Dietary administration impact of olive pulp on growth performance, metabolic profile, immune status, and antioxidant potential of gilthead seabream (Sparus aurata L.)
- 1Department of Food Sciences, College of Agriculture and Veterinary Medicine, United Arab Emirates University, Al Ain, Abu Dhabi, United Arab Emirates
- 2Immunobiology for Aquaculture Group, Department of Cell Biology and Histology, Faculty of Biology, Campus Regional de Excelencia Internacional “Campus Mare Nostrum”, University of Murcia, Murcia, Spain
The use of natural immunostimulants in aquaculture is intended to foster overall health and bolster resilience against diseases in farmed fish populations. It constitutes a crucial strategy that can contribute to securing the sustainability of the aquaculture industry, and it is an area that warrants ongoing exploration and development. Therefore, the aim of the present work was to evaluate the effect of dietary administration of olive pulp on growth rate, metabolic profile, serum antioxidant potential, and humoral and cellular innate immune parameters of gilthead seabream (Sparus aurata L.). For this, fish were fed control diet or olive pulp enriched diets (50, 100, and 200 mg kg feed−1) for 4 weeks. Our results demonstrated that the highest inclusion level improved the growth rates and the biological antioxidant potential in the serum of fish. However, after 4 weeks of feeding, most of the assayed metabolic parameters (Ca2+, TP, ALB, K+, and Na+) were increased in the serum of fish fed with a diet containing the lowest level of olive pulp (50 mg kg feed−1). Regarding the innate immune parameters, the IgM levels decreased in the serum of fish fed 50′s diet after 2 and 4 weeks of trial. However, the serum of fish fed with diets containing 100 and 200 showed an increase in hemolytic complement activity after 2 weeks whilst this increase was only sustained in the 200′s group after 4 weeks. After 2 weeks of feeding, the serum of the fish showed an increase in peroxidase activity due to the highest olive inclusion. Concerning cellular innate parameters, peroxidase activity, respiratory burst, and phagocytic ability were increased in head-kidney leucocytes of fish fed 100′s diet at 2 weeks compared to values from control fish. These results suggest that the administration of olive pulp-enriched diets can benefit fish growth, antioxidant, and immune status of gilthead seabream.
1 Introduction
The intensification of production systems in the fast-growing aquaculture field has increased the demand for eco-friendly and high-quality feed stuff to improve fish health with fewer side effects and without any environmental or hazardous problems (Zlaugotne et al., 2022). In the last decades, the biggest challenge for food industries and scientists is to develop novel approaches combining the provision of all the required nutrients of fish feeds with the economic benefits and fish welfare (Sioriki et al., 2015). Considerable research efforts have thus been devoted to find environmentally sustainable procedures of food production reinsuring food security and suppressing environmental pollution. Consequently, it has emerged a great demand for natural substances as additives in commercial fish feeds from the aqua-feeds industry and fish farmers since it is safer, cost effective and eco-friendly (Vijayan et al., 2021). Moreover, these tendencies are also widely accepted by consumers, who increase their sensitivity and demand for eco-friendly farming practices and chemically uncontaminated food products (Marimuthu et al., 2022).
Recently, prophylactic administration of immunostimulants as natural compounds strengthening the immunocompetency of fish, is proposed to be an effective alternative approach of antibiotics and chemotherapeutic molecules, to prevent infectious disease outbreaks in a sustainable aquaculture (Bricknell and Dalmo, 2005; Guardiola et al., 2016; Guerreiro et al., 2016; Ringø and Song, 2016; Vijayaram et al., 2023). Among natural immunostimulants, medicinal plants, plant derivatives or extracts are often locally available, inexpensive, easily biodegradable, and hence without any environmental or hazardous problems (Ali et al., 2022). In addition to their important activity against a broad spectrum of fish pathogens, these natural products showed an important modulation potency of the innate and adaptive immune responses in fish as well as on the antioxidant defenses, attributed to their high contents of several active compounds such as alkaloids, terpenoids, tannins and saponins, among others (Mandujano-Tinoco et al., 2021; Sakai et al., 2021). Moreover, due to their potential health benefits, the efficacy of many natural antioxidants of plant origin, have been demonstrated in several fish species (Hoseinifar et al., 2020b; Tadese et al., 2022; Ng et al., 2023). Research revealed that more than 60 different medicinal-plant species can enhance immune responses and disease resistance of cultured fish and they can be used as a possible therapeutic measure and disease management in aquaculture (Elumalai et al., 2020; Tadese et al., 2022).
The plant used in this study, Olea europaea belongs to the family Oleaceae of the genus Olea, and it is known commonly as the olive tree which is one of the widely consumed and studied fruit trees in Mediterranean countries (Ray et al., 2015). Several researchers evaluated olive pomace as a sustainable source of nutrients for different animal feedings such as for poultry, rabbits, swine (de Oliveira et al., 2021; Tzamaloukas et al., 2021; Sánchez et al., 2022), and fish (Hoseinifar et al., 2020a; Sokooti et al., 2021; Fazio et al., 2022; Hazreen-Nita et al., 2022). However, limited information is available on the use of olive pulp (OP), olive pomace or olive by-products in fish diets. These studies had focused mainly on the effects of olive oil or its by-products on growth performance, feed utilization, chemical composition, and some immune parameters in few fish species (Hazreen-Nita et al., 2022), such as common carp (Cyprinus carpio; Zemheri-Navruz et al., 2019; Sokooti et al., 2021; Assar et al., 2023), Nile tilapia (Oreochromis niloticus; Fazio et al., 2022), large yellow croaker (Larimichthys crocea; Li et al., 2019), rainbow trout (Oncorhynchus mykiss; Hoseinifar et al., 2020a), and gilthead seabream (Sparus aurata; Nasopoulou et al., 2011, 2013; Sioriki et al., 2016). Taking into consideration the benefits of olive by-products as a dietary supplement in fish feeds and the limited information about their physiological and immunological effects on gilthead seabream, one of the most important aquacultured fish species in the Mediterranean area, the aim of the present paper was to evaluate the impact of variable OP inclusions in diets on the growth performance, metabolic parameters, antioxidant status as well as on the humoral and cellular immune responses on this fish species.
2 Materials and methods
2.1 Animals
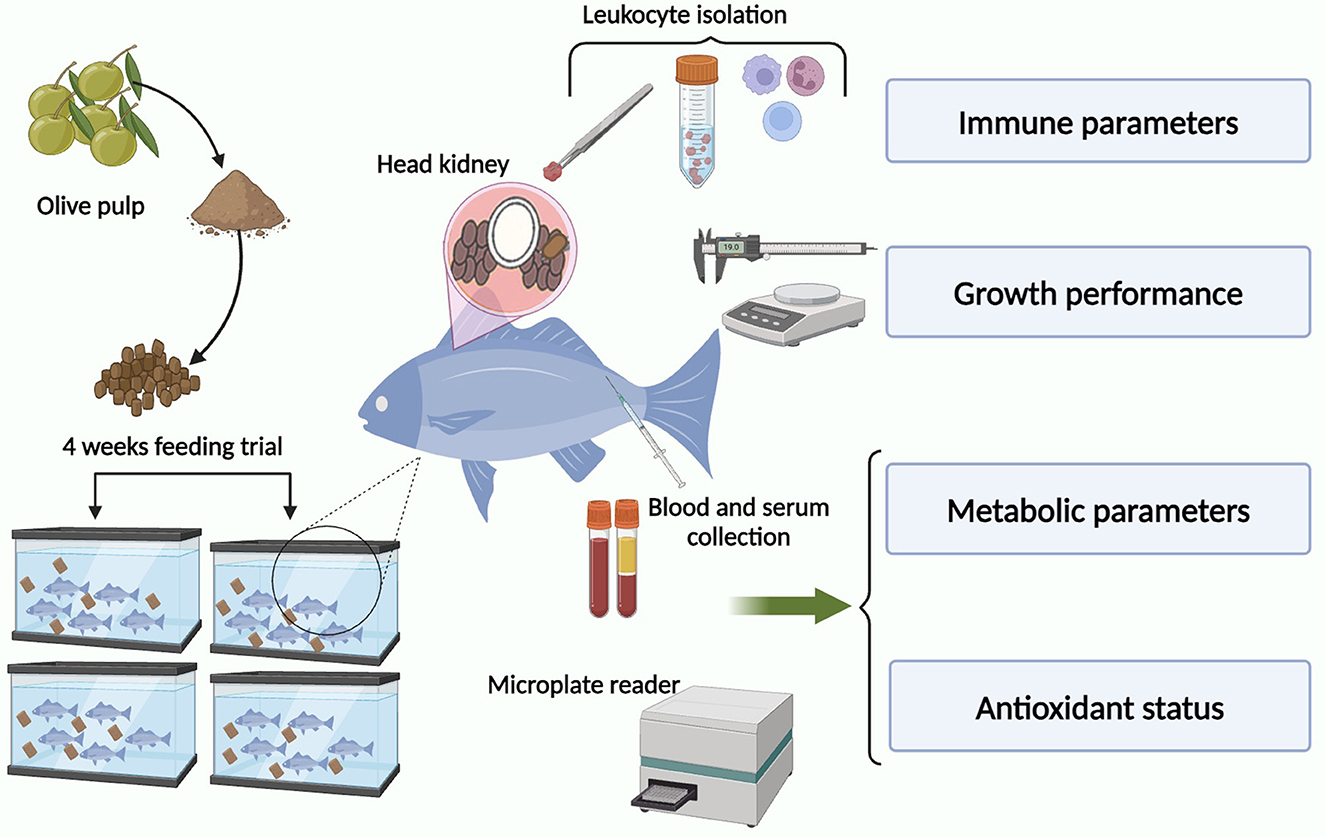
Sixty-four specimens (31.12 ± 7.05 g weight and 18.88 ± 0.91 cm length) of teleost gilthead seabream (Sparus aurata L.), obtained from the local farm (Murcia, Spain), were kept in re-circulating seawater aquaria (250 L) in the Marine Fish Facility at the University of Murcia. The water temperature was maintained at 20 ± 2°C with a flow rate of 900 L h−1 and 28‰ salinity. The photoperiod was 12 h light: 12 h dark and fish were fed with a commercial pellet diet (Skretting, Spain) at a rate of 2% body weight day−1. Fish were allowed to acclimatize for 15 days before the start of the experimental trial. Ammonia and nitrite levels in the water were measured twice a week using commercial kits and never exceeded 0.025 and 0.3 mg L−1, respectively. All experimental protocols were approved by the Ethical Committee of the University of Murcia following the guidelines of the European Union for animal handling (2010/63/EU).
2.2 Experimental diets
Dry OP was collected from a local industry (Granada, Spain) by BIOTHESAN S.L. and crushed. Briefly, the commercial pellet diet cited above was also crushed and mixed with distilled water containing the adequate amount of OP powder (50, 100, and 200 mg kg feed−1) or not supplemented (control diet). The final products were introduced into a meat grinder at room temperature and re-pelleted. The resulting pellets were dried in a forced-air oven at 37°C for 24 h before packaging in polypropylene bags and storing at 4°C until use.
2.3 Experimental design
Fish were randomly distributed into eight identical tanks (eight fish per tank) where the following groups were established in duplicate: (i) control (non-supplemented diet, 0 mg OP kg feed−1); (ii) 50 (50 mg OP kg feed−1); (iii) 100 (100 mg OP kg feed−1); and (iv) 200 (200 mg OP kg feed−1). Four specimens were sampled from each tank (eight fish per experimental group) following 2 or 4 weeks of feeding, after starving them for 24 h before sampling. All specimens were sacrificed by using an overdose of MS-222 (Sandoz, 100 mg L−1 water) and sampled.
2.4 Sample collection
Fish were weighed and measured. Blood samples were collected from the caudal vein with an insulin syringe and the head-kidney (HK) was dissected. The blood samples were left to clot at 4°C for 4 h and later the serum was collected after centrifugation (10,000 x g, 5 min, 4°C) and stored at −20°C until use. The excised HKs were cut in small fragments and transferred to 8 mL of sRPMI [RPMI-1640 culture medium (Gibco) supplemented with 0.35% sodium chloride (to adjust the medium's osmolarity to gilthead seabream plasma osmolarity of 353.33 mOs), 3% fetal calf serum (FCS; Gibco), 2 mM L-glutamine (Gibco), 100 i.u. mL−1 penicillin (Flow) and 100 μg mL−1 streptomycin (Flow)] for leucocyte isolation (Esteban et al., 1998). Cell suspensions were obtained by forcing fragments of the organ through a nylon mesh (mesh size 100 μm) and washed twice (400 x g, 10 min). HK cell suspensions were layered over a 34–51% Percoll density gradient (Pharmacia), centrifuged (400 x g, 40 min, 4°C) and the band of leucocytes above the 34–51% interface was collected, counted (Bio-Rad TC20 Cell Counter) and adjusted to 107 cells mL−1 in sRPMI. Cell viability was higher than 98%, as determined by the trypan blue exclusion test. All the assays were performed only in immediately isolated viable cells.
2.5 Growth performance
Body weight and length of each fish was measured before the trial. Growth was monitored by obtaining the weight gain (WG %), specific growth rate (SGR), and condition factor (CF), which were calculated for each of the treatments: WG% = [(Wf – Wi) Wi−1] × 100; SGR = [(Ln final weight – Ln initial weight) number of days−1] × 100 and CF = (weight length−3) × 100. Wi: initial weight; Wf: final weight.
2.6 Determination of metabolic parameters
The presence of aspartate aminotransferase (AST), creatine kinase (CK), uric acid (UA), glucose (GLU), calcium (CA+2), phosphorus (PHOS), total protein (TP), albumin (ALB), globulin (GLOB), potassium (K+), and sodium (Na+) were determined in the serum of seabream gilthead specimens at the end of the trial (after 4 weeks of feeding) using samples of 100 μL of serum in an automated analyzer (VetScan, Abaxis Veterinary Diagnostics) and rotor (VetScan Avian-Reptilian Profile Plus) according to the manufacturer's instructions.
2.7 Antioxidant status
Antioxidant status was measured spectrophotometrically (BOECO, S-22 UV/VIS) by BAP (biological antioxidant potential) test from serum according to the procedure outlined by the manufacturer (Diacron International S.R.L., Grosseto, Italy). Briefly, 50 μL of R2 reagent (ferric salt) was transferred to 1 mL of R1 reagent (a particular thiocyanate derivative), mixed gently and read on a photometer at a wavelength of 505 nm. Then, 10 μL of reagent blank, calibrator or serum sample were added to the cuvette, mixed gently, and read on the previous photometer. The values were expressed as μmoL antioxidant substance L−1 of vitamin C used as an iron-reducing agent reference.
2.8 Immune parameters
2.8.1 Immunoglobulin M level
Total serum IgM levels were analyzed using the enzyme-linked immunosorbent assay (ELISA; Cuesta et al., 2004). For this, 20 μL per well of 1/100 diluted serum were placed in flat-bottomed 96-well plates in triplicate and the proteins were coated by overnight incubation at 4°C with 200 μL of carbonate-bicarbonate buffer (PBT, 35 mM NaHCO3 and 15 mM Na2CO3, pH 9.6). After three rinses with PBT (20 mM Tris-HCl, 150 mM NaCl and 0.05% Tween 20, pH 7.3), the plates were blocked for 2 h at room temperature with blocking buffer containing 3% bovine serum albumin (BSA, Sigma) in PBT, followed by three rinses with PBT. The plates were then incubated for 1 h with 100 μL per well of mouse anti-seabream IgM monoclonal antibody (Aquatic Diagnostics Ltd.; 1/100 in blocking buffer), washed and incubated with the secondary antibody anti-mouse IgG-HRP (1/1,000 in blocking buffer, Sigma). After exhaustive rinsing with PBT the plates were developed using 100 μL of a 0.42 mM TMB solution, prepared daily in a 100 mM citric acid/sodium acetate buffer, pH 5.4, containing 0.01% H2O2. The reaction was allowed to proceed for 10 min and stopped by the addition of 50 μL of 2M H2SO4 and the plates were read at 450 nm in a microplate reader (BMG labtech). Negative controls consisted of samples without serum or without primary antibody, whose optical density (OD) values were subtracted for each sample value.
2.8.2 Natural hemolytic complement activity
Natural hemolytic complement activity was measured in serum according to Sunyer and Tort (1995) with slight modifications. The following buffers were used: GVB (Isotonic veronal buffered saline), pH 7.3, containing 0.1% gelatin; EDTA-GVB, as previous one but containing 20 mM EDTA; and Mg-EGTA-GVB, which is GVB with 10 mM Mg+2 and 10 mM EGTA. Rabbit red blood cells (RaRBC; ProbiologicaLda, Portugal) were used for natural hemolytic complement determination. RaRBC were washed four times in GVB and resuspended in GVB to a concentration of 2.5 x 108 cells mL−1. Twenty μL of RaRBC suspension were then added to 40 μL of serially diluted serum in Mg-EGTA-GVB buffer. The values of maximum (100%) and minimum (spontaneous) hemolysis were obtained by adding 40 μL of distilled water or Mg-EGTA-GVB buffer to 20 μL samples of RaRBC, respectively. Samples were incubated at room temperature for 100 min with regular shaking every 20 min. The reaction was stopped by adding 150 μL of cold EDTA-GVB. Samples were then centrifuged, and the extent of haemolysis was estimated by measuring the optical density of the supernatant at 414 nm in a microplate reader (BMG labtech). The degree of haemolysis (Y) was estimated and the lysis curve for each specimen was obtained by plotting Y (1-Y)−1 against the volume of serum added (μmL) on a log-log scaled graph. The volume of serum producing 50% hemolysis (ACH50) was determined and the number of ACH50 units mL−1 obtained for each experimental fish.
2.8.3 Protease activity
Protease activity was quantified using the azocasein hydrolysis assay according to the previous reported method of Guardiola et al. (2014). Briefly, aliquots of 100 μL of serum (previously diluted 1/10 in 100 mM ammonium bicarbonate buffer) was incubated with 125 μL of 100 mM ammonium bicarbonate buffer containing 2% azocasein (Sigma) for 24 h at 30°C. The reaction was stopped by adding 10% trichloro acetic acid (TCA). The mixture was then centrifuged (10,000 x g, 10 min) and the supernatants were transferred to a 96-well plate in triplicate containing 100 μL well−1 of 1 N NaOH, and the OD read at 450 nm using a microplate reader (BMG labtech). Serum was replaced by trypsin (5 mg mL−1, Sigma), as positive control (100% of protease activity), or by buffer, as negative controls (0% activity).
2.8.4 Antiprotease activity
Total antiprotease activity was determined by the ability of serum to inhibit trypsin activity (Guardiola et al., 2014). Briefly, 10 μL of serum samples were incubated (10 min, 22°C) with the same volume of standard trypsin solution (5 mg ml−1). After adding 100 μL of 100 mM ammonium bicarbonate buffer and 125 μL of buffer containing 2% azocasein (Sigma), samples were incubated (2 h, 30°C) and, following the addition of 250 μL of 10% TCA, a new incubation (30 min, 30°C) was done. The mixture was then centrifuged (10,000 x g, 10 min) being the supernatants transferred to a 96-well plate in triplicate containing 100 μL well−1 of 1 N NaOH, and the OD read at 450 nm using a microplate reader (BMG labtech). For a positive control, buffer replaced serum and trypsin, and for a negative control, buffer replaced the serum. The antiprotease activity was expressed in terms of percentage trypsin inhibition according to the formula: % Trypsin inhibition = (Trypsin OD – Sample OD) Trypsin OD−1 x 100.
2.8.5 Serum and leucocyte peroxidase activity
The peroxidase activity in serum or leucocytes was measured according to Quade and Roth (1997). Briefly, 15 μL of serum were diluted with 135 μL of HBSS (Hanks' Balanced Salt Solution) without Ca+2 or Mg+2 in flat-bottomed 96-well plates and 50 μL of 20 mM 3,3′,5,5′- tetramethylbenzidine hydrochloride (TMB, Sigma) and 5 mM H2O2 were added. To determine the leucocyte peroxidase content, immediately isolated viable 107 HK leucocytes (HKLs), in sRPMI were lysed with 0.002% cetyltrimethylammonium bromide (Sigma) and, after centrifugation (400 x g, 10 min), 150 μL of the supernatants were transferred to a fresh 96-well plate containing 25 μL of 10 mM TMB and 5 mM H2O2. In both cases, the color-change reaction was stopped after 2 min by adding 50 μL of 2 M sulphuric acid and the OD was read at 450 nm in a microplate reader (BMG labtech). Standard samples without serum or leucocytes, respectively, were used as blanks. All measurements were performed in three triplicates.
2.8.6 Respiratory burst activity
The respiratory burst activity of gilthead seabream HKLs was studied by a chemiluminescence method (Bayne and Levy, 1991). Briefly, samples of, immediately isolated viable 107 leucocytes in sRPMI were placed in the wells of a flat-bottomed 96-well microtiter plate, to which 100 μL of HBSS containing 1 mg mL−1 phorbol myristate acetate (PMA, Sigma) and 10−4 M luminol (Sigma) were added. The plate was shaken, and luminescence was immediately read in a microplate reader (BMG labtech) for 1 h at 2 min intervals. The kinetics of the reactions were analyzed, and the maximum slope of each curve was calculated. Luminescence backgrounds were calculated using reagent solutions containing luminol but not PMA. All measurements were performed in three triplicates.
2.8.7 Phagocytic activity
The phagocytosis of Saccharomyces cerevisiae (strain S288C) by gilthead seabream HKLs was studied by flow cytometry (Rodríguez et al., 2003). Heat-killed and lyophilized yeast cells were labeled with fluorescein isothiocyanate (FITC, Sigma), washed, and adjusted to 5 x 107 cells mL−1 of sRPMI. Phagocytosis samples consisted of 125 μL of labeled yeast cells and 100 μL of, immediately isolated viable HKLs in sRPMI (6.25 yeast cells:1 leucocyte). Samples were mixed, centrifuged (400 x g, 5 min, 22°C), resuspended, and incubated at 22°C for 30 min. At the end of the incubation time, samples were placed on ice to stop phagocytosis, and 400 mL ice-cold PBS was added to each sample. The fluorescence of the extracellular yeasts was quenched by adding 40 mL ice-cold trypan blue (0.4% in PBS). Standard samples of FITC-labeled S. cerevisiae or HKLs were included in each phagocytosis assay.
All samples were analyzed in a flow cytometer (Becton Dickinson) with an argon-ion laser adjusted to 488 nm. Analyses were performed on 3,000 cells, which were acquired at a rate of 300 cells s−1. Data were collected in the form of two-parameter side scatter (granularity; SSC) and forward scatter (size; FSC), and green fluorescence (FL1) dot plots or histograms were made on a computerized system. The fluorescence histograms represented the relative fluorescence on a logarithmic scale. The cytometer was set to analyze the phagocytic cells, showing the highest SSC and FSC values. Phagocytic ability was defined as the percentage of cells with one or more ingested bacteria (green-FITC fluorescent cells) within the phagocytic cell population whilst the phagocytic capacity was the mean fluorescence intensity. The quantitative study of the flow cytometric results was made using the statistical option of the Lysis Software Package (Becton Dickinson). All measurements were performed in three triplicates.
2.9 Bacteriostatic activity
Three opportunist marine pathogenic bacteria (Vibrio harveyi, V. anguillarum, and Photobacterium damselae subsp. piscicida) and one non-pathogenic bacterium for fish (Escherichia coli) were used to determine the bacteriostatic activity present in serum samples. Bacteria were grown in agar plates at 25°C in the adequate media: tryptic soy (TSB, Sigma) for V. harveyi, V. anguillarum, and P. damselae, and Luria (LB, Sigma) for E. coli. Then, fresh single colonies of 1–2 mm were diluted in 5 mL of appropriate liquid culture medium and cultured for 16 h at 25°C on an orbital incubator at 200–250 rpm. The serum antimicrobial activity was determined by evaluating their effects on the bacterial growth curves using the method of Sunyer and Tort (1995) with some modifications. Aliquots of 100 μL of each one of the bacterial dilutions (1/10) were placed in flat-bottomed 96-well plates and cultured with equal volumes of serum samples. The OD of the samples was measured at 620 nm at 30 min intervals during 24 h at 25°C in a microplate reader (BMG labtech). Samples without bacteria were used as blanks (negative control). Samples without serum were used as positive controls (100% growth or 0% bacteriostatic activity).
2.10 10. Statistical analysis
All measurements were performed in three replicates. The results are expressed as mean ± standard error of the mean (S.E.M.). Data were statistically analyzed by one-way analysis of variance (ANOVA) followed by a Tukey post-hoc test to determine differences between groups. Normality of the data was previously assessed using a Shapiro-Wilk test and homogeneity of variance was also verified using the Levene test. Statistical analyses were conducted using the computer package SPSS (25.0 version; SPSS Inc., Chicago, IL, USA) for WINDOWS and GraphPad Prism 7. The level of significance used was P < 0.05 for all statistical tests.
3 Results
3.1 Growth performance
After 2 and 4 weeks of feeding, the fish fed diet with the higher OP inclusion (200 mg kg−1) showed a significant increase in the SGR and the percentage of WG compared to fish fed other experimental diets (Table 1). Contrarily, the CF did not show any variations between any experimental groups.
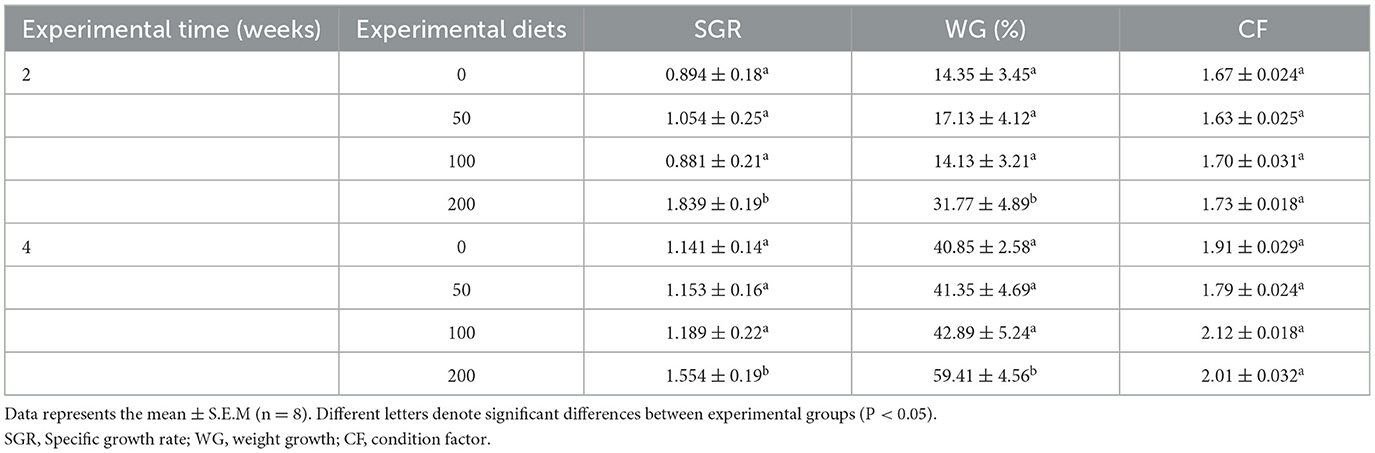
Table 1. Growth performance of gilthead seabream fed different diets [control diet: non-supplemented diet (0 mg olive pulp kg feed−1); 50: 50 mg olive pulp kg feed−1; 100: 100 mg olive pulp kg feed−1; and 200: 200 mg olive pulp kg feed−1] for 2 and 4 weeks.
3.2 Serum metabolic parameters
Most of the metabolic parameters analyzed in serum were increased in fish fed the diet with the lowest inclusion level (50 mg OP kg feed−1; Table 2). Concretely, uric acid, calcium, potassium and sodium were increased in serum of fish fed 50′s diet compared to all experimental groups. Similarly, total protein and albumin were increased in the serum of fish fed 50′s diet but regarding to values found in serum of fish from control group (non-supplemented diet). In the case of glucose, the values increased in the serum of fish fed the lowest and highest supplemented diets (50 and 200 diets) compared to the other experimental groups.
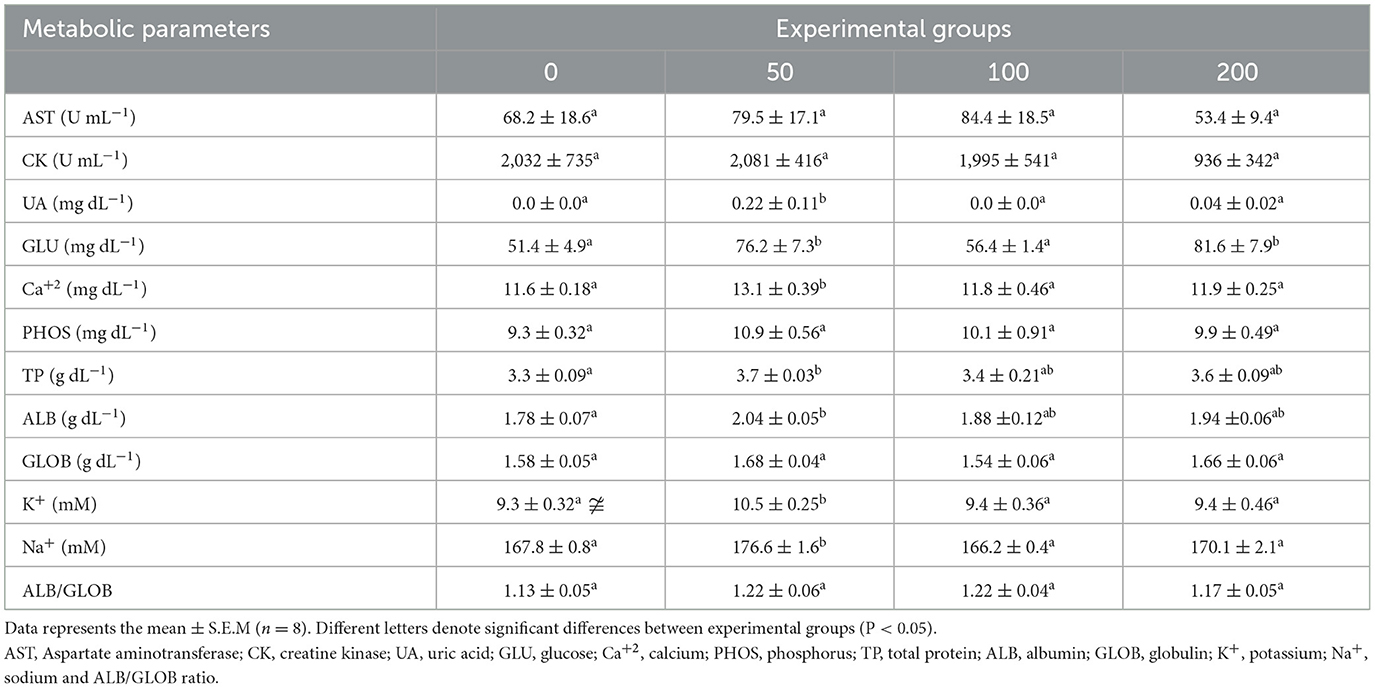
Table 2. Metabolic parameters in the serum of gilthead seabream fed different diets [control diet: non-supplemented diet (0 mg olive pulp kg feed−1); 50: 50 mg olive pulp kg feed−1; 100: 100 mg olive pulp kg feed−1; and 200: 200 mg olive pulp kg feed−1] for 4 weeks.
3.3 Antioxidant status
Biological antioxidant potential was analyzed in serum of gilthead seabream specimens fed diets with different inclusion levels of OP (Figure 1). The results revealed a significant increase of biological antioxidant potential in the serum of fish fed diet with the highest inclusion of OP respect to control group (non-supplemented diet) after 4 weeks of feeding whereas no variation was observed among experimental groups at the first experimental sampling (2 weeks).
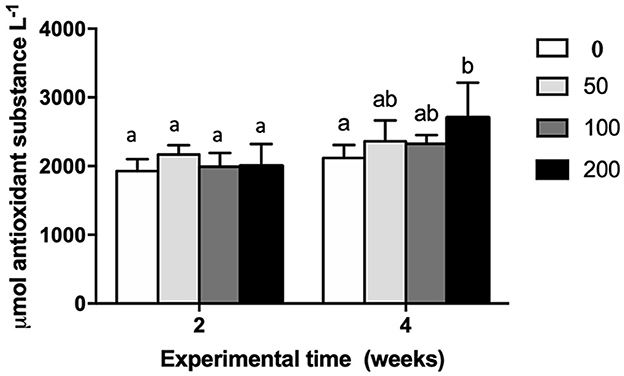
Figure 1. Biological antioxidant potential (expressed as μmol antioxidant substance L−1) in serum of gilthead seabream fed different diets [control diet: non-supplemented diet (0 mg olive pulp kg feed−1); 50: 50 mg olive pulp kg feed−1; 100: 100 mg olive pulp kg feed−1; and 200: 200 mg olive pulp kg feed−1] for 2 and 4 weeks. Bars represent the mean ± S.E.M (n = 8). Different letters denote significant differences between experimental groups (one-way ANOVA; P < 0.05).
3.4 Humoral immune parameters
The humoral immune parameters of gilthead seabream fed supplement diets were differently affected (Figures 2–4). The IgM levels were reduced in the serum from fish fed diet with the lowest inclusion (50 mg kg feed−1) of OP compared to control group at 2 and 4 weeks of feeding, being also reduced compared to values found in fish fed the 100′s diet at 2 weeks (Figure 2). However, the natural haemolytic complement registered an increase at both 100 and 200 groups at 2 weeks whilst only the fish from 200 group kept a high level at 4 weeks by respect to the control group (Figure 3A). In the case of peroxidase activity, the values increased in the fish fed 200 diet's at 2 weeks of feeding compared to other experimental groups (Figure 3B). Contrarily, protease (Figure 4A) and antiprotease (Figure 4B) activities were unaffected by dietary OP inclusion after 2 and 4 weeks of feeding trial.
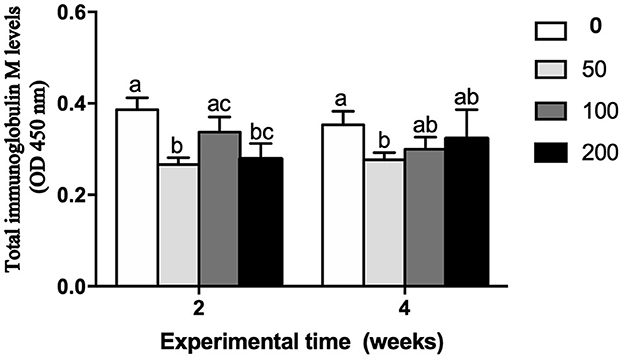
Figure 2. Total immunoglobulin M levels (OD 450 nm) in serum of gilthead seabream fed different diets [control diet: non-supplemented diet (0 mg olive pulp kg feed−1); 50: 50 mg olive pulp kg feed−1; 100: 100 mg olive pulp kg feed−1; and 200: 200 mg olive pulp kg feed−1] for 2 and 4 weeks. Bars represent the mean ± S.E.M (n = 8). Different letters denote significant differences between experimental groups (one-way ANOVA; P < 0.05).
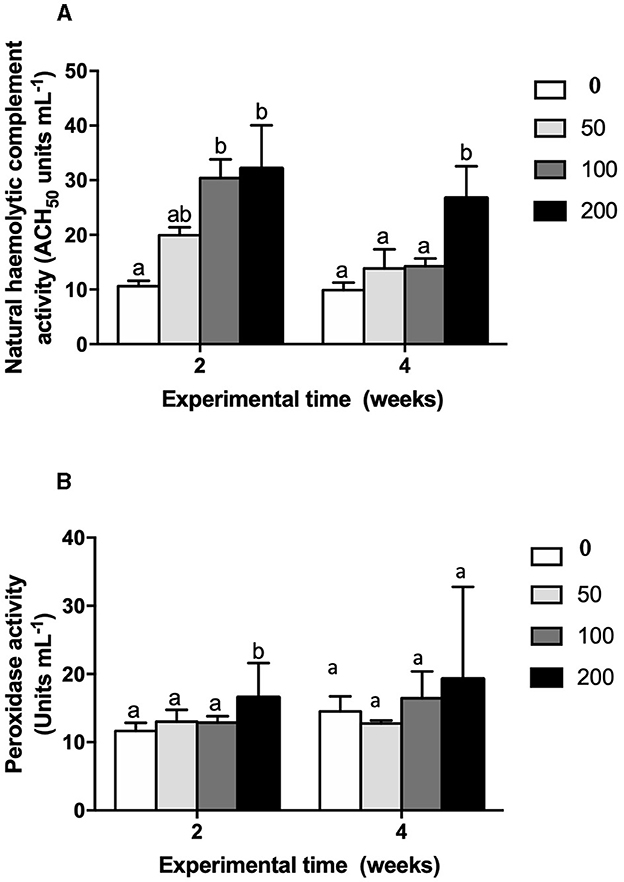
Figure 3. (A) Natural hemolytic complement activity (ACH50 units mL−1) and (B) peroxidase activity (units mL−1) in serum of gilthead seabream fed different diets [control diet: non-supplemented diet (0 mg olive pulp kg feed−1); 50: 50 mg olive pulp kg feed−1; 100: 100 mg olive pulp kg feed−1; and 200: 200 mg olive pulp kg feed−1] for 2 and 4 weeks. Bars represent the mean ± S.E.M (n = 8). Different letters denote significant differences between experimental groups (one-way ANOVA; P < 0.05).
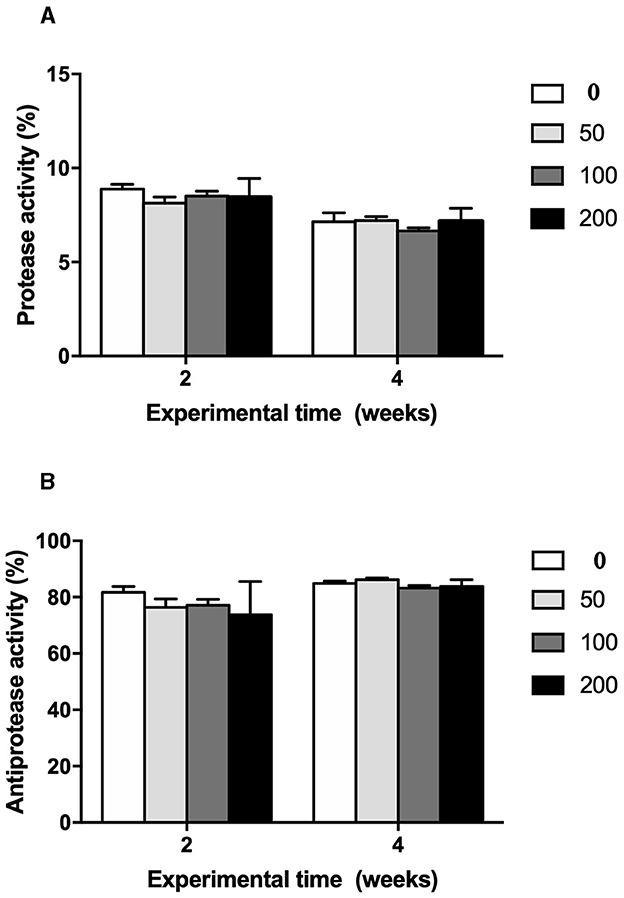
Figure 4. (A) Protease (%) and (B) antiprotease (%) activities in serum of gilthead seabream fed different diets [control diet: non-supplemented diet (0 mg olive pulp kg feed−1); 50: 50 mg olive pulp kg feed−1; 100: 100 mg olive pulp kg feed−1; and 200: 200 mg olive pulp kg feed−1] for 2 and 4 weeks. Bars represent the mean ± S.E.M (n = 8).
3.5 Cellular immune parameters
Cellular immune parameters were modulated by the supplement diets. Peroxidase (Figure 5A) and respiratory burst (Figure 5B) activities of HKLs were increased at 2 weeks in fish fed the 100 diet's compared to values found in fish from the control and 50 groups whilst no variations were observed at 4 weeks of trial. Similarly, phagocytic activity of HKLs of gilthead seabream fed 100 diet's was improved in both terms: phagocytic ability (Figure 6A) and phagocytic capacity (Figure 6B) after 2 and 4 weeks of feeding, respectively. These variations were significant compared to the values found in HKLs from control (non-supplement diet) and 50 groups in the case of phagocytic ability whilst the values of phagocytic capacity were compared to those found in the control and 200 groups.
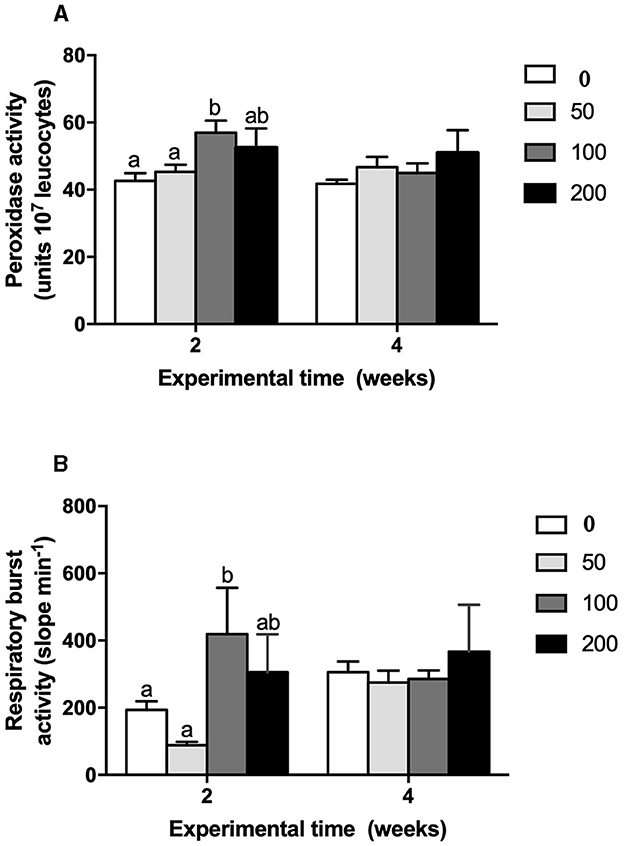
Figure 5. (A) Peroxidase activity (units 107 leucocytes) and (B) respiratory burst (slope a.u. luminescence min−1) in head-kidney leucocytes from gilthead seabream fed different diets [control diet: non-supplemented diet (0 mg olive pulp kg feed−1); 50: 50 mg olive pulp kg feed−1; 100: 100 mg olive pulp kg feed−1; and 200: 200 mg olive pulp kg feed−1] for 2 and 4 weeks. Bars represent the mean ± S.E.M (n = 8). Different letters denote significant differences between experimental groups (P < 0.05).
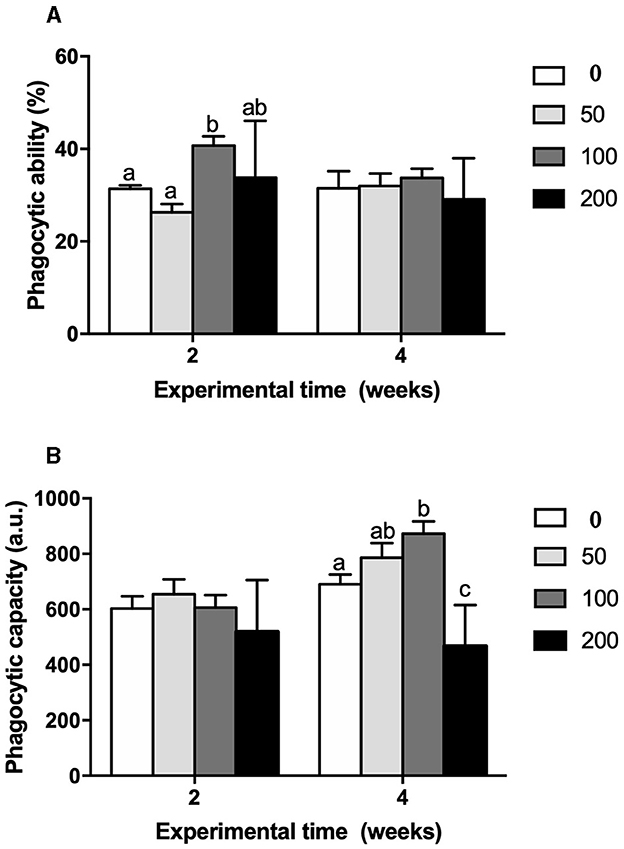
Figure 6. (A) Phagocytic capacity (%) and (B) phagocytic ability (a.u.) in head-kidney leucocytes from gilthead seabream fed different diets [control diet: non-supplemented diet (0 mg olive pulp kg feed−1); 50: 50 mg olive pulp kg feed−1; 100: 100 mg olive pulp kg feed−1; and 200: 200 mg olive pulp kg feed−1] for 2 and 4 weeks. Bars represent the mean ± S.E.M (n = 8). Different letters denote significant differences between experimental groups (P < 0.05).
3.6 Bacteriostatic activity
The bacteriostatic activity against the pathogenic and non-pathogenic bacteria tested did not show variations in the serum of fish fed experimental diets at any sampling time (Table 3).
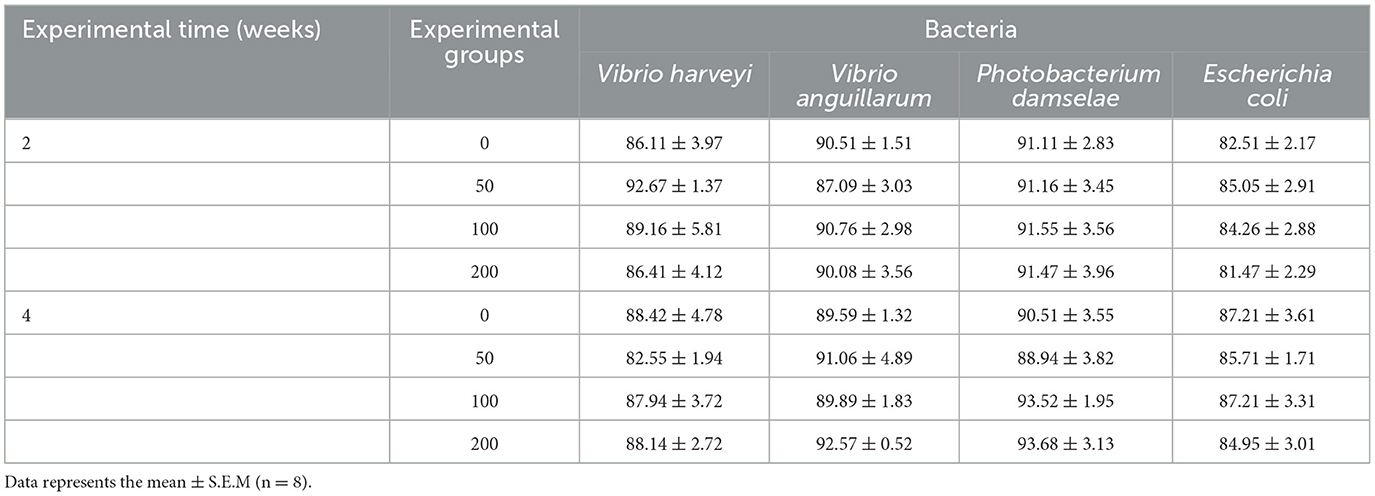
Table 3. Bacterial growth in the serum of gilthead seabream fed different diets [control diet: non-supplemented diet (0 mg olive pulp kg feed−1); 50: 50 mg olive pulp kg feed−1; 100: 100 mg olive pulp kg feed−1; and 200: 200 mg olive pulp kg feed−1] for 2 and 4 weeks.
4 Discussion
The nutritional status of fish is considered an important factor in determining the ability of fish to resist disease. In fact, several authors have identified clear correlations among nutrition and immunity parameters in fish (Awad et al., 2015; Cerezuela et al., 2016; Guardiola et al., 2016; Bahi et al., 2017; Marimuthu et al., 2022; Tadese et al., 2022). Therefore, in the last years, there has been a clear need to find a proper diet to improve growth performance, and immune status and to prevent outbreaks of disease. Interestingly, the present results evidence that the higher inclusion level of OP in experimental diets led to improve the growth parameters, particularly the SGR and WG without negatively affecting the CF. These data suggested that the gilthead seabream fed the supplemented diets exhibited a satisfactory growth performance. These results agree with a previous study performed by Gisbert et al. (2017) in which they demonstrated that 0.17 and 0.42% of olive oil bioactive extract feeding trial for 90 days enhanced gilthead seabream growth performances. Moreover, Hoseinifar et al. (2020a) showed that the use of olive waste at a concentration of 2.5 g kg−1, improved growth rate and feed utilization of rainbow trout after a trial of 6 weeks. In another study, olive leaf extract at a concentration of 200 mg kg−1 of diet for 75 days, resulted in a significant enhancement of WG, SGR and PER values in common carp (Sokooti et al., 2021). Recently, the effects of dietary incorporation of olive leaf extract (OLE) on antioxidant defense of common carp for 8 weeks, at a dose of 0.1% OLE exhibited an increase and decrease of WG and SGR, respectively, compared to the other groups (Assar et al., 2023).
Concerning seric metabolic parameters of fish fed OP supplement diets were evaluated in order to know the metabolic routes or activities which could be involved in the effects of this supplement. The AST and CK are ones of the important non-specific plasma enzymes that they, also, exist in different organs like gills, liver, kidney, and heart of fish where their activity possesses a clinical relevance to specify any organ dysfunction (liver disease, muscle damage and renal health indicators; Nabi et al., 2022). Regarding UA, it is one of the main water-soluble antioxidants in fish blood plasma (Nabi et al., 2022). Clinical relevance of serum uric acid concentrations is indicated in studies investigating impacts of toxicity on fish blood chemistry (Abdel-Tawwab et al., 2013; Mutlu et al., 2015). In the present work, the UA was significantly accumulated only in the serum of fish fed diet with the lowest concentration of OP after 4 weeks of feeding. On the other hand, the parameters related to liver disease and muscle damage (AST and CK) didn't exhibit any variations in the serum of fish from any experimental group. The most of increments observed on the analyzed metabolic parameters of fish fed the diet 50 are related with different metabolic reactions concerning renal status (Ca+2, TP, and ALB) and fluid balance (K+, Na+). In a previous study, serum globulin, albumen, total protein contents and albumin/globulin ratio showed significant increment in Nile tilapia specimens fed on a fortified feed with the higher used concentrations (1.5 and 2%) of olive leaves extract (Fazio et al., 2022). Serum glucose level is one of the indicators of stress in aquatic animals (Sokooti et al., 2021). In our current study, blood glucose amounts showed that using olive pulp (50 and 200 mg kg feed−1) in the diet lead to a significant increment. Similarly, Zemheri-Navruz et al. (2019) observed a considerable increase in the amount of glucose of common carp fed by diets containing 0.5 and 1% olive leaf extracts. Nevertheless, the serum glucose amount was not affected by diets containing various levels of olive leaf extract in Nile tilapia (Baba et al., 2018) and common carp (Sokooti et al., 2021). Moreover, in the present study, calcium, potassium and sodium amounts were increased in the serum of fish fed with the lowest OP concentration (50 mg kg feed−1). Sokooti et al. (2021) found that the calcium amount increased in fish fed with 200 mg kg feed−1 of olive leaf extract while the amounts of magnesium, phosphorus and chlorine were not affected. These authors suggested that since olive leaves are a source of calcium, the increase in the amount of serum calcium in the groups fed with diet containing olive leaf extracts was predictable (Sokooti et al., 2021).
Dietary antioxidants are non-enzymatic antioxidants which act as a branch of defense mechanisms that prevent or delay the oxidative damage from reactive oxygen species (Albertos et al., 2017). These diet derived antioxidants are primarily synthesized by plants as secondary products principally phenolic compounds (Hoseinifar et al., 2020a). Several studies have reported the positive effect of natural antioxidants from plants in farmed fish health by enhancing their performance and antioxidant activity (Elumalai et al., 2020; Hoseinifar et al., 2020a,b; Tadese et al., 2022). Likewise, in the current work, the BAP test revealed the effectiveness of the highest inclusion level of OP (200 mg kg feed−1) in enhancing the antioxidant potential in fish after 4 weeks of feeding. Therefore, this effect could be associated to the high antioxidant capacity of olive pomace which contains many substances with potent antioxidant properties, above all polyphenols and tocopherols (Gisbert et al., 2017; Hazreen-Nita et al., 2022). In a similar study, Albertos et al. (2017) showed that olive leaves powder was able to reduce the lipid and protein oxidation in Atlantic horse mackerel (Trachurus trachurus) muscle and suggested its association to the high antioxidant capacity of olive leaves and the presence of different polyphenolic compounds. In a further study, Hoseinifar et al. (2020a) demonstrated that dietary 2.5 g kg−1 of olive waste supplementation had beneficial influence on liver antioxidant enzymes of rainbow trout, where it exhibited the highest liver SOD and GST in comparison with the other treatments, after 6 weeks feeding trial. Besides, the dose 0.1% of OLE promoted the common carp's antioxidant capacity through an increase in the activity of superoxide dismutase and glutathione peroxidase and decreased hepatic lipid peroxidation end products (malondialdehyde-MDA), both pre- and post-infection (Assar et al., 2023). However, in a previous study, large yellow croaker specimens fed diet with 100% olive oil for 10 weeks, had registered significant decrease of the SOD activity and capacity T-AOC compared with the control group (Li et al., 2019).
Generally, in studies investigating the effects of dietary supplements on fish health status, immune responses are important issues to take into consideration. Thus, the modulation of the immune response, using plant products or extracts as a possible therapeutic measure has become the focus of extensive scientific investigation (Elumalai et al., 2020; Tadese et al., 2022; Ng et al., 2023). In our study, the IgM levels were reduced significantly in serum of fish after dietary administration of 50 diet at both sampling times regarding to the non-supplemented fish. Regarding the complement system, which constitutes a powerful non-specific defense mechanism in fish against a wide range of potentially invasive organisms (Burgos-Aceves et al., 2019), the values were enhanced in the serum of fish fed diets with the highest inclusion rates of OP (100 and 200 mg kg feed−1) at 2 and 4 weeks of feeding trial. In a previous study, feeding rainbow trout specimens diets containing 2.5 and 5 g olive waste cake (OWC) per kg increased serum and mucosal lysozyme activities as well as total Ig levels in skin mucus. However, OWC at concentrations of 0, 0.5, 2.5, and 5 g kg−1 had no impact on serum total Ig levels and ACH50 activity (Hoseinifar et al., 2020a). In one of the most recent papers, the supplementation of common carp diet with 0.1% of OLE registered an increment of its immune response, with higher immunoglobulin and lysosome activity, than non-supplemented fish, both before and after the Aeromonas hydrophila challenges (Assar et al., 2023).
Peroxidase is an essential enzyme with microbicidal properties where it uses one of the oxidative radicals (H2O2) to produce hypochlorous acid, in a process that is very important as a way of killing foreign microorganisms and maintaining the redox balance of immune cells (Johnston, 1978; Guardiola et al., 2014; Elumalai et al., 2020). The present results showed that, at the serine level and after 2 weeks of feeding, only the highest inclusion level of OP (200 mg kg feed−1) improved the peroxidase activity in gilthead seabream specimens. However, the peroxidase activity in HKLs increased only in the fish fed 100 diet's also after 2 weeks of trial. This activity was unaffected by the same inclusion rates after 4 weeks of feeding at humoral and cellular levels which could suggest that the peroxidase activity is affected by the dietary supplements in both dose and time-dependent manner. Zemheri-Navruz et al. (2019) demonstrated that in the OLE 0.1% treatment group the serum myeloperoxidase activity was significantly increased, however, it was significantly decreased in fish fed with higher doses (OLE 1% diet). Furthermore, serum radical scavenging and peroxidase activities of the Convict cichlid (Amatitlania nigrofasciata) receiving the 2.0 g kg−1 PMIX treatment [polyphenols mixture extracted from chest nutwood (CW) and olive mill wastewater (OMWW) (9:1, CW: OMWW)], for 8 weeks, were significantly higher than the other treatments (Hoseinifar et al., 2020a).
Interestingly, our results demonstrated an enhancement of HKLs' respiratory burst in the fish from group 100 compared to the control and 50 groups at 2 weeks in agreement with the results obtained in peroxidase and phagocytic activities measured in HKLs. As it is known, the respiratory burst is the rapid release of many forms of reactive oxygen species (ROS) from phagocytosis process, which are considered toxic to bacterial fish pathogens (Biller and Takahashi, 2018; Mandujano-Tinoco et al., 2021; Sakai et al., 2021). Concerning phagocytic activity, which is a primitive defense mechanism, and a main characteristic of fish innate cellular immune response (Vijayaram et al., 2023), our results evidenced that the administration of 100 mg kg feed−1 of OP was the unique effective dose able to enhance the phagocytic ability after 2 weeks and the phagocytic capacity after 4 weeks in HKLs of seabream. Contrarily, the 200 mg kg feed−1 diet caused a reduction in the HKLs' phagocytic capacity after 4 weeks with respect to control and other experimental groups. Previously, common carp groups fed diets containing 200 or 400 mg kg−1 OLE registered significant enhancement on their nitro blue tetrazolium activity (NBT; respiratory burst; Sokooti et al., 2021). Recently, Assar et al. (2023) found that feeding common carp with 0.1% OLE significantly improved cellular (phagocytic activity and respiratory burst) and humoral (lysozyme activity) immune parameters after challenge with A. hydrophila, However, the same researchers recorded that higher doses (0.2 and 0.3%) led to a notable decrease in these immune parameters, either pre- or post-challenge test compared to the control group (Assar et al., 2023). According to a previous paper published by Baba et al. (2018), il-1β gene expression was up-regulated in liver tissue of rainbow trout fed with 1 g kg−1 OLE in diets at 60 days of feeding. Similarly, in a study published by Zemheri-Navruz et al. (2019), OLE diet supplementation effectively up-regulated the expression of immune related genes such as tnf-α, il-1β, and il-8 in liver, spleen and HK of common carp, particularly with 1 and 2 g kg−1 OLE supplemented diets after 60 days of feeding.
According to Sakai (1999), the effect of an immunostimulant on innate immune cells, improves the function of phagocytic cells and augments their fungicidal and bactericidal activities. Nevertheless, in the present paper, although the recorded enhancements in the majority of the evaluated humoral (the serum ACH50 and peroxidase activities) and cellular innate immune responses (the HKL's peroxidases, respiratory burst, and phagocytic activities), the bacteriostatic activity against the pathogenic and non-pathogenic bacteria tested did not show variations in the serum of fish fed experimental diets at any sampling time. Serum bactericidal activity of different fish species was enhanced with several plant parts/extracts (Dossou et al., 2018; Yonar et al., 2019; Tadese et al., 2022). Furthermore, the survival rate of the common carp fed the 0.1% OLE-supplemented diets had a noticeably higher survival rate compared to the control group and the groups supplied with higher OLE concentrations (0.2 and 0.3%) after 2 weeks of challenge with A. hydrophila (Assar et al., 2023).
To summarize, by incorporating agricultural by-products such as olive oil by-products into fish diets, aquaculture feed costs can be reduced, waste management expenses can be reduced, and animal nutrition can be improved (Nasopoulou and Zabetakis, 2013; Nunes et al., 2016). These by-products offer high nutritional and physiological advantages and can significantly contribute to fish growth and development (Gisbert et al., 2017; Parrillo et al., 2017; Fazio et al., 2022). In the current study, the higher incorporation rate of olive pulp resulted in significant improvement in fish growth parameters. Olive pulp is considered a great component for animal consumption because of its high digestibility and nutrient availability (Hazreen-Nita et al., 2022). Actually, olive pulp contains lower levels of fiber than other olive by-products which can be difficult for fish to digest, especially in large amounts. Olive pulp also has a finer texture, making it easier for fish to ingest and digest. In addition, olive pulp contains valuable nutrients such as sugars, proteins, antioxidants, and essential fatty acids which fish can easily digest and absorb (Sioriki et al., 2016). These nutrients help meet the energy needs and promote the growth of fish. Overall, olive pulp may offer advantages in terms of digestibility, potentially leading to better growth and overall health of the fish. While the highest dosage administration of olive pulp significantly impacted the growth performance of gilthead seabream, it's noteworthy that most metabolic parameters were affected by the lowest dosage. This discrepancy could be due to various factors. One possible explanation is that growth performance in gilthead seabream is influenced by factors such as nutrient availability and energy intake, which may have been enhanced by the highest dosage of olive pulp. On the other hand, metabolic parameters in gilthead seabream may be more sensitive to changes in specific nutrients or compounds present in the lowest dosage of olive pulp (antioxidants and anti-inflammatory factors). Likewise, the present study has shown that higher antioxidant activity was observed in fish when exposed to a higher dose of olive pulp compared to a lower dose. This effect can be attributed to several factors, including, the increased concentration of antioxidants in the higher dose, the synergistic effects of these antioxidants, the longer duration of exposure to antioxidants, the stronger oxidative stress response in fish, and the increased bioavailability of antioxidants from the higher dose (Sokooti et al., 2021). Moreover, our study found that while the IgM level was reduced, other humoral parameters registered an increment or at least no changes compared to the control group. The reduction in certain humoral parameters can be attributed to several factors related to the composition and effects of this dietary supplement on fish physiology (Lee et al., 2016; Hazreen-Nita et al., 2022). Olive pulp contains bioactive compounds such as phenolic compounds and tocopherols, which possess anti-inflammatory properties, thus decreasing the production of certain humoral factors associated with inflammation (Sokooti et al., 2021). Additionally, dietary supplementation with olive pulp may lead to metabolic adaptations in fish, affecting metabolic pathways, nutrient utilization, and energy metabolism, which could indirectly influence the production or release of humoral factors (Nasopoulou et al., 2013; Habotta et al., 2022). Furthermore, the rich antioxidant content of olive pulp can mitigate oxidative stress in fish, which is associated with inflammation and immune dysregulation (Luciano et al., 2013; Nunes et al., 2016; Parrillo et al., 2017).
Based on the abovementioned observation that different dosages of olive pulp appear to have varying effects on different parameters, we need to consider optimizing the dosage of olive pulp based on the specific outcomes desired. For example, in the present paper, higher dosages are more effective in promoting growth performance and antioxidant parameters, but lower dosages are preferable for modulating metabolic parameters, a tailored approach to dosage selection may be warranted. The study found that the experimental group, which received a higher dosage, did not show any significant differences in metabolic parameter patterns compared to the control group. The results were within normal ranges, similar to the control group. Therefore, it cannot be considered that a high dosage is not beneficial for gilthead seabream, as long as it does not cause any controversial effects. In fact, higher dosages are recommended as they have been shown to have more beneficial effects on fish growth performance, antioxidant and immunological parameters, without causing any harmful effects. In brief, tailoring dosage selection based on specific outcomes, conducting further dose-response studies, considering individual variation, and monitoring responses are key recommendations for optimizing olive pulp use in fish diets. We should also, recognize that factors such as species, size, age, and health status can influence the optimal dosage for achieving desired outcomes.
To conclude, till to date, aquaculture studies on the use of olive by-products as dietary supplement in fish have not investigated properly the issue of minimum and maximum effective doses, optimal dietary intake timing, the accurate effect on immune and antioxidant systems, and disease resistance against pathogenic bacteria, among others. For that reason, the present study showed the positive effects of OP dietary intake on growth performance, metabolic parameters, antioxidant and immune status in gilthead seabream. According to these aforementioned effects, this paper expands the information on the immunomodulatory effects of olive oil industry by-products as possible enhancers of fish immunity, providing valuable, useful scientific data and a potential tool for further application in gilthead seabream farming and in general aquaculture. However, a better understanding of mode of actions, dosage, optimum administration time, and many other topics require further investigations which may lead to effective and appropriate uses of OP in the aquaculture sector.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Ethical Committee of the University of Murcia following the guidelines of the European Union for animal handling (2010/63/EU). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
AB: Conceptualization, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing—original draft, Writing—review & editing. RB: Funding acquisition, Validation, Writing—original draft, Writing—review & editing. ME: Validation, Visualization, Writing—original draft, Writing—review & editing. FG: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Fundación Séneca de la Región de Murcia (Grupo de Excelencia. Grant no. 19883/GERM/15). Also, this research is part of a grant project from UAEU-AUA Grant Code 00003747 supported Research Center (National Water and Energy Center, UAEU), United Arab Emirates.
Acknowledgments
The authors are grateful to L. Vilchez-Gómez from BIOTHESAN S.L. for the generous supply of the olive pulp.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Tawwab, M., Mousaad, M. N., Sharafeldin, K. M., and Ismaiel, N. E. (2013). Changes in growth and biochemical status of common carp, Cyprinus carpio L. exposed to water-born zinc toxicity for different periods. Int. Aquat. Res. 5:11. doi: 10.1186/2008-6970-5-11
Albertos, I., Martín-Diana, A. B., Jaime, I., Avena-Bustillos, R. J., McHugh, T. H., Takeoka, G. R., et al. (2017). Antioxidant effect of olive leaf powder on fresh Atlantic horse mackerel (Trachurus trachurus) minced muscle. J. Food Process Preserv. 42:e13397. doi: 10.1111/jfpp.13397
Ali, M. F. Z., Nakahara, S., Otsu, Y., Ido, A., Miura, C., and Miura, T. (2022). Effects of functional polysaccharide from silkworm as an immunostimulant on transcriptional profiling and disease resistance in fish. J. Insects Food Feed 8, 1221–1233. doi: 10.3920/JIFF2021.0108
Assar, D. H., Ragab, A. E., Abdelsatar, E., Salah, A. S., Salem, S. M. R., Hendam, B. M., et al. (2023). Dietary olive leaf extract differentially modulates antioxidant defense of normal and aeromonas hydrophila-infected common carp (Cyprinus carpio) via Keap1/Nrf2 pathway signaling: a phytochemical and biological link. Animals 13:2229. doi: 10.3390/ani13132229
Awad, E., Cerezuela, R., and Esteban, M. Á. (2015). Effects of fenugreek (Trigonella foenum graecum) on gilthead seabream (Sparus aurata L.) immune status and growth performance. Fish Shellfish Immunol. 45, 454–464. doi: 10.1016/j.fsi.2015.04.035
Baba, E., Acar, Ü., Yilmaz, S., Zemheri, F., and Ergün, S. (2018). Dietary olive leaf (Olea europea L.) extract alters some immune gene expression levels and disease resistance to Yersinia ruckeri infection in rainbow trout Oncorhynchus mykiss. Fish Shellfish Immunol. 79, 28–33. doi: 10.1016/j.fsi.2018.04.063
Bahi, A., Guardiola, F. A., Messina, C., Mahdhi, A., Cerezuela, R., Santulli, A., et al. (2017). Effects of dietary administration of fenugreek seeds, alone or in combination with probiotics, on growth performance parameters, humoral immune response and gene expression of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 60, 50–58. doi: 10.1016/j.fsi.2016.11.039
Bayne, C. J., and Levy, S. (1991). Modulation of the oxidative burst in trout myeloid cells by adrenocorticotropic hormone and catecholamines: mechanisms of action. J. Leukoc. Biol. 50, 554–560. doi: 10.1002/jlb.50.6.554
Biller, J. D., and Takahashi, L. S. (2018). Oxidative stress and fish immune system: phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Ciênc. 90, 3403–3414. doi: 10.1590/0001-3765201820170730
Bricknell, I., and Dalmo, R. A. (2005). The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol. 19, 457–472. doi: 10.1016/j.fsi.2005.03.008
Burgos-Aceves, M. A., Lionetti, L., and Faggio, C. (2019). Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total Environ. 670, 1170–1183. doi: 10.1016/j.scitotenv.2019.03.275
Cerezuela, R., Guardiola, F. A., Cuesta, A., and Esteban, M. Á. (2016). Enrichment of gilthead seabream (Sparus aurata L.) diet with palm fruit extracts and probiotics: effects on skin mucosal immunity. Fish Shellfish Immunol. 49, 100–109. doi: 10.1016/j.fsi.2015.12.028
Cuesta, A., Meseguer, J., and Esteban, M. A. (2004). Total serum immunoglobulin M levels are affected by immunomodulators in seabream (Sparus aurata L.). Vet. Immunol. Immunopathol. 101, 203–210. doi: 10.1016/j.vetimm.2004.04.021
de Oliveira, C. O., Roll, A. A. P., Medeiros Gonçalves, F. M., Lopes, D. C. N., and Xavier, E. G. (2021). Olive pomace for the feeding of commercial poultry: effects on performance, meat and eggs quality, haematological parameters, microbiota and immunity. Worlds Poult. Sci. J. 77, 363–376. doi: 10.1080/00439339.2021.1894409
Dossou, S., Koshio, S., Ishikawa, M., Yokoyama, S., Dawood, M. A. O., El Basuini, M. F., et al. (2018). Growth performance, blood health, antioxidant status and immune response in red sea bream (Pagrus major) fed Aspergillus oryzae fermented rapeseed meal (RM-Koji). Fish Shellfish Immunol. 75, 253–262. doi: 10.1016/j.fsi.2018.01.032
Elumalai, P., Kurian, A., Lakshmi, S., Faggio, C., Esteban, M. A., and Ringø, E. (2020). Herbal immunomodulators in aquaculture. Rev. Fish Sci. Aquac. 2020, 1–25. doi: 10.1080/23308249.2020.1779651
Esteban, M. A., Mulero, V., Muñoz, J., and Meseguer, J. (1998). Methodological aspects of assessing phagocytosis of Vibrio anguillarum by leucocytes of gilthead seabream (Sparus aurata L.) by flow cytometry and electron microscopy. Cell Tissue Res. 293, 133–141. doi: 10.1007/s004410051105
Fazio, F., Habib, S. S., Naz, S., Filiciotto, F., Cicero, N., Rehman, H. U., et al. (2022). Effect of fortified feed with olive leaves extract on the haematological and biochemical parameters of Oreochromis niloticus (Nile tilapia). Nat. Prod. Res. 36, 1575–1580. doi: 10.1080/14786419.2021.1883606
Gisbert, E., Andree, K. B., Quintela, J. C., Calduch-Giner, J. A., Ipharraguerre, I. R., and Pérez-Sánchez, J. (2017). Olive oil bioactive compounds increase body weight and improve gut health and integrity in gilthead sea bream (Sparus aurata). Br. J. Nutr. 117, 351–363. doi: 10.1017/S0007114517000228
Guardiola, F. A., Cuesta, A., Arizcun, M., Meseguer, J., and Esteban, M. A. (2014). Comparative skin mucus and serum humoral defence mechanisms in the teleost gilthead seabream (Sparus aurata). Fish Shellfish Immunol. 36, 545–551. doi: 10.1016/j.fsi.2014.01.001
Guardiola, F. A., Porcino, C., Cerezuela, R., Cuesta, A., Faggio, C., and Esteban, M. A. (2016). Impact of date palm fruits extracts and probiotic enriched diet on antioxidant status, innate immune response and immune-related gene expression of European seabass (Dicentrarchus labrax). Fish Shellfish Immunol. 52, 298–308. doi: 10.1016/j.fsi.2016.03.152
Guerreiro, I., Couto, A., Machado, M., Castro, C., Pousão-Ferreira, P., Oliva-Teles, A., et al. (2016). Prebiotics effect on immune and hepatic oxidative status and gut morphology of white sea bream (Diplodus sargus). Fish Shellfish Immunol. 50, 168–174. doi: 10.1016/j.fsi.2016.01.023
Habotta, O. A., Dawood, M. A. O., Kari, Z. A., Tapingkae, W., and Van Doan, H. (2022). Antioxidative and immunostimulant potential of fruit derived biomolecules in aquaculture. Fish Shellfish Immunol. 130, 317–322. doi: 10.1016/j.fsi.2022.09.029
Hazreen-Nita, M. K., Abdul Kari, Z., Mat, K., Rusli, N. D., Mohamad Sukri, S. A., Che Harun, H., et al. (2022). Olive oil by-products in aquafeeds: opportunities and challenges. Aquac. Rep. 22:100998. doi: 10.1016/j.aqrep.2021.100998
Hoseinifar, S. H., Shakouri, M., Yousefi, S., Van Doan, H., Shafiei, S., Yousefi, M., et al. (2020a). Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea L.) waste. Fish Shellfish Immunol. 100, 171–178. doi: 10.1016/j.fsi.2020.02.067
Hoseinifar, S. H., Yousefi, S., Van Doan, H., Ashouri, G., Gioacchini, G., Maradonna, F., et al. (2020b). Oxidative stress and antioxidant defense in fish: the implications of probiotic, prebiotic, and synbiotics. Rev. Fish Sci. Aquac. 2020, 1–20. doi: 10.1080/23308249.2020.1795616
Johnston, R. B. (1978). Oxygen metabolism and the microbicidal activity of macrophages. Fed. Proc. 37, 2759–2764.
Lee, S. M., Mohammadi Azarm, H., and Chang, K. H. (2016). Effects of dietary inclusion of fermented soybean meal on growth, body composition, antioxidant enzyme activity and disease resistance of rockfish (Sebastes schlegeli). Aquaculture 459, 110–116. doi: 10.1016/j.aquaculture.2016.03.036
Li, X., Cui, K., Fang, W., Chen, Q., Xu, D., Mai, K., et al. (2019). High level of dietary olive oil decreased growth, increased liver lipid deposition and induced inflammation by activating the p38 MAPK and JNK pathways in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 94, 157–165. doi: 10.1016/j.fsi.2019.08.062
Luciano, G., Pauselli, M., Servili, M., Mourvaki, E., Serra, A., Monahan, F. J., et al. (2013). Dietary olive cake reduces the oxidation of lipids, including cholesterol, in lamb meat enriched in polyunsaturated fatty acids. Meat Sci. 93, 703–714. doi: 10.1016/j.meatsci.2012.11.033
Mandujano-Tinoco, E. A., Sultan, E., Ottolenghi, A., Gershoni-Yahalom, O., and Rosental, B. (2021). Evolution of cellular immunity effector cells; perspective on cytotoxic and phagocytic cellular lineages. Cells 10:1853. doi: 10.3390/cells10081853
Marimuthu, V., Shanmugam, S., Sarawagi, A. D., Kumar, A., Kim, I. H., and Balasubramanian, B. (2022). A glimpse on influences of feed additives in aquaculture. eFood 3:e6. doi: 10.1002/efd2.6
Mutlu, E., Aydin, S., and Kutlu, B. (2015). Alterations of growth performance and blood chemistry in Nile tilapia (Oreochromis nuoticus) affected by copper sulfate in long-term exposure. Turk. J. Fish. Aquat. Sci. 15, 481–488. doi: 10.4194/1303-2712-v15_2_35
Nabi, N., Ahmed, I., and Wani, G. B. (2022). Hematological and serum biochemical reference intervals of rainbow trout, Oncorhynchus mykiss cultured in Himalayan aquaculture: morphology, morphometrics and quantification of peripheral blood cells. Saudi J. Biol. Sci. 29, 2942–2957. doi: 10.1016/j.sjbs.2022.01.019
Nasopoulou, C., Gogaki, V., Stamatakis, G., Papaharisis, L., Demopoulos, C. A., and Zabetakis, I. (2013). Evaluation of the in vitro anti-atherogenic properties of lipid fractions of olive pomace, olive pomace enriched fish feed and gilthead sea bream (Sparus aurata) fed with olive pomace enriched fish feed. Mar. Drugs 11, 3676–3688. doi: 10.3390/md11103676
Nasopoulou, C., Stamatakis, G., Demopoulos, C. A., and Zabetakis, I. (2011). Effects of olive pomace and olive pomace oil on growth performance, fatty acid composition and cardio protective properties of gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax). Food Chem. 129, 1108–1113. doi: 10.1016/j.foodchem.2011.05.086
Nasopoulou, C., and Zabetakis, I. (2013). Agricultural and aquacultural potential of olive pomace a review. J. Agri. Sci. 5, 116–127. doi: 10.5539/jas.v5n7p116
Ng, J. J. Y., Yusoff, N. A. H., Elias, N. A., Norhan, N. A. S., Harun, N. A., Abdullah, F., et al. (2023). Phytotherapy use for disease control in aquaculture: a review of the last 5 years. Aquac. Int. 2023, 1–26. doi: 10.1007/s10499-023-01292-4
Nunes, M. A., Pimentel, F. B., Costa, A. S. G., Alves, R. C., and Oliveira, M. B. P. P. (2016). Olive by-products for functional and food applications: challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 35, 139–148. doi: 10.1016/j.ifset.2016.04.016
Parrillo, L., Coccia, E., Volpe, M. G., Siano, F., Pagliarulo, C., Scioscia, E., et al. (2017). Olive mill wastewater-enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish, Astacus leptodactylus. Aquaculture 473, 161–168. doi: 10.1016/j.aquaculture.2017.02.013
Quade, M. J., and Roth, J. A. (1997). A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 58, 239–248. doi: 10.1016/S0165-2427(97)00048-2
Ray, N. B., Lam, N. T., Luc, R., Bonvino, N. P., and Karagiannis, T. C. (2015). “Cellular and molecular effects of bioactive phenolic compounds in olives and olive oil,” in Olive and Olive Oil Bioactive Constituents, ed. D. Boskoud (Amsterdam: Elsevier Inc.), 53–91.
Ringø, E., and Song, S. K. (2016). Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture. Aquac. Nutr. 22, 4–24. doi: 10.1111/anu.12349
Rodríguez, A., Esteban, M. Á., and Meseguer, J. (2003). Phagocytosis and peroxidase release by seabream (Sparus aurata L.) leucocytes in response to yeast cells. Anatom. Rec. 272, 415–423. doi: 10.1002/ar.a.10048
Sakai, M. (1999). Current research status of fish immunostimulants. Aquaculture 172, 63–92. doi: 10.1016/S0044-8486(98)00436-0
Sakai, M., Hikima, J., and Kono, T. (2021). Fish cytokines: current research and applications. Fish Sci. 87, 1–9. doi: 10.1007/s12562-020-01476-4
Sánchez, C. J., Barrero-Domínguez, B., Martínez-Miró, S., Madrid, J., Baños, A., Aguinaga, M. A., et al. (2022). Use of olive pulp for gestating iberian sow feeding: influence on performance, health status indicators, and fecal microbiota. Animals 12:3178. doi: 10.3390/ani12223178
Sioriki, E., Nasopoulou, C., Demopoulos, C. A., and Zabetakis, I. (2015). Comparison of sensory and cardioprotective properties of olive-pomace enriched and conventional gilthead sea bream (Sparus aurata): the effect of grilling. J. Aquat. Food Prod. Technol 24, 782–795. doi: 10.1080/10498850.2013.813100
Sioriki, E., Smith, T. K., Demopoulos, C. A., and Zabetakis, I. (2016). Structure and cardioprotective activities of polar lipids of olive pomace, olive pomace-enriched fish feed and olive pomace fed gilthead sea bream (Sparus aurata). Int. Food Res. J. 83, 143–151. doi: 10.1016/j.foodres.2016.03.015
Sokooti, R., Chelemal Dezfoulnejad, M., and Javaheri Baboli, M. (2021). Effects of olive leaf extract (Olea europaea Leecino) on growth, haematological parameters, immune system and carcass composition in common carp (Cyprinus carpio). Aquac. Res. 52, 2415–2423. doi: 10.1111/are.15091
Sunyer, J. O., and Tort, L. (1995). Natural hemolytic and bactericidal activities of sea bream Sparus aurata serum are affected by the alternative complement pathway. Vet. Immunol. Immunopathol. 45, 333–345. doi: 10.1016/0165-2427(94)05430-Z
Tadese, D. A., Song, C., Sun, C., Liu, B., Liu, B., Zhou, Q., et al. (2022). The role of currently used medicinal plants in aquaculture and their action mechanisms: a review. Rev. Aquac. 14, 816–847. doi: 10.1111/raq.12626
Tzamaloukas, O., Neofytou, M. C., and Simitzis, P. E. (2021). Application of olive by-products in livestock with emphasis on small ruminants: implications on rumen function, growth performance, milk and meat quality. Animals 11:531. doi: 10.3390/ani11020531
Vijayan, A., Sivaraman, G. K., Visnuvinayagam, S., Mothadaka, M. P., Vijayan, A., Sivaraman, G. K., et al. (2021). Role of natural additives on quality and shelf-life extension of fish and fishery products. Nat. Food Additiv. 2021:99436. doi: 10.5772/intechopen.99436
Vijayaram, S., Ringø, E., Zuorro, A., van Doan, H., and Sun, Y. (2023). Beneficial roles of nutrients as immunostimulants in aquaculture: a review. Aquac. Fish 2:1. doi: 10.1016/j.aaf.2023.02.001
Yonar, M. E., Mişe Yonar, S., Ispir, Ü., and Ural, M. S. (2019). Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish Shellfish Immunol. 89, 83–90. doi: 10.1016/j.fsi.2019.03.038
Zemheri-Navruz, F., Acar, Ü., and Yilmaz, S. (2019). Dietary supplementation of olive leaf extract increases haematological, serum biochemical parameters and immune related genes expression level in common carp (Cyprinus carpio) juveniles. Fish Shellfish Immunol. 89, 672–676. doi: 10.1016/j.fsi.2019.04.037
Keywords: olive pulp, natural immunostimulants, metabolic profile, antioxidant status, immune response, gilthead seabream (Sparus aurata L.)
Citation: Bahi A, Bhaskaracharya R, Esteban MA and Guardiola FA (2024) Dietary administration impact of olive pulp on growth performance, metabolic profile, immune status, and antioxidant potential of gilthead seabream (Sparus aurata L.). Front. Sustain. Food Syst. 8:1395436. doi: 10.3389/fsufs.2024.1395436
Received: 03 March 2024; Accepted: 15 April 2024;
Published: 01 May 2024.
Edited by:
Roberto Anedda, Porto Conte Ricerche, Parco Scientifico e Tecnologico della Sardegna, ItalyReviewed by:
Xiaohong Liu, Southwest University, ChinaRoberta Marcoli, James Cook University, Australia
Copyright © 2024 Bahi, Bhaskaracharya, Esteban and Guardiola. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisco A. Guardiola, faguardiola@um.es; Abir Bahi, abirbahi@uaeu.ac.ae; Raman Bhaskaracharya, ramankumarb@uaeu.ac.ae
 Abir Bahi
Abir Bahi Raman Bhaskaracharya
Raman Bhaskaracharya Maria Angeles Esteban
Maria Angeles Esteban Francisco A. Guardiola
Francisco A. Guardiola