Clinical evaluation of paraspinal mini-tubular technique vs. laminoplasty for spinal intradural extramedullary tumors: Study protocol for a multicenter, randomized controlled trial
- Department of Neurosurgery, Fujian Medical University Union Hospital, Fuzhou, China
The development of minimally invasive surgery has promoted the use of the paraspinal mini-tubular technique (PMTT) for spinal tumors. The similarity of the efficacy of PMTT to that of traditional surgery remains unclear; randomized controlled trials (RCTs) have not been conducted to investigate this technique for spinal tumors. The conventional surgery used for such tumors is laminoplasty (LP). To compare the differences between the two surgical techniques, an RCT is significantly required. Therefore, a prospective, multi-center, non-inferiority RCT was designed to compare the safety and effectiveness of LP and PMTT for treating spinal intradural extramedullary (IDEM) tumors. Moreover, the availability of PMTT, including its advantages and disadvantages, surgical indications, procedures, complications, and prognosis, would be explored. Overall, 280 patients will be randomly allocated to the PMTT and LP groups in a 1:1 ratio. The trial hypothesis is that PMTT has superior or equivalent efficacy and cost-effectiveness to LP. The primary outcome is the Japanese Orthopedic Association score. The non-inferiority margin for the primary outcome is five. The Ethics Committee of Fujian Medical University Union Hospital, Fuzhou, China, has approved this study (project number: FJMUUH05). Any results of the trial will be published in international peer-reviewed journals and disseminated through presentations at scientific conferences.
Trial registration number: ChiCTR2100047582
1. Introduction
Although spinal intradural extramedullary (IDEM) tumors grow slowly, the spinal canal is limited by its small volume, and the spinal cord and nerve roots are compressed or invaded by the tumor, which causes sensory, motor, and autonomic dysfunction (1). The treatment of spinal IDEM tumors focuses on removing tumor tissue and decompressing the nerve root and spinal cord to improve nerve function (2, 3). Traditional surgical methods include laminectomy (total laminectomy and hemilaminectomy), laminotomy, and laminoplasty (LP) (4). Among the available spinal surgeries with different indications, a “standard surgical procedure” for treating spinal IDEM tumors has not been determined. Owing to the development of minimally invasive spine surgery (MISS), the paraspinal mini-tubular technique (PMTT) has been introduced for treating IDEM tumors (5–7). Most studies reported that tubular spine surgeries reduced the volume of estimated blood loss (EBL) and shortened the length of hospital stay. The postoperative outcome of the patients was good, with fewer complications. These together promoted the development of MISS. A microscope-assisted PMTT has a larger operating space than endoscopy and a relatively wide range of indications, including various types of intervertebral disc herniation, spinal stenosis, spinal tumors, and spinal cord injuries (8). With the popularization and application of PMTT, the safety and effectiveness of treating spinal-related diseases have been increasingly recognized. Compared with traditional surgery, the microscope-assisted microtubular technique has the advantages of less tissue damage, fewer complications, shorter length of hospital stay, reduced postoperative pain, improved surgical efficiency, and reduced risk of spinal instability (9–11). To the best of our knowledge, prospective, randomized controlled trial studies with a large sample comparing the safety and effectiveness of the PMTT and LP in the treatment of spinal IDEM tumors, have not been conducted. Therefore, this study aims to explore the difference in the therapeutic effect of the PMTT and LP in the treatment of patients with spinal IDEM tumors through a prospective, multi-centered, non-inferiority randomized clinical trial. The main hypothesis is that PMTT has advantages in cost-effectiveness, and it is equivalent in safety and efficacy to LP.
2. Methods and analysis
2.1. Study description
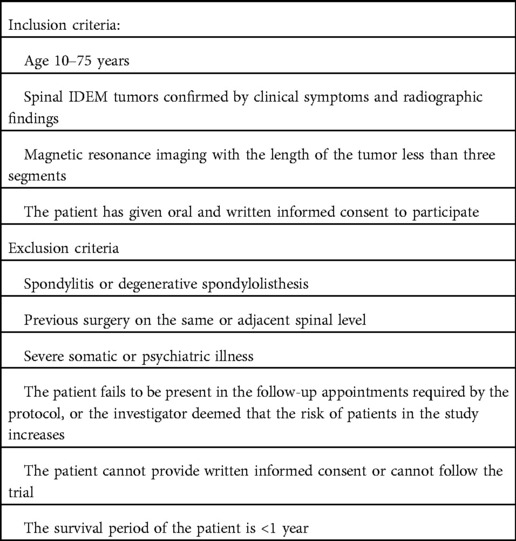
The FJMUUH05 trial is designed as a prospective, multi-center, non-inferiority RCT with two parallel groups in eight hospitals from seven provinces in China. A total of 280 patients aged 10–75 years with confirmed radiographic diagnosis and IDEM tumor lengths of less than three segments will be enrolled in this study. After the patients sign a written informed consent to participate, they will be randomized to one of the two groups: the PMTT group, which will receive paravertebral approach and microtubular technique treatment, and the LP group, which will receive laminoplasty treatment. The follow-up period will last for two years. The study is registered at https://www.chictr.org.cn, which can be accessed online (ChiCTR2100047582). Consent (version date January 21, 2021, V.1.0) will be obtained by an investigator who will comply with applicable regulatory requirements and adhere to the ethical principles of the Declaration of Helsinki. The time points, randomization, treatment allocation, and assessment are summarized in Figure 1.
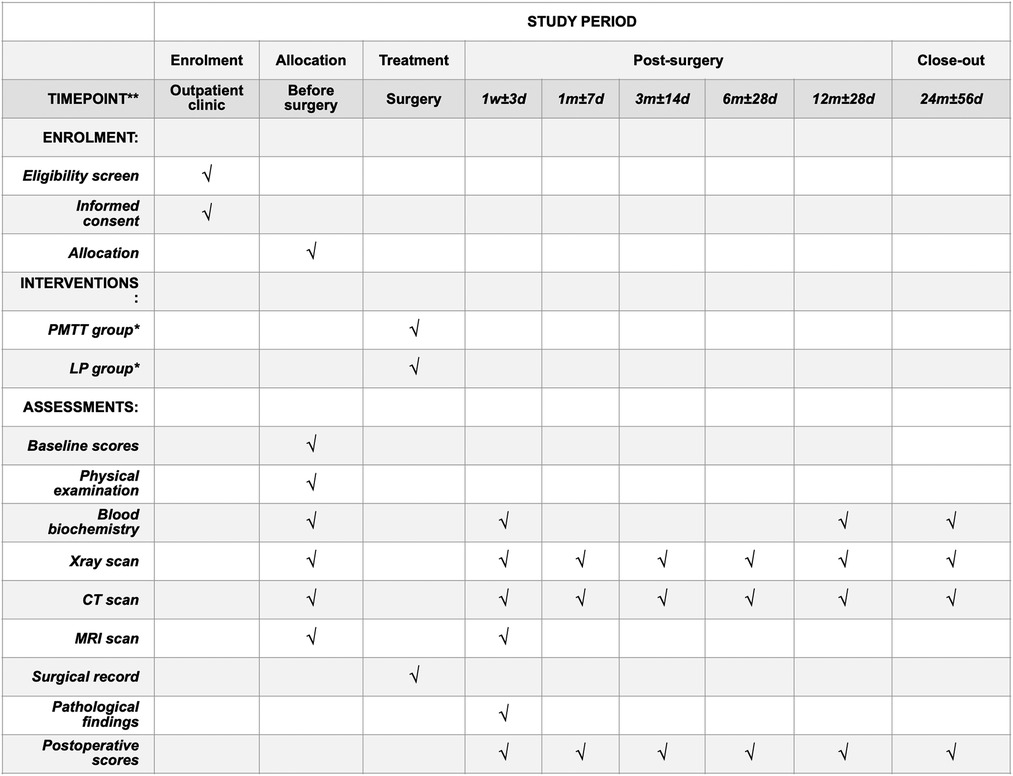
Figure 1. The schedule of enrolment, interventions, and assessments. *PMTT group, paraspinal mini-tubular technique; LP group, laminoplasty group.
2.2. Participant eligibility and recruitment
Participating surgeons and hospitals must satisfy the following criteria to prevent bias: surgeons must have experience performing >100 surgeries for spinal tumors, participating hospitals should have performed >40 spinal tumor surgeries annually, and they must have completed the Fujian Medical University Union Hospital spinal training program.
Eligible patients with spinal IDEM tumors will be referred by the participating surgeons, following a formal outpatient assessment. This study follows the informed consent principle; thus, each patient must provide written informed consent prior to group randomization. Detailed inclusion and exclusion criteria are listed in Table 1.
2.3. Patient and public involvement statement
The patients and public are not involved in the design, conduction, reporting, or dissemination plans of this research.
2.4. Randomization and blinding
Once written informed consent is obtained, patients would be randomized to undergo either the paravertebral approach or microtubular technique, or LP in a ratio of 1:1. The patients will be randomized using a block randomization model (block size: six). Computer-generated random number tables will be prepared by an experienced statistician. After baseline data are obtained, and a physical examination has been conducted, allocation of treatment will be performed by the computer system, and the results of the allocation will be provided to the surgeon in a concealed envelope one day before surgery. Due to the noticeable differences between the PMTT and LP procedures, blinding of the patients will not be strictly required. However, all researchers and data analysts will be blinded to the allocated intervention during the follow-up period of 2 years.
2.5. Treatment
Patients will be randomized to receive either PMTT or LP treatments. The tumor's location will be verified using a mobile image intensifier with fluoroscopy (anteroposterior and lateral views). The PMTT and LP will be performed under general anesthesia. All the patients will be placed in a standard prone position. The surgeons involved in this study would have had extensive experience with both procedures.
2.5.1. (A) intervention: PMTT
After inducing general anesthesia, the patients will be placed in the prone position to minimize lumbar lordosis or thoracic kyphosis and to avoid compressing the abdomen. A 20–25 mm skin incision will be made approximately 20–35 mm lateral to the midline (adjusted according to the patient's body habitus), and the accurate target level will be confirmed by lateral fluoroscopy. The paravertebral approach will involve the paraspinous muscles, and the muscles will be bluntly dissected by a muscle-splitting technique. After the smallest dilator is inserted to reach the lamina or peripheral bone structure, the dilators will be piled sequentially, and the working tubule (diameter, 14 or 16 mm) will be inserted over the dilators. The dilators will be removed, and a tubular surgical channel will be established. The tubule will be fixed using a flexible fixed arm mounted on the operating table. The tubule could be angulated to expand the operating field because of the flexible fixed arm. The subsequent procedure will be performed under a microscope (OPMI Pentero, Carl Zeiss, Germany). Part of the lamina will be removed using high-speed drills and Kerrison rongeurs. After the dura mater is incised, the IDEM tumor will be separated and removed piece by piece using a microsurgical technique. The dura mater will be sutured tightly. After the tubule is withdrawn, the paravertebral muscles will be repositioned, and the fascia, subcutaneous tissues, and skin will be sutured in sequence. Thereafter, a surgical drain will be placed in the resection site for 24–48 h.
2.5.2. (B) intervention: LP
A skin incision will be made, and subcutaneous tissue, lower back fascia, and muscle will be incised in layers to expose the spinous process and lamina of the spinal segment where the tumor is located. The retractor will be used to expose the bone structures, and the complex of the spinous process and lamina will be removed using an ultrasonic bone knife combined with a high-speed drill. The ligamentum flavum will be removed, and the dura will be sharply incised. The tumor will be separated and removed with the aid of a microscope, and the dura will be closed with a 5.0 or 6.0 suture. The spinous process and lamina will be repositioned, and the laminoplasty will be performed with titanium screws and connecting plates. The paravertebral muscles will be repositioned, and the fascia, subcutaneous tissue, and skin will be sutured one after the other. A drain will be placed at the resection site for 24–48 h.
2.6. Baseline assessment
The demographics, medical history, family history of tumors, results of a physical examination, body mass index, tumor location, visual analog scale (VAS) scores, and Japanese Orthopedic Association (JOA) scores will be recorded at baseline. Data will be collected prior to randomization.
2.7. Withdrawal
All patients can decide to withdraw at any time. If a patient withdraws, information will not be recorded in the study. However, the research team can still collect the outcome data from the healthcare records.
2.8. Outcome measurements
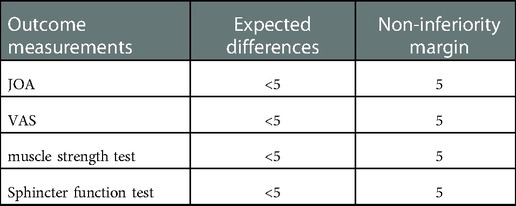
Patients must have an indication for the surgery. The primary outcome variable is the JOA score. The secondary outcome variables include the VAS score, physical examination findings, length of hospital stay, expenses, and complications. Outcomes will be measured on the first postoperative day, and thereafter at the 1st week, and at 1, 3, 6, 12, and 24 months after surgical treatment. A physical examination will be conducted after 1 week, 1 month, and 12 months from the day of the surgery (Table 1). The non-inferiority margin for the primary outcome is five.
2.9. Primary outcome
We will use the JOA score to assess the primary outcome (12, 13). It is divided into the cervical and JOA scores, including subjective symptoms, clinical signs, daily activities, and bladder function. Each item had one or more problems. The total score of the JOA was 29, and the total JOA score of the cervical spine was 17. The lower the score, the more obvious the dysfunction. The outcome parameters will be assessed using validated questionnaires and physical examination. Data from the questionnaires will be collected at 1 week and at 1, 3, 6, 12, and 24 months after the surgery by the research nurse.
2.10. Secondary outcome
Secondary outcomes include the gross tumor resection(GTR) rate, tumor recurrence rate, VAS, assessment of back and leg pain at rest, physical examination, length of hospital stay, expenses, and complications. One of the secondary outcomes was the VAS of pain. Perceived pain intensity was measured using the VAS score [pain scale ranging from 0 (no pain) to 100 mm (the worst pain imaginable)] (14). The pain will be assessed because most patients have pain in different regions. A physical examination will be conducted at 1, 6, and 12 months after the surgery. This will include the sphincter function test, muscle strength exercise, patellar and Achilles tendon reflex assessment, and sensory assessment of the affected spinal region. Muscle strength will be scored on a scale ranging from zero (no contraction) to five (normal muscle strength) (15).
The cost-effectiveness of the hospital stay will be evaluated by summarizing the cost of the surgical procedure and the number of days before discharge. The cost of the additional procedures in case of complications will be added to the total cost of the patient's hospital stay. The primary cost of treatment will include the cost of hospital admission, surgery, medication, rehabilitation, and other healthcare utilization. The details of these charges will be registered in a diary. Immediately after the operation, a systematic assessment of complications (including cerebrospinal fluid leakage, venous thromboembolism, wound infection, urinary tract infection, hematoma, and progressive neurological deficit) will be conducted by the surgeon and research nurse until patient discharge. Surgical data will include intraoperative spinal cord and nerve root injury, operative time, and intraoperative blood loss.
2.10.1. Adverse and serious adverse events
All adverse events related or not to the interventions will be reported during the complete study period. The list of adverse effects is adjacent segment instability, surgical site infection, worsening neurological symptoms, pain recurrence, dural tears, and CSF leakage.
2.11. Sample size calculation
The sample size for this study was calculated based on the JOA scores. Based on the preliminary data of our department, the mean difference and SD for the JOA used in the sample size calculation were as follows: mean 21.7, SD 5.8. The sample size for non-inferiority trials was calculated using the following: (1) significance level (alpha) of 0.05 and (2) power (beta) of 90%. We estimated that 112 patients would need to be included in each group. Accounting for 10% attrition and the actual situation of each participating center, 280 patients will be recruited in total. We intend to recruit patients within two years. Recruitment for the study began in October 2021.
2.12. Statistical analysis
All data will be analyzed according to the intention-to-treat principle (16, 17). The baseline data will be compared and analyzed using descriptive statistics [mean (SD), proportion, or median (range)] to determine whether balanced groups were obtained after randomization. Student's t-test or Mann–Whitney's U test will be used to compare continuous variables. Categorical variables will be compared using χ2 or Fisher's exact tests. All comparative analyses will be reported with point estimates [means (SD) or odd ratios (ORs)], 95% CIs, and p-values. Statistical significance will be set at p < 0.05. Non-inferiority margins will be set as listed in Table 2. Statistical analysis will be performed using appropriate statistical software (e.g., SPSS version 22.0 or Stata).
2.13. Ethics and dissemination
The study will be performed in accordance with the Declaration of Helsinki. This article describes a protocol for a prospective, non-inferiority randomized controlled trial to examine the safety, efficacy, and cost-effectiveness of PMTT vs. LP in treating spinal IDEM tumors. This study was approved by the Ethical Committee of Fujian Medical University Union Hospital, Fuzhou, China, in June 2021(2021YF022-01). Informed consent will be obtained from all eligible participants prior to randomization. The results of the research will be published in international peer-reviewed journals and disseminated through presentations at scientific conferences.
3. Discussion
The FJMUUH05 trial is a multicenter RCT, the first RCT of surgery for spinal IDEM tumors. To date, similar RCTs have not been reported on this topic. The trial was approved by the Chinese Clinical Trial Registry (ChiCTR, www.chictr.org.cn). The trial hypothesis is that PMTT has superior cost-effectiveness and is equivalent in safety and efficacy to LP for treating spinal IDEM tumors.
Tubular spinal surgery has gained significant prominence because of the development of minimally invasive technologies and instruments. The tubular retractor is inserted to reach the operation field by separating the paravertebral muscle and exposing the pathological lesions. This technology allows surgeons to remove tumors with two-handed operations and simultaneously use multiple microscopic instruments. Moreover, intraoperative three-dimensional imaging is another massive shift from endoscopic technology, which lets the operator conveniently operate the imaging apparatus, thereby reducing the complication rates of dural rupture. Compared with traditional surgery, fewer clinical studies have been conducted on the selection of surgical indications. The safety and effectiveness of the operation and the treatment of complications during and after the operation lack a unified opinion. The patients will be randomized to the PMTT or LP groups after tumor resection. The trial aims to study the clinical results of PMTT vs. LP for spinal IDEM tumors. This RCT study provides high-quality evidence for the efficacy of tubular spine surgery for spinal IDEM tumors. We believe that the FJMUUH05 trial will provide useful information on the long-term effects of the two treatments for spinal IDEM tumors. To date (October 2021), 11 patients have been included in the trial.
In conclusion, the FJMUUH05 trial is a multicenter RCT investigating the cost-effectiveness, safety, and effectiveness of PMTT and LP in spinal IDEM tumors. When this trial is completed with the hypothesis confirmed, it will popularize the application of PMTT and improve patient outcomes.
Author contributions
Conceptualization: RW, ZYL, YC, and CC. Funding acquisition: RW. Methodology: RW and ZYL. Visualization: RW, ZYL, and YC. Writing – original draft: RW and ZYL. Writing – review & editing: RW., ZYL., YC, and CC. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Fujian Provincial Department of Science and Technology Agency, China (grant NO. 2021Y0021 to RW).
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chotai S, Zuckerman SL, Parker SL, Wick JB, Stonko DP, Hale AT, et al. Healthcare resource utilization and patient-reported outcomes following elective surgery for intradural extramedullary spinal tumors. Neurosurgery. (2017) 81:613–9. doi: 10.1093/neuros/nyw126
2. Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine (Phila Pa 1976). (2007) 32(16):1735–47. doi: 10.1097/BRS.0b013e3180bc2431
3. Caspar W, Campbell B, Barbier DD, Kretschmmer R, Gotfried Y. The Caspar microsurgical discectomy and comparison with a conventional standard lumbar disc procedure. Neurosurgery. (1991) 28:78–86; discussion -7. doi: 10.1097/00006123-199101000-00013
4. Jacobs WC, van Tulder M, Arts M, Rubinstein SM, van Middelkoop M, Ostelo R, et al. Surgery versus conservative management of sciatica due to a lumbar herniated disc: a systematic review. Eur Spine J. (2011) 20(4):513–22. doi: 10.1007/s00586-010-1603-7
5. Hernandez RN, Wipplinger C, Navarro-Ramirez R, Soriano-Solis S, Kirnaz S, Hussain I, et al. Ten-step minimally invasive cervical decompression via unilateral tubular laminotomy: technical note and early clinical experience. Oper Neurosurg (Hagerstown). (2020) 18:284–94. doi: 10.1093/ons/opz156
6. Wang R, Chen Y, Liang Z, Yang W, Chen C. Efficacy of one-stage paravertebral approach using a micro-tubular technique in treating thoracic dumbbell tumors. Orthop Surg. (2021) 13:1227–35. doi: 10.1111/os.12991
7. Zhuang Y, Cai G, Fu C, Zhang W, Zhao W, Wang R, et al. Novel combination of paraspinal keyhole surgery with a tubular retractor system leads to significant improvements in lumbar intraspinal extramedullary schwannomas. Oncol Lett. (2017) 14:7873–9. doi: 10.3892/ol.2017.7203
8. Ahn Y. Current techniques of endoscopic decompression in spine surgery. Ann Transl Med. (2019) 7:S169. doi: 10.21037/atm.2019.07.98
9. Greiner-Perth R, Böhm H, El Saghir H. Microscopically assisted percutaneous nucleotomy, an alternative minimally invasive procedure for the operative treatment of lumbar disc herniation: preliminary results. Neurosurg Rev. (2002) 25:225–7. doi: 10.1007/s10143-002-0220-2
10. Ryang YM, Oertel MF, Mayfrank L, Gilsbach JM, Rohde V. Standard open microdiscectomy versus minimal access trocar microdiscectomy: results of a prospective randomized study. Neurosurgery. (2008) 62:174–81; discussion 81–2. doi: 10.1227/01.Neu.0000311075.56486.C5
11. Arts MP, Brand R, van den Akker ME, Koes BW, Bartels RH, Peul WC. Tubular diskectomy vs conventional microdiskectomy for sciatica: a randomized controlled trial. JAMA. (2009) 302:149–58. doi: 10.1001/jama.2009.972
12. Kato S, Oshima Y, Oka H, Chikuda H, Takeshita Y, Miyoshi K, et al. Comparison of the Japanese Orthopaedic Association (JOA) score and modified JOA (mJOA) score for the assessment of cervical myelopathy: a multicenter observational study. PLoS One. (2015) 10:e0123022. doi: 10.1371/journal.pone.0123022
13. Fujimori T, Okuda S, Iwasaki M, Yamasaki R, Maeno T, Yamashita T, et al. Validity of the Japanese Orthopaedic Association scoring system based on patient-reported improvement after posterior lumbar interbody fusion. Spine J. (2016) 16:728–36. doi: 10.1016/j.spinee.2016.01.181
14. MacDowall A, Skeppholm M, Robinson Y, Olerud C. Validation of the visual analog scale in the cervical spine. J Neurosurg Spine. (2018) 28:227–35. doi: 10.3171/2017.5.Spine1732
15. Dyck PJ, Boes CJ, Mulder D, Millikan C, Windebank AJ, Dyck PJ, et al. History of standard scoring, notation, and summation of neuromuscular signs. A current survey and recommendation. J Peripher Nerv Syst. (2005) 10:158–73. doi: 10.1111/j.1085-9489.2005.0010206.x
16. Abraha I, Cherubini A, Cozzolino F, De Florio R, Luchetta ML, Rimland JM, et al. Deviation from intention to treat analysis in randomised trials and treatment effect estimates: meta-epidemiological study. Br Med J. (2015) 350:h2445. doi: 10.1136/bmj.h2445
Keywords: paraspinal minitubular technique, laminoplasty, randomized controlled trial, intradural extramedullary, multicenter
Citation: Wang R, Liang Z, Chen Y and Chen C (2023) Clinical evaluation of paraspinal mini-tubular technique vs. laminoplasty for spinal intradural extramedullary tumors: Study protocol for a multicenter, randomized controlled trial. Front. Surg. 9:1053885. doi: 10.3389/fsurg.2022.1053885
Received: 29 September 2022; Accepted: 8 December 2022;
Published: 5 January 2023.
Edited by:
Ziya Levent Gokaslan, Brown University, United StatesReviewed by:
Vadim Byvaltsev, Irkutsk State Medical University, RussiaTianyi Niu, University of California, Los Angeles, United States
© 2023 Wang, Liang, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Mei Chen cmchen2009@sina.com
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Abbreviations CI, confidence interval; EBL, estimated blood loss; IDEM, intradural extramedullary; JOA, Japanese Orthopedic Association; LP, laminoplasty; OR, odd ratio; PMTT, paraspinal mini-tubular technique; RCT, randomized controlled trial; SD, standard deviation; VAS, visual analog scale.
 Rui Wang†
Rui Wang†  Ze-Yan Liang
Ze-Yan Liang Yan Chen
Yan Chen Chun-Mei Chen
Chun-Mei Chen