First Robotic Hepatectomy With Middle Hepatic Vein Reconstruction Using ePTFE Graft for Hepatic Adenoma: A Case Report
- Department of Hepatobiliary Surgery, the First Affiliated Hospital of Guangxi Medical University, Nanning, China
Surgical resection remains the best choice for the treatment of liver tumors. Hepatectomy combined with artificial vascular reconstruction has been proven as an alternative to treating tumors involving the main hepatic veins. As the cutting-edge surgical technique, robotic liver surgery is a novel procedure expanding the field of minimally invasive approaches, especially in complex reconstruction. This study reports, for the first time, on a robotic hepatectomy with middle hepatic vein (MHV) reconstruction using an expanded polytetrafluoroethylene (ePTFE) graft for a patient with hepatic adenoma. The tumor, which was located in segment 8, was adjacent to the MHV. Robot-assisted resection of segment 4 and partial segment 8, and MHV reconstruction using a ePTFE graft were performed. During the post-operative examination and follow-up, the blood flow of the ePTFE graft was patent, and liver function recovered well. Thus, robotic hepatectomy with MHV reconstruction is a safe, minimally invasive, and precise surgery that may provide a novel approach for patients with liver tumors that are invading or adjacent to the main hepatic veins.
Introduction
Surgical resection remains the best treatment for liver tumors (1). Hepatic vena cava confluence, vascular involvement of inferior vena cava (IVC), portal vein, and hepatic artery have long been considered contraindications to hepatectomy (2). In recent years, complex hepatectomy combined with vascular reconstruction has become increasingly common due to the development of vascular reconstruction (3). The removed blood vessels can now be replaced with various materials, such as autologous veins, allogeneic blood vessels, and artificial blood vessels. Meanwhile, the development of the robot-assisted hepatectomy technique further promotes the development of precise surgical techniques (4). A robot-assisted hepatectomy with hepatic vein reconstruction has never been reported on. The present study reports, for the first time, on a patient who underwent robot-assisted resection of liver segment 4 (S4) and partial segment 8 (S8) with reconstruction of the middle hepatic vein (MHV) through the successful use of a ringed expanded polytetrafluoroethylene (ePTFE) graft.
Case Report
Patient Base Condition and Preoperative Evaluation
The patient was a 29-year-old female. Routine physical examination and abdominal CT showed a hepatic space-occupying lesion of approximately 1.6 cm × 1.0 cm × 1.3 cm. The patient had a previous history of polycystic ovary syndrome and pituitary microadenomas, and virological examination showed that she did not suffer from liver viruses, such as hepatitis B or hepatitis C. Peripheral blood was biochemically examined for a range of tumor markers, and they were within normal values. Liver MRI plain scan and enhanced examination were further refined, which suggested a lesion in S8 (Figures 1A–C). The lesion in the artery phase showed non-circular high enhancement, the portal vein phase and delay phase were decreased and lower than the liver parenchyma, and the hepatobiliary phase was decreased significantly. The lesion was suspected to be a hepatic adenoma (HCA) or small hepatocarcinoma (HCC).
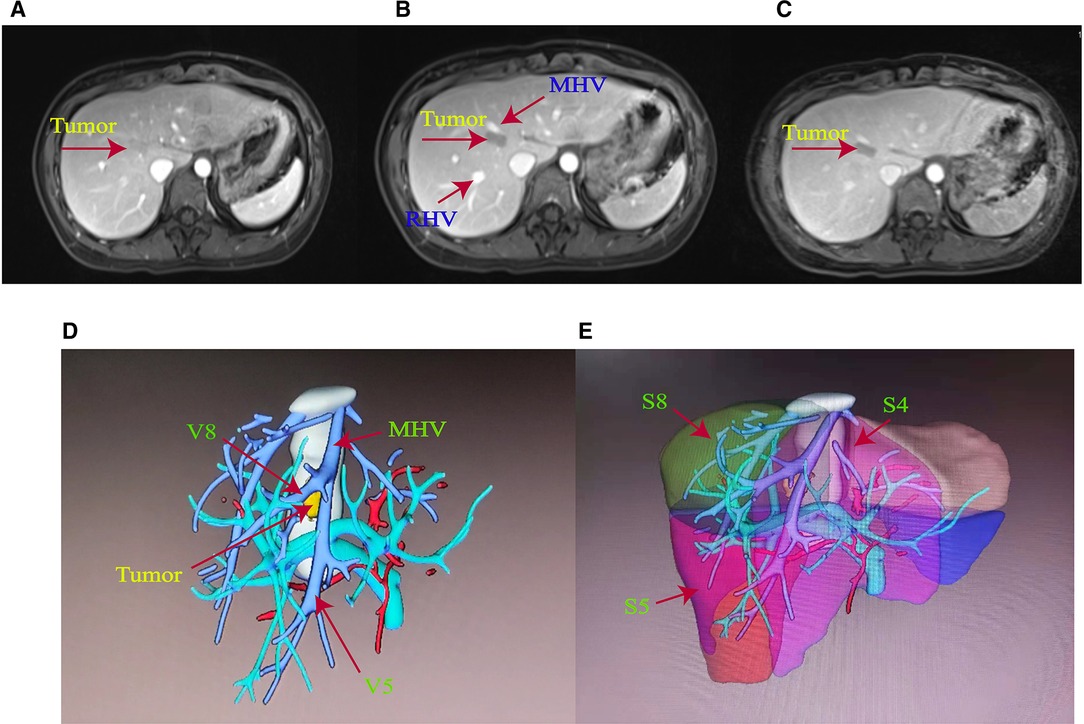
Figure 1. (A) Preoperative MRI of the patient with enhanced arterial phase. (B) Preoperative MRI of the patient with enhanced venous phase. (C) Delayed phase of preoperative MRI enhancement in the patient. (D) Main hepatic vein (MHV) trunk and V5 and V8 branches were labeled in accordance with the three-dimensional stereo images of hepatic vessels reconstructed by imaging. (E) S4, S5, and S8 reconstruction images of liver segments.
An IQQA system (EDDA Health Technologies, Inc., Princeton, USA) was used to perform 3D reconstruction. As shown in Figures 1D, E, the tumor was located in S8, near S4 and adjacent to the main trunk of MHV. Liver function was Child–Pugh A, and the liver stiffness score, as examined by the liver elasticity measurement technique, was F1. The retention at 15 min was less than 10% for the indocyanine green test. Liver volume was calculated by the IQQA-3D system. The standard liver volume was 840 ml based on the West China formula (5), and the residual liver volume after resection of S4 and S8 segments was 588 ml, accounting for 70% of the standard liver volume. The reflux areas of the V5 and V8 segments of MHV were 153.7 and 158 ml, respectively.
Surgical Procedure
A da Vinci Xi robot system was used. The patient was positioned in the 12° reverse Trendelenburg position. The procedure was performed with a second surgeon positioned between the patient’s legs. A 12 mm incision was made at the umbilicus, and a trocar was placed for the assistant surgeon. The camera port (8 mm) was inserted in the right abdomen, approximately 10 cm away from the umbilicus. Another three 8 mm ports for the robot were placed in the right hypochondrium, 2 cm below the xiphoid process and left hypochondrium. The first, second, third, and fourth robotic arms were docked on the right hypochondrium, on the camera, 2 cm below the xiphoid process, and on the left hypochondrium ports, respectively (Figure 2A).
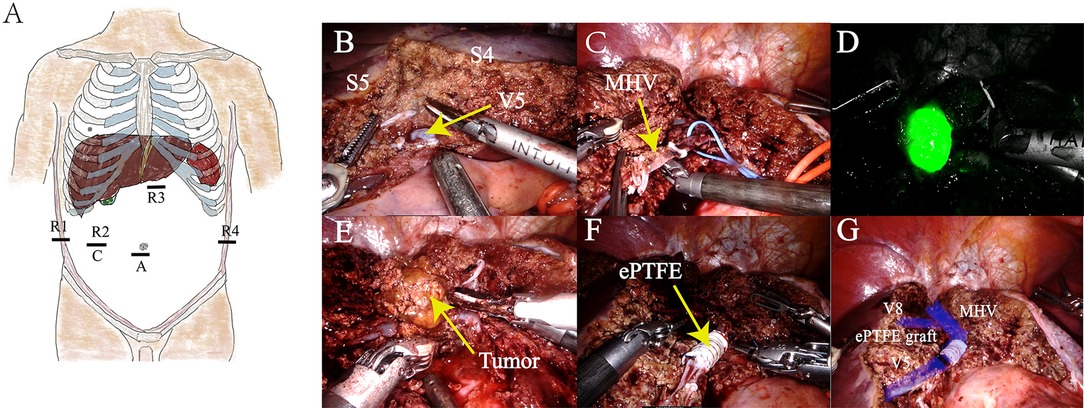
Figure 2. (A) Human distribution position of Xi trocar. (B) S4 and S5 segments were isolated, revealing the V5 branch of the MHV. (C) Trunk of the MHV was cut. (D) Intraoperative fluorescence imaging of the tumor was performed to facilitate complete resection of the tumor. (E) Isolation and resection of tumors. (F) MHV reconstruction using artificial blood vessels of expanded polytetrafluoroethylene material. (G) Schematic of the completion of MHV reconstitution.
The exploration showed pathological adhesion of the upper abdominal bowel and liver cirrhosis on the surface of the liver. No abnormality was found in the gall bladder. Enlarged lymph nodes were found in the first hepatic hilum, with a soft texture. No signs of tumor metastasis were found in the abdominal cavity. Intraoperative ultrasonography revealed a tumor, which was located in S8, adhered with MHV.
The first hepatic hilum was exposed, and a vascular blocking band was preset around the hepatic pedicle. Parenchymal liver transection was achieved using robotic fenestrated bipolar forceps and a harmonic scalpel. The Glissonean pedicles of S4 were ligated and cut. S4 resection was completed. MHV was exposed and stripped, and then, partial segment 8 was resected. As the tumor was close to the V8 branch of MHV, MHV was cut off to completely remove the tumor and avoid MHV bleeding. Following this, the Glissonean pedicles of the tumor were ligated and cut. The tumor was successfully resected under the guidance of intraoperative fluorescence imaging.
The MHV could not be anastomosed directly without tension. An 8-mm-internal ringed ePTFE graft (W.L. Gore & Associates, Inc., Arizona, USA) was used for hepatic vein reconstruction. The MHV was blocked using vascular clamps, and the length of the required ePTFE graft was measured (it was approximately 4 cm). Then, the ePTFE graft was anastomosed to both ends of the MHV using a running suture of 5-0 Prolene. Intermittent irrigation of heparin saline was performed during the anastomoses.
After the reconstruction was completed, the MHV was patent. The operating time was 300 minutes. The intraoperative blood loss was 230 mL. The patient recovered uneventfully and was discharged on the 14th postoperative day. Pathological examination confirmed that the lesion was adenoma, and the subtype was inflammatory. Surgery videos were presented as a supplementary document (Supplementary Video S1 and S2).
Follow Up
The patient received rivaroxaban for 3 months after the procedure, and her coagulation profile was checked regularly (Figure 3). Ultrasound blood-flow examinations were performed at 1 week and 3 months after surgery to confirm the patency of the reconstructed vessels. The results showed that the reconstructed MHV was patent.
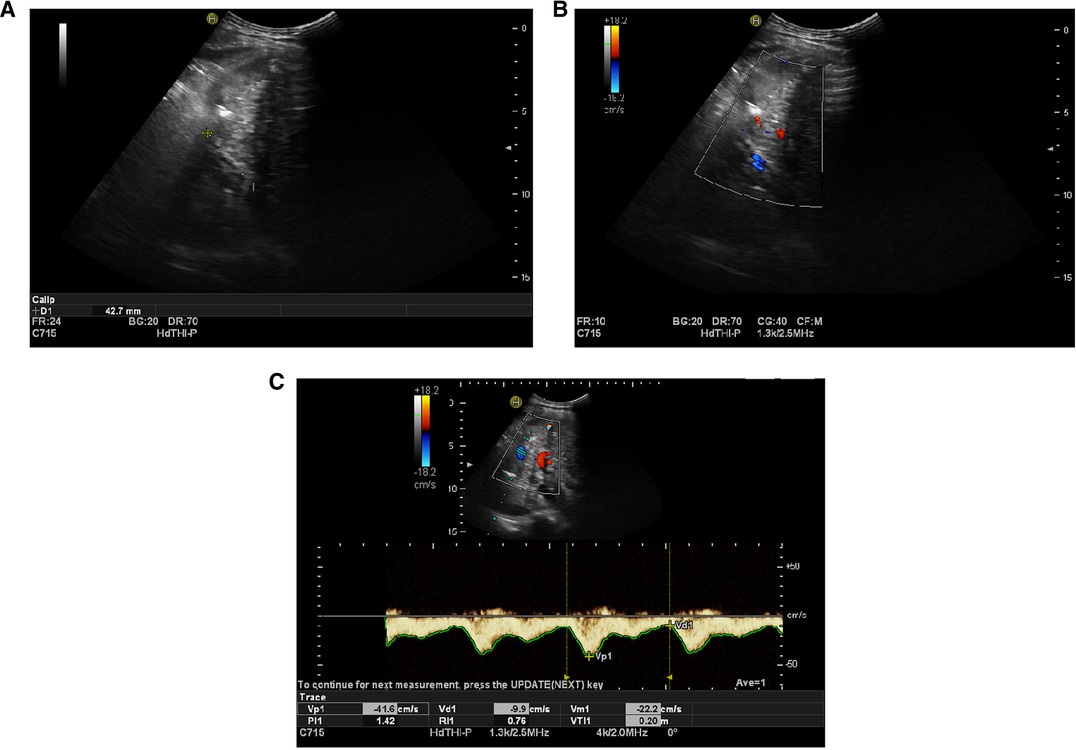
Figure 3. (A) Artificial vessels, which were visualized by ultrasound one week after operation, were approximately 42.7 mm in length. (B) Blood flow of artificial blood vessels displayed by ultrasound one week after operation (blue). (C) Ultrasound blood flow spectrogram of artificial vessels one week after operation, showing blood flow patency.
At the latest follow-up time, which was the ninth postoperative month, the patient’s re-examination showed a good quality of life. The ePTFE graft for the MHV was patent.
Discussion
Liver resection remains the optimal treatment modality for most liver tumors. Robotic artificial vascular reconstruction combined with hepatectomy can play an important role in the secondary surgery of patients with residual and recurrent tumors, especially when the tumor invades the main hepatic vein or needs to remove the great vein. This fine technique can help these patients avoid liver congestion caused by outflow tract obstruction, which can accelerate the recovery of liver function, improve the quality of life and long-term prognosis (6). Many limitations for liver surgery have been overcome in recent decades thanks to multimodal treatment concepts with staged liver resections, interventional and systemic therapies, and refinement of surgical techniques (7, 8).
Vascular invasion is often considered a contraindication to liver surgery, and such patients often receive only palliative treatment. This is because of the poor prognosis, and the technical difficulties and risks of resection of multiple vessels involved in tumor infiltration. The main goal of tumor surgery is to achieve complete tumor clearance while ensuring patients’ safety (9). The resectability of liver tumors has increased due to the continuous development of new adjuvant treatment modalities and new surgical techniques. Vascular reconstruction combined with hepatic resection is an update in the surgical management of tumors. The progress of techniques and comprehensive treatment has reduced the morbidity and mortality during the perioperative period, and has improved the long-term prognosis of the patients. Nowadays, vascular reconstruction is more frequent (10). Autologous materials are generally the first choice for hepatic vein reconstruction (11, 12). However, the advantage of artificial grafts is the range of length and diameters that can be easily obtained. Therefore, in some cases, artificial grafts have become the ideal substitute. In liver surgery, the artificial grafts mainly used for vascular reconstruction are polyethylene (PTE) grafts and porous polytetrafluoroethylene (PTFE) (13). PTFE has been proven to be a feasible option, with the following advantages over autologous venous grafts: (1) flexibility in complex reconstruction; (2) exact orientation and size calibration for proper fit; and (3) significantly shorter back-table time and anhepatic time (14).
Robot-assisted hepatectomy is becoming increasingly common, and the introduction of robotics in the field of liver surgery may make technically difficult, minimally invasive liver approaches more feasible (15). Robotic liver surgery enables more difficult and refined surgical manipulation through robotic arm advantages, which provide an important basis for complex vascular reconstruction operations. Despite the rapid growth and expansion of robotic liver surgery with promising perioperative outcomes, reports on vascular reconstruction combined with hepatic resection remain limited (16). In several experienced robotic centers, great progress has been made in the manipulation of the hepatic vasculature, and the portal vein, inferior vena cava, and hepatic artery have been safely and successfully reconstructed (17–19). The present paper reports the first case of robot-assisted reconstruction of MHV with artificial blood vessels, combined with a hepatectomy.
HCAs are rare benign tumors of the liver, and the majority (70%–80%) of HCAs are solitary and usually located in the right hepatic lobe (20). At present, according to the molecular biological genotype and pathological characteristics of HCAs, HCAs can be roughly divided into four subtypes: HNF1A inactivated adenoma (H-HCA), β-catenin activated adenoma (β-HCA), inflammatory adenoma, and unclassified adenoma. The inflammatory type is the most common subtype, and β-HCA is the most likely subtype of malignant transformation (21). HCAs are difficult to differentiate clinically from focal nodular hyperplasia and well-differentiated HCC, and the misdiagnosis rate is high. Given that bleeding and malignant transformation are common complications of HCA, surgical treatment is currently advised as early as possible after discovery (22). In the present study, the tumor was close to the junction of the MHV branch and main trunk, and although the volume was small, ablation therapy was not chosen after considering the risk of bleeding and the individual wishes of the patient. Resection of S4 and partial S8 was beneficial to expose the MHV and control hepatic vein hemorrhage, thus providing an adequate perspective to reveal the tumor. Finally, the bleeding volume was decreased.
In liver surgery, maintaining proper blood outflow is as important as maintaining sufficient blood inflow. MHVs are normally responsible for the refluxing of the paracentral segment of the liver, and they have a limited role in the refluxing of S4 (23). Therefore, MHV reconstruction in left hemihepatectomies may be important (24, 25). Reconstruction of the MHV could avoid severe congestion of the refluxing segment, and studies have reported that it could increase the non-hyperemic FLR from 36.5% to 73.8%, which could benefit liver regeneration and avoid the occurrence of postoperative liver failure (26). Severely congested liver segments may cause postoperative liver failure, massive ascites, and impaired liver regeneration (27).
Different criteria for hepatic vein reconstruction in hepatectomies have been published (28, 29). In the authors’ center, the indication for reconstructing the hepatic vein was based on the size (diameter ≥ 5 mm) and depth (superficial or deep) of the hepatic vein. Superficial and deep veins were defined as veins draining 1–2 cm and >2 cm of parenchyma, respectively (30). In the present case, the branch of the MHV (diameter = 7 mm, deep vein) was reconstructed for S5. The MHV could not be anastomosed directly without tension. Thus, an ePTFE graft was used for reconstruction.
Meanwhile, the use of robot technology provided a clear and complete perspective of deep tumor resection and hepatic vein reconstruction. It helps to reconstruct the hepatic vein precisely and prevent the occurrence of postoperative complications related to vascular anastomosis.
Conclusions
This study reported the first robotic MHV reconstruction combined with a hepatectomy using an ePTFE graft. The patient recovered well, and the reconstructed venous blood flow was patent, which showed that hepatic vein reconstruction combined with a robotic hepatectomy is feasible in an experienced robotic surgery center. The advantages of robotic hepatectomy combined with hepatic vein reconstruction could provide a minimal surgical approach for patients with deep tumors adjacent to large blood vessels.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the medical ethics committee of the First Affiliated Hospital of Guangxi Medical University, Guangxi, China. The patients/participants provided their written informed consent to participate in this study.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
JW and ZJ contributed equally to this work. ZJ and JW collected and analyzed data and wrote the manuscript. BX, HZ, JZ, and WC contributed to the conception and design of the work. YG, LZ, and TL revised the manuscript. ZW was responsible for the design of research ideas and the accuracy of all aspects of the work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81902983 and 82160128), the Guangxi Natural Science Foundation of China (No. 2018GXNSFBA050030), the “Medical Excellence Award” funded by the creative research development grant from the First Affiliated Hospital of Guangxi Medical University (No. 180327), the Guangxi Medical and Health Technology Development and Application Project (Nos. S2019097 and S2018100), and the project fund for improving the basic scientific research ability of young and middle-aged teachers in colleges and universities in Guangxi (2019KY0123).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fsurg.2022.904253/full#supplementary-material.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen PD, Wu CY, Hu RH, Chen CN, Yuan RH, Liang JT, et al. Robotic major hepatectomy: is there a learning curve? Surgery. (2017) 161(3):642–9. doi: 10.1016/j.surg.2016.09.025
2. Bismuth H, Castaing D, Garden OJ. Major hepatic resection under total vascular exclusion. Ann Surg. (1989) 210(1):13–9. doi: 10.1097/00000658-198907000-00002
3. Azoulay D, Eshkenazy R, Andreani P, Castaing D, Adam R, Ichai P, et al. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg. (2005) 241(2):277–85. doi: 10.1097/01.sla.0000152017.62778.2f
4. Ohwada S, Kawashima Y, Ogawa T, Ohya T, Takeyoshi I, Saito A, et al. Extended hepatectomy with ePTFE graft vena caval replacement and hepatic vein reconstruction: a case report. Hepatogastroenterology. (1999) 46(26):1151–5.10370683
5. Shi ZR, Yan LN, Li B, Wen TF. Evaluation of standard liver volume formulae for Chinese adults. World J Gastroenterol. (2009) 15(32):4062–6. doi: 10.3748/wjg.15.4062
6. Dimitroulis D, Tsaparas P, Valsami S, Mantas D, Spartalis E, Markakis C, et al. Indications, limitations and maneuvers to enable extended hepatectomy: current trends. World J Gastroenterol. (2014) 20(24):7887–93. doi: 10.3748/wjg.v20.i24.7887
7. Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. (2005) 241(5):715–22, discussion 22-4. doi: 10.1097/01.sla.0000160703.75808.7d
8. Gregoire E, Hoti E, Gorden DL, de la Serna S, Pascal G, Azoulay D. Utility or futility of prognostic scoring systems for colorectal liver metastases in an era of advanced multimodal therapy. Eur J Surg Oncol. (2010) 36(6):568–74. doi: 10.1016/j.ejso.2010.03.009
9. Alikhanov RB, Kubishkin VA, Dubrovskiy AV, Efanov MG. Reconstruction of hepatic veins in liver resection. technique and possibility of prophylaxis of posthepatectomy liver failure. Khirurgiia. (2016) 3:56–8. doi: 10.17116/hirurgia2016356-58
10. Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. (1999) 230(6):808–18. discussion 19. doi: 10.1097/00000658-199912000-00010
11. Orimo T, Kamiyama T, Yokoo H, Kakisaka T, Wakayama K, Tsuruga Y, et al. Usefulness of artificial vascular graft for venous reconstruction in liver surgery. World J Surg Oncol. (2014) 12(113):1–8. doi: 10.1186/1477-7819-12-113
12. Kato Y, Sugioka A, Tanahashi Y, Kojima M, Nakajima S, Yasuda A, et al. Recipient hepatic vein as an autologous vascular graft for hepatic vein reconstruction in living donor liver transplantation. Liver Transpl. (2021) 27(2):291–5. doi: 10.1002/lt.25905
13. Roll S, Müller-Nordhorn J, Keil T, Scholz H, Eidt D, Greiner W, et al. Dacron vs. PTFE as bypass materials in peripheral vascular surgery–systematic review and meta-analysis. BMC Surg. (2008) 8:8–22. doi: 10.1186/1471-2482-8-22
14. Singhal A, Makki K, Chorasiya V, Khan AA, Mohamed Q, Ahmad F, et al. Venous outflow reconstruction using a polytetrafluoroethylene (PTFE) graft in right lobe living donor liver transplantation: a single center study. Surgery. (2021) 169(6):1500–9. doi: 10.1016/j.surg.2021.01.011
15. Croner RS, Perrakis A, Hohenberger W, Brunner M. Robotic liver surgery for minor hepatic resections: a comparison with laparoscopic and open standard procedures. Langenbecks Arch Surg. (2016) 401(5):707–14. doi: 10.1007/s00423-016-1440-1
16. Wang JM, Li JF, Yuan GD, He SQ. Robot-assisted versus laparoscopic minor hepatectomy: a systematic review and meta-analysis. Medicine. (2021) 100(17):e25648. doi: 10.1097/MD.0000000000025648
17. Yamamoto M, Omori T, Shinno N, Hara H, Mukai Y, Sugase T, et al. Robotic total gastrectomy with thrombectomy and portal vein reconstruction for gastric cancer and portal vein tumor thrombus. World J Surg Oncol. (2022) 20(1):36. doi: 10.1186/s12957-022-02502-8
18. Davila VJ, Velazco CS, Stone WM, Fowl RJ, Abdul-Muhsin HM, Castle EP, et al. Robotic inferior vena cava surgery. J Vasc Surg. (2017) 5(2):194–9. doi: 10.1016/j.jvsv.2016.08.003
19. Yin SM, Peng CH, Jin JB, Qian JF. Robotic pancreaticoduodenectomy with hepatic artery reconstruction is feasible and safe for type 9 hepatic arterial anatomy: a case report. Asian J Surg. (2020) 43(5):644–6. doi: 10.1016/j.asjsur.2020.01.003
20. Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. (2009) 137(5):1698–705. doi: 10.1053/j.gastro.2009.07.061
21. Dharmana H, Saravana-Bawan S, Girgis S, Low G. Hepatocellular adenoma: imaging review of the various molecular subtypes. Clin Radiol. (2017) 72(4):276–85. doi: 10.1016/j.crad.2016.12.020
22. Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). (2010) 12(8):509–22. doi: 10.1111/j.1477-2574.2010.00222.x
23. Sadamori H, Hioki M, Monden K, Kobatake C, Kanehira N, Ohno S, et al. Right hepatic vein reconstruction with an autologous jugular vein graft to expand the surgical indications for liver tumors. J Gastrointest Surg. (2019) 23(12):2467. doi: 10.1007/s11605-019-04349-z
24. Sano K, Makuuchi M, Miki K, Maema A, Sugawara Y, Imamura H, et al. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg. (2002) 236(2):241–7. doi: 10.1097/00000658-200208000-00013
25. Kishi Y, Sugawara Y, Akamatsu N, Kaneko J, Matsui Y, Kokudo N, et al. Sharing the middle hepatic vein between donor and recipient: left liver graft procurement preserving a large segment VIII branch in donor. Liver Transpl. (2004) 10(9):1208–12. doi: 10.1002/lt.20226
26. Miyata A, Sakamoto Y, Yamamoto S, Akamatsu N, Arita J, Kaneko J, et al. Aggressive hemihepatectomy combined with resection and reconstruction of middle hepatic vein for intrahepatic cholangiocarcinoma. Ann Surg Oncol. (2016) 23(Suppl 4):494–500. doi: 10.1245/s10434-016-5384-z
27. Fortea JI, Puente Á, Cuadrado A, Huelin P, Pellón R, González Sánchez FJ, et al. Congestive hepatopathy. Int J Mol Sci. (2020) 21(24):1–23. doi: 10.3390/ijms21249420
28. Mise Y, Hasegawa K, Satou S, Aoki T, Beck Y, Sugawara Y, et al. Venous reconstruction based on virtual liver resection to avoid congestion in the liver remnant. Br J Surg. (2011) 98(12):1742–51. doi: 10.1002/bjs.7670
29. Wu CC, Peng CM, Cheng SB, Yeh DC, Lui WY, Liu TJ, et al. The necessity of hepatic vein reconstruction after resection of cranial part of the liver and major hepatic veins in cirrhotic patients. Surgery. (2012) 151(2):223–31. doi: 10.1016/j.surg.2010.10.014
Keywords: case reports, robotic surgical procedures, hepatectomy, hepatic veins, vascular grafting, liver cell adenoma
Citation: Wang J, Jin Z, Xu B, Chen W, Zhang J, Zhu H, Lu T, Zhang L, Guo Y and Wen Z (2022) First Robotic Hepatectomy With Middle Hepatic Vein Reconstruction Using ePTFE Graft for Hepatic Adenoma: A Case Report. Front. Surg. 9:904253. doi: 10.3389/fsurg.2022.904253
Received: 25 March 2022; Accepted: 20 May 2022;
Published: 14 June 2022.
Edited by:
Ka-Chun Siu, University of Nebraska Medical Center, United StatesCopyright © 2022 wang, Jin, xu, cheng, zhang, zhu, lu, zhang, guo and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Wen wenzgxmu@163.com
These authors have contributed equally to this work
Specialty section: This article was submitted to Visceral Surgery, a section of the journal Frontiers in Surgery
 Jilong Wang
Jilong Wang Zongrui Jin
Zongrui Jin Banghao Xu
Banghao Xu  Ling Zhang
Ling Zhang Zhang Wen
Zhang Wen