traditional care. In this study, similar to many researchers (18–20), incomplete ERAS was defined as compliance <70%.
The aim of this study (Research Registration Unique Identifying Number: clinicaltrials NCT05412355) was to evaluate the perioperative complications of incomplete ERAS in patients undergoing laparoscopic radical colorectomy by a multicenter propensity-matched analysis. In addition, we analyzed the impact of incomplete ERAS in subgroups of patients with different clinicopathological characteristics.
Methods
Compliance calculation
Compliance scores were calculated according to the ERAS protocol for colorectal cancer, as shown in Table 1. We calculated the compliance score for each patient by reviewing the compliance of each of the 20 nodes of the protocol, with a score of 5 for a node that was fully complied with and 0 for a node that was not.
Patient selection and study design
The clinical data of 1,258 patients undergoing laparoscopic radical colorectomy at four large university hospitals between August 2008 and December 2020 were collected for this study. After excluding cases that did not meet the criteria for inclusion, eligible cases were divided into the non-ERAS and incomplete ERAS groups. The ERAS Society Guidelines for colorectal resection were established in 2012, and since then, ERAS has been gradually accepted by Chinese surgeons. The non-ERAS group included patients who had never completed an ERAS protocol, most of whom were hospitalized from 2008 to 2012. The incomplete ERAS group included patients who had ERAS compliance <70% and were hospitalized from 2012 to 2020. The inclusion criteria were 1. age ≥18 years; 2. pathological diagnosis of colorectal cancer; and 3. radical laparoscopic surgery was performed. The exclusion criteria were 1. ERAS compliance ≥70%; 2. open surgery; 3. conversion to laparotomy after laparoscopic surgery; 4. severe mental illness; 5. pregnancy or lactation; 6. simultaneous malignant tumors in multiple organs; 7. history of other malignant tumors; and 8. emergency radical colorectomy. All included cases were completed after the surgeon mastered the learning curve, and the surgical technique and skills of the surgeon at each institution were recognized and confirmed by the surgeons at the other institutions. Postoperative complications were defined as surgery-related complications during the postoperative hospital stay and surgery-related readmission or death within 30 days after surgery.
Matching
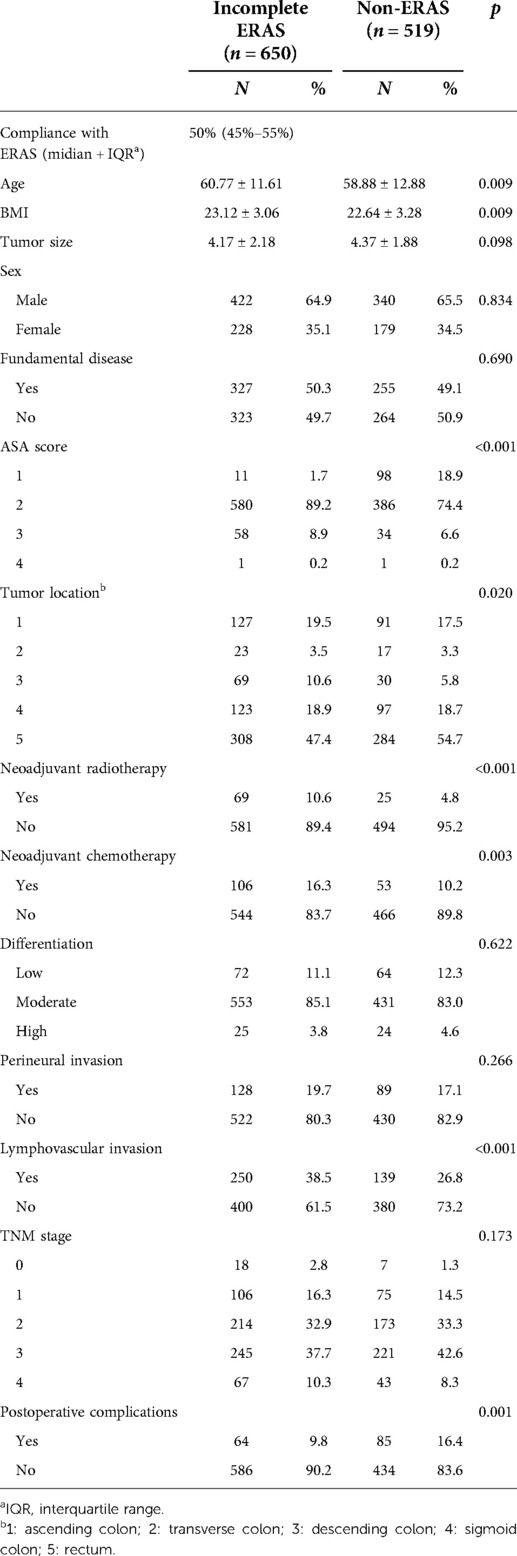
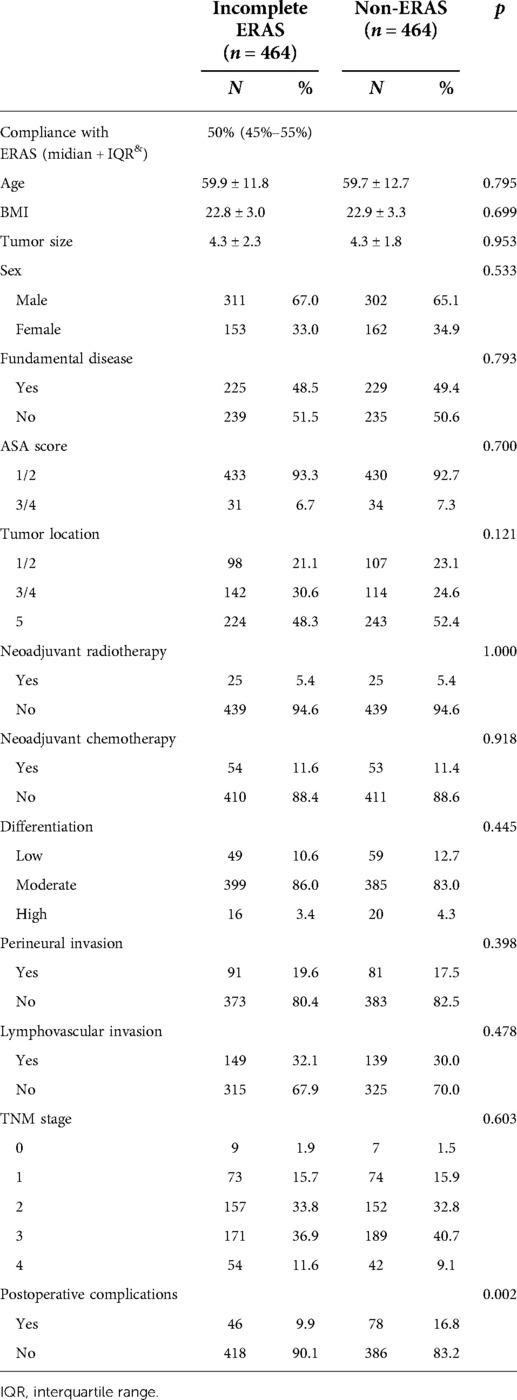
PSM was used to balance the following factors between the two groups: sex, age, BMI (body mass index), fundamental diseases, ASA score (American Society of Anesthesiologists ASA score), tumor location, tumor size, tumor differentiation, perineural invasion, lymphovascular invasion, tumor stage, and neoadjuvant chemoradiotherapy. After calculating the propensity score for each patient, we matched the patients 1:1 with an SD set caliper width of 0.01. Some items in the clinical data of the two groups showed significant differences before PSM (shown in Table 1), but they were adjusted and confirmed to be balanced after matching (Table 2).
Statistical analysis
SPSS 25.0 software was used for analysis. Categorical and continuous variables were compared with the x2 test and Student's t-test, respectively. For subgroup analysis, Fisher's exact test was used to analyze two factors because of the small number of cases. A p value < 0.05 was considered statistically significant.
Results
Patient characteristics
Data from 1,258 patients were collected, and 1,169 were screened for study inclusion. Of these patients, 650 were assigned to the incomplete ERAS group, and 519 were assigned to the non-ERAS group (Figure 1), which had unbalanced clinical characteristics, such as age (p = 0.009), BMI (p = 0.009), ASA score (p < 0.001), tumor location (p = 0.020), neoadjuvant radiotherapy (p < 0.001), neoadjuvant chemotherapy (p = 0.003), and lymphovascular invasion (p < 0.001), as shown in Table 2. After propensity score matching, 464 pairs of well-matched patients were generated for comparative study.
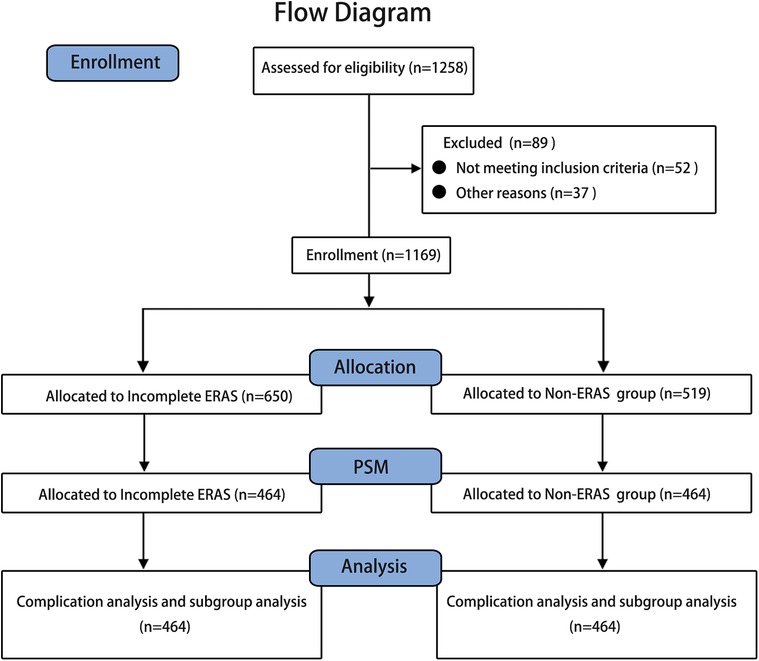
Figure 1. Flow diagram the clinical data of 1,258 patients undergoing laparoscopic radical colorectomy at four large university hospitals between August 2008 and December 2020 were collected for this study, and 1,169 were screened for study inclusion. Of these patients, 650 were assigned to the incomplete ERAS group, and 519 were assigned to the non-ERAS group. After PSM (propensity score matching), 464 pairs of well-matched patients were generated for analysis.
Postoperative complications
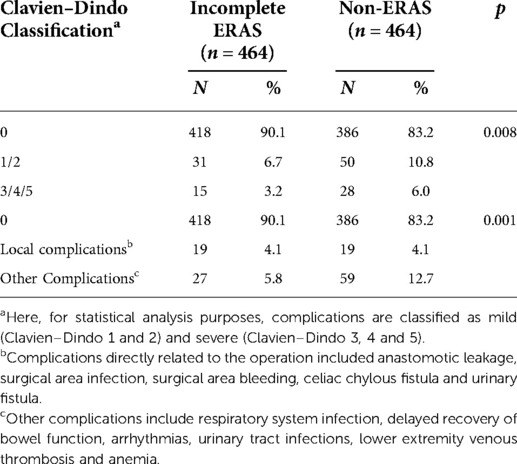
It can also be seen that after matching, patients in the incomplete ERAS group still had the advantage of fewer postoperative complications compared with those in the non-ERAS group (p = 0.002), as shown in Table 3. It can be seen from Table 4 that the incomplete ERAS protocol reduced the occurrence of postoperative complications (p = 0.008), among which the incidence of mild complications (Clavien–Dindo classification 1/2) was 6.7% vs. 10.8% (incomplete ERAS vs. non-ERAS), while the incidence of serious complications (Clavien–Dindo classification 3/4/5) was 3.2% vs. 6.0% (incomplete ERAS vs. non-ERAS). Then we used an alternative classification for complications: One is directly related to surgery, such as anastomotic leakage, surgical area infection, surgical area bleeding, celiac chylous fistula and urinary fistula. The other is the indirect complications of surgery, including respiratory system infection, delayed recovery of bowel function, arrhythmias, urinary tract infections, lower extremity venous thrombosis and anemia. Statistically,incomplete ERAS reduced indirect surgical complications (27,5.8% vs. 59, 12.7) but not direct complications (19,4.1% vs. 19, 4.1%).
Subgroup analysis of postoperative complications
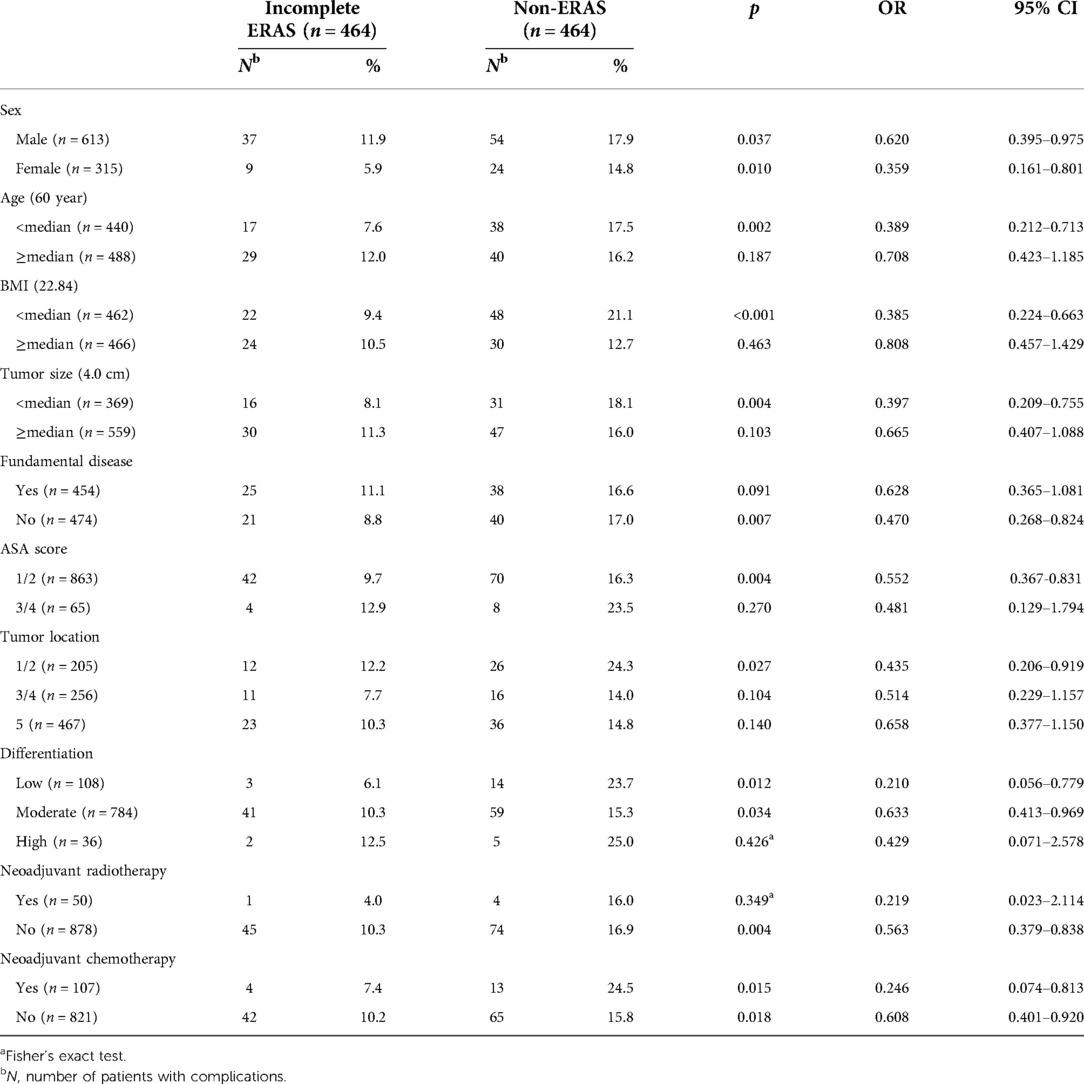
To identify who might benefit from the incomplete ERAS protocol, different clinical characteristics of the two groups were subjected to subgroup analyses, as shown in Table 5. The subgroup analysis of postoperative complications revealed that all patients benefited regardless of sex (male, p = 0.037,11.9% vs. 17.9%; female, p = 0.010, 5.9% vs. 14.8%) and whether neoadjuvant chemotherapy was treated (neoadjuvant chemotherapy, p = 0.015, 7.4% vs. 24.5%; no neoadjuvant chemotherapy, p = 0.018,10.2% vs. 15.8%). However, subgroup analysis of other factors showed different results. Younger patients (<60 year, p = 0.002, 7.6% vs. 17.5%) had an advantage over older patients (≥60 year, p = 0.187, 12.0% vs. 16.2%). Patients with a low BMI (<22.84, p < 0.001, 9.4% vs. 21.1%) received a significant benefit, while those with a high BMI (≥22.84, p = 0.463, 10.5% vs. 12.7%) did not. Patients with a smaller tumor size (<4.0 cm, p = 0.004, 8.1% vs. 18.1%) were better than those with a larger tumor size (≥4.0 cm, p = 0.103, 11.3% vs. 16.0%). There was a significant difference in the incidence of complications between the two groups among those without fundamental diseases (p = 0.007, 8.8% vs. 17.0%), but the same result was not found among patients with fundamental diseases (p = 0.091, 11.1% vs. 16.6%). Patients with a low ASA score (1/2, p = 0.004, 9.7% vs. 16.3%) were more likely to benefit from the incomplete ERAS protocol, while no significant differences were found for patients with high scores (3/4, p = 0.270, 12.9% vs. 23.5%). A statistically significant difference was observed for patients with proximal colon tumors (ascending/transverse colon, p = 0.027, 12.2% vs. 24.3%), but not for patients with distal colon tumors (descending/sigmoid colon, p = 0.104, 7.7% vs. 14.0%) and rectal tumors (p = 0.140, 10.3% vs. 14.8%). Subgroup analysis of differentiation status showed that patients with poor (p = 0.012, 6.1% vs. 23.7%) and moderate (p = 0.034, 10.3% vs. 15.3%) differentiation in the incomplete ERAS group had fewer postoperative complications than those in the non-ERAS group. However, for the patients with high differentiation (p = 0.426, 12.5% vs. 25.0%), no statistically significant difference was found between the two groups. Patients without neoadjuvant radiotherapy significantly benefited from an incomplete ERAS protocol and had fewer postoperative complications (p = 0.004, 10.3% vs. 16.9%), whereas patients who received neoadjuvant radiotherapy did not benefit (p = 0.349, 4.0% vs. 16.0%).
Discussion
There have been many studies on the effects of ERAS on colorectal cancer surgery, and the vast majority of those studies have focused on the perioperative effects of highly compliant ERAS and the differences between ERAS and traditional care (21–24). However, in clinical practice, overall compliance with the ERAS protocol varies from hospital to hospital and depends on the hospital's teamwork, the medical staff's acceptance of the protocol (25), and patient cooperation. The perioperative benefits of complete ERAS do not need to be confirmed by more studies, according to Dr. Kehlet H., the proposer of the ERAS protocol, and the core elements of ERAS should be clarified for further improvement (26). Looking for such key factors in patients with lower ERAS compliance seems to be one possible route. To this end, we focused our research on exploring the perioperative complications of colorectal cancer patients who had low compliance with the ERAS protocol. To the best of our knowledge, to date, there are no large-scale comparisons of perioperative complications between incomplete ERAS and non-ERAS patients.
PSM is a statistical analysis method used to reduce the impact of data bias and confounding variables. In this study, we used PSM to balance the two sets of data. After PSM, the following imbalanced factors were well balanced: age (p = 0.795), BMI (p = 0.699), ASA score (p = 0.700), tumor location (p = 0.121), neoadjuvant radiotherapy (p = 1.000), neoadjuvant chemotherapy (p = 0.795), and lymphovascular invasion (p = 0.398). To better match the scoring and guide clinical practice, we regrouped patients according to the ASA score and tumor location. For ASA score, patients were classified according to a score of 1/2 and 3/4. For tumor location, patients were classified into the proximal colon group (including ascending colon/transverse colon) and distal colon (including descending colon and sigmoid colon) and rectum group according to the actual surgical judgment. Interestingly, with or without PSM, the NR group showed fewer postoperative complications than the conventional care group, but after PSM, the two groups were more comparable, as shown in Tables 2, 3.
Here, for statistical analysis purposes, complications are classified as mild (Clavien–Dindo 1 and 2) and severe (Clavien–Dindo 3, 4 and 5). Further analysis of the postoperative complications showed that even without high compliance to the ERAS protocol, the program still reduced the incidence of postoperative complications compared to traditional care, with fewer mild complications (6.7% vs. 10.8%) and severe complications (3.2% vs. 6.0%) observed. Fewer complications indirectly related to surgery were observed in the incomplete ERAS group, and as most surgeons know, Local surgical complications were related to presence of drain, the depth of the tumor, including T4b, operation time, and blood loss. This result suggests that ERAS affects other aspects of recovery, rather than the surgery itself. Based on this result, it is reasonable to further ask whether some elements in ERAS protocols are more clinically significant protective factors than others. Our study seems to serve as a support for such investigations, and our next step is to identify the key elements of ERAS and make the ERAS protocol easier to implement to help provide patients with better perioperative recovery.
In the sex subgroup analysis, the incomplete ERAS group had fewer complications than the non-ERAS group, regardless of sex (male, OR 0.620, 95% CI, 0.395–0.975; female, OR 0.359, 95% CI, 0.161–0.801). Similar results were observed in the neoadjuvant chemotherapy subgroup analysis. Thus, it was not deemed a factor that affected the reduction in perioperative complications of ERAS (neoadjuvant chemotherapy, OR 0.246, 95% CI, 0.074–0.813; no neoadjuvant chemotherapy, OR 0.608, 95% CI, 0.401–0.920). Although the OR values here indicate that neoadjuvant therapy is advantageous, this advantage should be considered with caution due to the small number of cases in this group. However, the subgroup analysis of several other factors presented biased options. The incomplete ERAS protocol was found to have a significant advantage for younger patients (<60 years, OR 0.389, 95% CI, 0.212–0.713) with a low BMI (<22.48,OR 0.385,95% CI, 0.224–0.663), small tumor size (<4.0 cm, OR 0.397,95% CI, 0.209–0.755), no fundamental diseases (OR 0.470, 95% CI, 0.268–0.824), low ASA score (OR 0.552, 95% CI, 0.367–0.831), ascending/transverse colon tumor (OR 0.435, 95% CI, 0.206–0.919), poor differentiation (OR 0.210, 95% CI, 0.056–0.779) or moderate differentiation (OR 0.633,95% CI, 0.413–0.969), and no neoadjuvant radiotherapy (OR 0.563, 95% CI, 0.379–0.838). In the statistical analysis, due to the small number of patients in the highly differentiated and neoadjuvant radiotherapy subgroups, Fisher's exact test was adopted to obtain more accurate results.
Based on these results, we believe it would be beneficial to attempt the ERAS protocol in as many patients as possible, especially young patients with a low BMI, small tumor size, low ASA score, proximal colon, poor/moderately differentiated tumor, and without fundamental diseases or neoadjuvant radiotherapy, because a reduction in postoperative complications, both mild and severe, was noted in this population. It is well known that patients with younger age, lower BMI, lower ASA, and no fundamental disease have a lower risk of complications. As to why such patients are more likely to benefit from ERAS programs, based on our experience, we believe that such patients are more likely to receive ERAS procedures and have better cooperation than older and frail patients who have more trouble getting postoperative ambulation early. The incidence of postoperative complications, surgical readmission or death within 30 days of surgery is extremely important for both health care workers and patients because more complications mean a longer total hospital stay, higher medicare payments (27), and more human resources.
Considering the impact of the introduction time of laparoscopic colorectal surgery on surgical complications, we selected those cases that went beyond the learning curve as the study subjects. Since the introduction of laparoscopic sigmoid resection in 1993, Chinese doctors have gradually accepted and mastered the technique. After 2000, laparoscopic colorectal resection has been performed in many medical centers in China. The four centers involved in the study were all university hospitals, and surgeons were well beyond the learning curve and proficient in laparoscopic surgery before the earliest cases were included. The incidence of postoperative complications was similar among the participating institutions.
There are some limitations to the study. First, although this is a multicenter study, the biases associated with retrospective studies are inevitable, so the results may be statistically biased. Second, most of the non-ERAS data we collected were from before the publication of ERAS for colorectal cancer surgery in 2012, while almost all the data in the incomplete ERAS group were from after 2012. This may have led to bias in the statistical analysis, thus affecting the reliability of the results. Therefore, an RCT cohort study must be completed in the future before the results of this study can be extended to a larger, more complex population.
Conclusions
An incomplete ERAS protocol reduces the incidence of postoperative complications compared to no ERAS protocol, especially for younger patients who have a low BMI, smaller tumor size, no fundamental diseases, low ASA score, proximal colon tumors, poor/moderate differentiation and no preoperative neoadjuvant radiotherapy. ERAS should be recommended to as many patients as possible, although some patients are unable to adhere well to the protocol. In the future, more core elements of ERAS need to be identified.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional and Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CJ, CY and XS contributed to the conception and study design and obtained funding. CJ, ZZ, SG and JF contributed to writing the article. JC, NZ, HB, XL, XC and WZ helped in the data collection, analysis, and interpretation. CJ and XS supervised the study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 82072744) and Natural Science Foundation of Fujian Province, China (No. 2021J011371).
Conflict of interest
The handling editor Z.L. declared a shared parent affiliation with the authors C.J., J.C., N.Z., H.B., X.L., X.C., W.Z, and X.S. at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Klek S, Salowka J, Choruz R, Cegielny T, Welanyk J, Wilczek M, et al. Enhanced recovery after surgery (ERAS) protocol is a safe and effective approach in patients with gastrointestinal fistulas undergoing reconstruction: results from a prospective study. Nutrients. (2021) 13:6. doi: 10.3390/nu13061953
2. Visioni A, Shah R, Gabriel E, Attwood K, Kukar M, Nurkin S. Enhanced recovery after surgery for noncolorectal surgery?: a systematic review and meta-analysis of major abdominal surgery. Ann Surg. (2018) 267(1):57–65. doi: 10.1097/SLA.0000000000002267
3. Haro GJ, Sheu B, Marcus SG, Sarin A, Campbell L, Jablons DM, et al. Perioperative lung resection outcomes after implementation of a multidisciplinary, evidence-based thoracic ERAS program. Ann Surg. (2021) 274(6):e1008–13. doi: 10.1097/SLA.0000000000003719
4. Williams SB, Cumberbatch M, Kamat AM, Jubber I, Kerr PS, McGrath JS, et al. Reporting radical cystectomy outcomes following implementation of enhanced recovery after surgery protocols: a systematic review and individual patient data meta-analysis. Eur Urol. (2020) 78(5):719–30. doi: 10.1016/j.eururo.2020.06.039
5. Kowalsky SJ, Zenati MS, Steve J, Esper SA, Lee KK, Hogg ME, et al. A combination of robotic approach and ERAS pathway optimizes outcomes and cost for pancreatoduodenectomy. Ann Surg. (2019) 269(6):1138–45. doi: 10.1097/SLA.0000000000002707
6. Jogerst K, Thomas O, Kosiorek HE, Gray R, Cronin P, Casey WR, et al. Same-day discharge after mastectomy: breast cancer surgery in the era of ERAS[(R)]. Ann Surg Oncol. (2020) 27(9):3436–45. doi: 10.1245/s10434-020-08386-w
7. Kiong KL, Vu CN, Yao C, Kruse B, Zheng G, Yu P, et al. Enhanced recovery after surgery (ERAS) in head and neck oncologic surgery: a case-matched analysis of perioperative and pain outcomes. Ann Surg Oncol. (2021) 28(2):867–76. doi: 10.1245/s10434-020-09174-2
8. Webb C, Day R, Velazco CS, Pockaj BA, Gray RJ, Stucky CC, et al. Implementation of an enhanced recovery after surgery (ERAS) program is associated with improved outcomes in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. (2020) 27(1):303–12. doi: 10.1245/s10434-019-07900-z
9. Aarts MA, Rotstein OD, Pearsall EA, Victor JC, Okrainec A, McKenzie M, et al. Postoperative ERAS interventions have the greatest impact on optimal recovery: experience with implementation of ERAS across multiple hospitals. Ann Surg. (2018) 267(6):992–7. doi: 10.1097/SLA.0000000000002632
10. Pang KH, Groves R, Venugopal S, Noon AP, Catto J. Prospective implementation of enhanced recovery after surgery protocols to radical cystectomy. Eur Urol. (2018) 73(3):363–71. doi: 10.1016/j.eururo.2017.07.031
11. Tian Y, Cao S, Liu X, Li L, He Q, Jiang L, et al. Randomized controlled trial comparing the short-term outcomes of enhanced recovery after surgery and conventional care in laparoscopic distal gastrectomy (GISSG1901). Ann Surg. (2022) 275(1):e15–21. doi: 10.1097/SLA.0000000000004908
12. Pisarska M, Pedziwiatr M, Malczak P, Major P, Ochenduszko S, Zub-Pokrowiecka A, et al. Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study. Int J Surg. (2016) 36(Pt A):377–82. doi: 10.1016/j.ijsu.2016.11.088
13. On behalf of the ERAS Compliance Group. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection. Ann Surg. (2015) 261(6):1153–9. doi: 10.1097/SLA.0000000000001029
14. Ripolles-Melchor J, Abad-Motos A, Diez-Remesal Y, Aseguinolaza-Pagola M, Padin-Barreiro L, Sanchez-Martin R, et al. Association between use of enhanced recovery after surgery protocol and postoperative complications in total hip and knee arthroplasty in the postoperative outcomes within enhanced recovery after surgery protocol in elective total hip and knee arthroplasty study (POWER2). JAMA Surg. (2020) 155(4):e196024. doi: 10.1001/jamasurg.2019.6024
15. Jian C, Fang J, Wu L, Zheng Z, Song Y, Liu W, et al. Failure of enhanced recovery programs after laparoscopic radical gastrectomy: a single-center retrospective study. Surg Endosc. (2021) 35(6):2629–35. doi: 10.1007/s00464-020-07683-5
16. You K, Sohn DK, Park SS, Park SC, Oh JH, Han KS, et al. Factors associated with diet failure after colon cancer surgery. Surg Endosc. (2022) 36(5):2861–8. doi: 10.1007/s00464-021-08575-y
17. Oh HK, Ihn MH, Son IT, Park JT, Lee J, Kim DW, et al. Factors associated with failure of enhanced recovery programs after laparoscopic colon cancer surgery: a single-center retrospective study. Surg Endosc. (2016) 30(3):1086–93. doi: 10.1007/s00464-015-4302-y
18. Lohsiriwat V. Learning curve of enhanced recovery after surgery program in open colorectal surgery. World J Gastrointest Surg. (2019) 11(3):169–78. doi: 10.4240/wjgs.v11.i3.169
19. Pisarska M, Torbicz G, Gajewska N, Rubinkiewicz M, Wierdak M, Major P, et al. Compliance with the ERAS protocol and 3-year survival after laparoscopic surgery for non-metastatic colorectal cancer. World J Surg. (2019) 43(10):2552–60. doi: 10.1007/s00268-019-05073-0
20. Viannay P, Hamy A, Jaouen R, Caroli-Bosc FX, Luel C, Vasseur S, et al. Does enhanced recovery improve the survival rates of patients 3 years after undergoing surgery to remove a tumor in the colon? Int J Colorectal Dis. (2019) 34(3):441–9. doi: 10.1007/s00384-018-3205-5
21. Lopez-Rodriguez-Arias F, Sanchez-Guillen L, Lillo-Garcia C, Aranaz-Ostariz V, Alcaide MJ, Soler-Silva A, et al. Assessment of body composition as an indicator of early peripheral parenteral nutrition therapy in patients undergoing colorectal cancer surgery in an enhanced recovery program. Nutrients. (2021) 13(9):3245. doi: 10.3390/nu13093245
22. Wang C, Feng H, Zhu X, Song Z, Li Y, Shi Y, et al. Comparative effectiveness of enhanced recovery after surgery program combined with single-incision laparoscopic surgery in colorectal cancer surgery (2019) a retrospective analysis. Front Oncol. (2021) 11:768299. doi: 10.3389/fonc.2021.768299
23. Silva-Velazco J, Dietz DW, Stocchi L, Costedio M, Gorgun E, Kalady MF, et al. Considering value in rectal cancer surgery: an analysis of costs and outcomes based on the open, laparoscopic, and robotic approach for proctectomy. Ann Surg. (2017) 265(5):960–8. doi: 10.1097/SLA.0000000000001815
24. Millan M, Renau-Escrig AI. Minimizing the impact of colorectal surgery in the older patient: the role of enhanced recovery programs in older patients. Eur J Surg Oncol. (2020) 46(3):338–43. doi: 10.1016/j.ejso.2019.12.018
25. Pearsall EA, Meghji Z, Pitzul KB, Aarts MA, McKenzie M, McLeod RS, et al. A qualitative study to understand the barriers and enablers in implementing an enhanced recovery after surgery program. Ann Surg. (2015) 261(1):92–6. doi: 10.1097/SLA.0000000000000604
26. Kehlet H. ERAS implementation-time to move forward. Ann Surg. (2018) 267(6):998–9. doi: 10.1097/SLA.0000000000002720
Keywords: incomplete ERAS protocol, colorectal cancer, traditional care, complications, propensity score-matched analysis
Citation: Jian C, Zhou Z, Guan S, Fang J, Chen J, Zhao N, Bao H, Li X, Cheng X, Zhu W, Yang C and Shu X (2022) Can an incomplete ERAS protocol reduce postoperative complications compared with conventional care in laparoscopic radical resection of colorectal cancer? A multicenter observational cohort and propensity score-matched analysis. Front. Surg. 9:986010. doi: 10.3389/fsurg.2022.986010
Received: 4 July 2022; Accepted: 9 August 2022;
Published: 26 August 2022.
Edited by:
Zeming Liu, Huazhong University of Science and Technology, ChinaReviewed by:
Shinya Abe, The University of Tokyo, JapanShenghui Huang, Fujian Medical University Union Hospital, China
© 2022 Jian, Zhou, Guan, Fang, Chen, Zhao, Bao, Li, Cheng, Zhu, Yang and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaogang Shu sxg678@yahoo.com; Chunkang Yang chunkang129@fjmu.edu.cn
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Chenxing Jian1,2,†
Chenxing Jian1,2,†  Zili Zhou
Zili Zhou Xukai Cheng
Xukai Cheng Xiaogang Shu
Xiaogang Shu