The Anthropology of Aquaculture
- 1School of Marine and Environmental Programs, University of New England, Biddeford, ME, United States
- 2Ecological Aquaculture Foundation, LLC, Biddeford, ME, United States
- 3Ecological Aquaculture Foundation, LLC, Ilha do Pico, Portugal
Aquaculture is nothing new. It has a long, fascinating history that stretches from antiquity at least 8,000 years ago. What is new is the evolution of aquaculture in modern times into highly intensive monocultures which arose in the 1970–1980's. Modern aquaculture production has grown worldwide but remains concentrated in Asia due to the: (1) increased demands for aquatic foods as explosive population growth occurred in coastal cities with increasing affluence, (2) expansion of scientific and engineering breakthroughs, (3) high export values of aquatic foods, and (4) sharp decline of costs of global to local transport/shipping. The pioneering anthropologist Claude Levi-Strauss brought the idea of “structuralism” to anthropology: the concept that societies throughout history followed universal patterns of behavior. A qualitative document analysis of the key anthropological literature to assess aquaculture developments from antiquity to the beginning of the modern era was conducted to evaluate if there was adequate evidence to support a theory of anthropological “structuralism” for aquaculture in human history. Seven case studies of the cultural/environmental history of aquaculture were reviewed in diverse parts of the world (China, Australia, Egypt, Europe, South America, Canada/USA, Hawai'i). Analysis supports the structural theory that whenever the demands of aquatic/seafood-eating peoples exceeded the abilities of their indigenous fishery ecosystems to provide for them, they developed aquaculture. Modern aquaculture concepts and new communities of practice in “restoration aquaculture” have beginnings in Indigenous anthropology and archeology in aquaculture and point the way for Indigenous nations to engage as leaders of the United Nations Food and Agriculture Organization (FAO) ecosystem approach to aquaculture worldwide. Bringing ancient knowledge of Indigenous aquaculture into the modern context is an essential part of an alternative, “radical transformation” of modern aquaculture. There is an urgent need to develop and promote locally designed and culturally appropriate aquaculture systems that fit into the livelihoods of communities as part of a larger, diverse portfolio of food security.
Introduction
Cultures practicing aquaculture in antiquity have been little examined comprehensively in the anthropological literature. As a result, no unifying theories exist to explain how aquaculture develops, evolves, and fits into human development. Cross cultural analysis tools such as the Standard Cross-Cultural Sample (SCCS) of Murdock and White (1969) and the EthnoAtlas (Gray, 1998) examine over 180 societies anthropologically using many variables but do not classify societies as aquaculture or mariculture-centric rather focus on hunting, gathering, fishing, and animal husbandry, reinforcing this lack of examination.
As aquaculture has grown to be one of the most important protein systems in the world (FAO, 2020) it is vital to examine its ancient environmental/cultural history. Understanding the ancient past will allow a modern appreciation of aquaculture as nothing “new” but as an important part of historical food production. Connections to the ancient past will allow a better appreciation of the diversity of development pathways possible for the future of aquaculture and the divergence of intensive aquaculture in modern times, especially to aquaculture development in the post-World War II era that is believed to be the beginning of the Anthropocene (Waters, 2016).
Modern, industrial, export-driven aquaculture is technologically complex as are its interactions with modern societies (Indigenous, urban, rural, rich, poor, etc.). Communities can either embrace change, develop new values, ceremonies and rituals, and accommodate social transformations, or reject these and continue their social, cultural, and economic evolutions without such disruptive interventions in society such as the rise of aquaculture. Nahuelhual et al. (2019) point to the recent feature of aquaculture in discussions of a “Blue Transition” especially how aquaculture has featured in policy discussions as relieving pressure on wild capture fisheries and contributing to food security and employment of the world's poor. International discussions incorporating aquaculture development as a “blue revolution” place aquaculture into foundational documents on the very nature of the future of food. In these reports aquaculture is connected strongly to the blue economy, blue growth, and blue carbon (Gentry et al., 2017; Hoegh-Guldberg et al., 2019; Willett et al., 2019; Costello et al., 2020). Nahuelhual et al. (2019) point out that a “Blue Transition” is akin to other environmental transitions and emphasize the need for such a transition to include social-ecological feedbacks citing Berkes and Folke (1998) who state that “a social-ecological feedback refers to a situation in which the ecological and the social systems (or components of the two) are connected together such that each system influences the other and their dynamics are thus strongly coupled.” In the case of aquaculture, Krause et al. (2015) point to cases of aquaculture development that are a “blue revolution without people.”
Many cultures that have relied historically on aquatic foods as their primary source of proteins. Recovering trajectories of Indigenous aquaculture development from antiquity and making connections to modernity opens the full social-ecological panoply of traditional knowledge to evolve alternative developmental pathways for aquaculture. Indigenous communities recovering their pasts are evolving new aquaculture communities of practice that have clear connections from ancestors to modernity. No matter the sources of knowledge used to chart alternative developmental pathways for aquaculture, whether traditional knowledge, scientific, corporate, or some blend, well-planned, transparent, participatory processes are required. Such development alternatives may drag out for longer than both Indigenous and other decision-makers want but, in the end, will lead to shared visions of more sustainable futures.
Since the Cultural Revolution China is recognized as the world's aquaculture leader in production for all forms of aquaculture from ocean to freshwater aquaculture, integrated and non-integrated systems, and for all fed to non-fed typologies (FAO, 2020). China is one of the only nations today where aquaculture production exceeds production from capture fisheries at a scale that is transformative of protein foods on Earth (Naylor et al., 2021). The evolution of aquaculture in China has occurred over thousands of years. China's historical scientific/cultural knowledge systems have contributed directly not only to its modern ascendancy in industrial and scientific areas but also more fundamentally to the adoption of aquaculture as culture.
Too many proposals for modern aquaculture developments post WWII, especially in the “new geographies for aquaculture” outside its traditional places in Asia (Costa-Pierce and Chopin, 2021) have been marketed to societies neglecting their anthropological and environmental histories and without considerations of their allied, cultural, and spiritual backgrounds. The modern case study of industrial aquaculture in Chile by Nahuelhual et al. (2019) demonstrates clearly that the promised blue transition in that country has not occurred as planned and advertised.
Without such knowledge of past cultural advances, societies worldwide will lose one of their greatest opportunities for more socially and environmentally sound forms of food production. The aquaculture profession will continue to limp along, especially in areas with great potential outside of Asia, losing opportunities for aggregating and delivering teachable moments due to the tiresome constraints repeated over and over about a lack of “social license” for aquaculture (Costa-Pierce, 2010; Zajicek et al., 2021). Cultural studies and historical backgrounds can serve as local, place-based ecological and social baselines to evolve the “blue revolution.” A “culture of aquaculture” needs to be built on historical foundations so that informed politicians, investors, and communities can make better decisions based upon complete information and timelines of this historically important food innovation that has arisen multiple times in antiquity.
Historical Evolution of Aquatic Species Management
It is widely accepted that aquaculture arose multiple times in societies as an evolution from capturing and trapping fish (Atlas et al., 2020), to holding and keeping fish, to reproducing, growing, and domesticating fish (Balon, 1995; Beveridge and Little, 2002; Nash, 2011). There are numerous anthropological and archeological studies of capture fisheries from antiquity (O'Connor et al., 2011). Hu et al. (2009) found freshwater fish were a part of the diet of ancient people near Beijing China 40,000 years ago. Steneck and Pauly (2019) describe the “kelp highway hypothesis” for colonizing the Americas from Northeast Asia. The hypothesis states that “saltwater people” (as defined by McNiven, 2003) from east Asia advanced north along western Pacific coasts then east across the sea to rapidly colonize the entire eastern Pacific coast. Archeological findings in Monte Verde in the south of Chile date to 14,500 years B.P. are “more consistent with the idea of a coastal rather than a land-based migration” (Steneck and Pauly, 2019).
The kelp highway hypothesis adds an ocean food systems component to accepted archeological findings on the importance of the “maritime highway” for human migrations in antiquity in Asia/Pacific. The oldest accepted evidence for open ocean crossings by modern humans is the migration to “Sahul,” the combined continent of Australia and New Guinea, 47,000 years B.P. (Davidson, 2013; Clarkson et al., 2017; O'Connell et al., 2018). Coastal cultures in the north Pacific relied primarily on the abundant marine resources of kelp ecosystems for their primary sustenance. In the south, Steneck and Pauly (2019) theorized that rich mangrove ecosystems could have sustained similar large-scale coastal colonization's in the Tropics. Erlandson and Braje (2015) assessed if mangrove ecosystems could have facilitated a larger scale maritime colonization of people from East Africa to Oceania.
These hypotheses are intriguing from an evolutionary perspective of ancient aquaculture development from wild capture fisheries, to fish trapping and holding, and onwards to “proto-aquaculture” (Beveridge and Little, 2002). Archeological and anthropological evidence of complex Indigenous knowledge of extensive fish traps, fish holding and onwards to “proto-aquaculture” exists from Taiwan, Japan, and the Ryukyu Islands, Japan, 35,000–17,000 years B.P. (Kaifu, 2015; Fujita et al., 2016). Kaifu et al. (2020) reported evidence that difficult maritime crossings from the north (via Kyushu) and south (via Taiwan) to the Ryukyu Islands of southwestern Japan occurred some 35,000–30,000 years B.P. They state that “migration to the Ryukyus is difficult because it requires navigation across one of the world's strongest currents, the Kuroshio, toward an island that lay invisible beyond the horizon.” Furthermore, “this suggests that the Paleolithic Island colonization occurred in a wide area of the western Pacific was a result of human's active and continued exploration, backed up by technological advancement.”
Capture fisheries are the capture and harvest of wild aquatic organisms where no interventions are made to manage or otherwise influence captured organisms by containment, feeding, or application of any aquaculture techniques. Historical fish trapping is the capture of wild aquatic organisms for direct harvest using sedentary, non-mobile gears. Beveridge and Little (2002) defined “proto-aquaculture” as “activities designed to extract more food from aquatic environments, such as: the transplantation of fertilized eggs, entrapment of fish in areas where they could thrive and be harvested as required, environmental enhancements, such as development of spawning areas, enhancement of food, exclusion of competitors or predators, etc., and the holding of fish and shellfish in systems (ponds, cages, pens) until they had increased in biomass or until their value had improved.” They distinguished “proto-aquaculture” from aquaculture due to the small degree of control over the life cycle of an aquatic species and the low impact of the intervention on aquatic production. One example cited is the “bundhs” of West Bengal India which are seasonal ponds that fill with water in during the Indian Ocean monsoon first rains and were used to stimulate spawning of Indian major carps over a 100 years ago (Sharma and Rana, 1986). Klinger et al. (2013) pointed out that even in the modern context it is difficult to separate fisheries and aquaculture or to define the various typologies noting there are ancient and modern practices of capture-based aquaculture (Lovatelli and Holthus, 2008). They state that “Numerous seafood species are produced for the global marketplace using a spectrum of methods and cannot be cleanly ascribed as either fisheries or aquaculture.”
Aquaculture is defined as the farming of aquatic species in water. Farming implies intervention in the rearing process to enhance production, such as the regular stocking, feeding, protection from predators, etc., plus the individual, community, organization, or corporate ownership of the stock being farmed (Rana, 1998). Modern aquaculture systems are remarkably diverse and comprise the farming of hundreds of species in fed or unfed systems growing domesticated and non-domesticated species with hatcheries having little to no connections to wild genetics. Domestication of plants and animals dates from the Neolithic about 14,000 years B.P. (Zeuner, 1963). Domestication of aquatic species to the level of their separation from wild ancestors, with selection and breeding to create “synthetic species” similar to those produced throughout millennia on land, and in closed aquaculture production networks, is “true” aquaculture (Costa-Pierce, 2003).
Live capture of aquatic organisms in traps for direct harvest is present throughout the ancient and modern world. Nelson (2017) describes the extensive knowledge of tides and fish behavior used to design sophisticated fish traps and weirs over large portion of coastal Taiwan. Traps incorporated curves to incorporate knowledge of the tendency of fish to turn when they hit a curve. At high tides, trap walls were submerged allowing fish to swim over them. At low tides fish were trapped and gathered and people scooped out thousands of anchovies, herrings and other species using simple gears. Hawaiian fish traps (loko 'umeiki) are good historical examples as similar designs are present throughout Oceania. They are stone structures built into the sea with low, semi-circular walls that were partially or wholly submerged at high tide and contained numerous openings (lanes) leading into or out of the trap (Kikuchi, 1973, 1976). Siting of fish traps was done by knowledge of longshore currents that transported fish along the shore. The Hawaiian island of Moloka'i had many fish traps owing to the favorable orientation of the island with regard to longshore currents. Lanes in the walls of traps connecting to the sea were used to catch fish migrating down the coastline who were attracted to the surge of water at the lane entrances. Nets laid facing the sea across the opening of the lane captured fish flowing into the trap on an incoming tide. When the tide reversed fishermen faced their nets toward the traps capturing fish as they swam out to sea. It was reported that the right to fish during different portions of the tidal cycle was divided among family groups. Timoteo Keaweiwi in 1853 stated, “Such was the case of Mikiawa Pond at Ka'amola, Moloka'i. When the tide was coming in, the people of Keawanui could set the lanes. When the sea ebbed, the fish belong to Ka'amola” (Summers, 1964).
The next evolution in aquatic species management involved short- to long-term live storage of catches as a form of food storage/banking. Social drivers of development were the provision of sufficient aquatic foods for consumption, ceremonies, increase reliability of supplies, and/or trade to markets which are common strategies found throughout antiquity and are common strategies today. Trapping and holding to ensure fish supplies dates to the Neolithic about 6,000 years B.P. in Europe (European Commission, 2018). Modified fish traps connected to excavated ponds, netted off or channelized shallow areas of lakes, bamboo cages in rivers and irrigation ditches (Costa-Pierce and Effendi, 1988) and traditional floating cages as used in Tonal Sap, Cambodia (Beveridge, 1996) remain worldwide. Detailed traditional knowledge of fish behaviors and tides to trap fish in cages and weirs is preserved in the knowledge systems and literatures of Indigenous cultures and island communities worldwide from the Americas (for salmon, Atlas et al., 2020), Europe to East Africa and throughout Oceania. In modern times, a capture fishery that live harvests wild organisms at early stages in their life cycle, then transports them to aquaculture systems for growout under confinement and management to mature, harvestable adults is referred to as “capture-based aquaculture” (Lovatelli and Holthus, 2008). FAO (2016) estimated that 20% of global aquaculture production today is CBA.
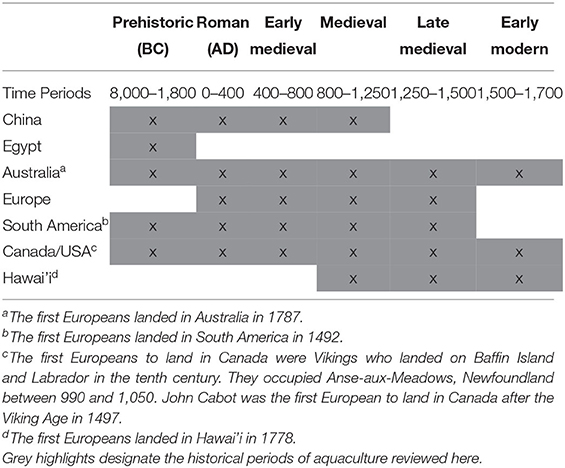
Methods
A narrative review of case studies from seven sites of historical aquaculture development was completed to gain knowledge on recurrent themes (Finfgeld, 2003). Sites were chosen from the historical and anthropological literature over a time span from antiquity ~8,000 years ago until the mid-1700's, or the Industrial Revolution, which is defined as the 100 years from about 1760 to 1860 when machines transformed industry and the concept of societies and work changed fundamentally (Horn et al., 2010; Table 1). A qualitative content analysis (Frankfort-Nachmias et al., 2015) was completed to find if there existed recurrent evidence in case studies of: (1) an aquatic/seafood-eating culture, (2) natural aquatic food resource scarcities, and (3) aquaculture development.
Case Studies
China−8,000 Years+
Historical records describe common carp (Cyprinus carpio) being raised widely in ponds and paddy fields in China by the first millennium BC and earlier. Nash (2011) stated that carp aquaculture dates to 2,600 B.P. Shijing pottery, the oldest surviving collection of ancient Chinese pottery, shows carp being reared in ponds around 1,140 B.C. Rice paddies in China have been dated to the fifth millennium BC. Nakajima et al. (2019) provide evidence from fish bones excavated from an early Neolithic site in Jiahu, Henan Province that carp aquaculture was practiced there between 6,200 and 5,700 B.C., making aquaculture in China at least 8,000 years old. They state that “a large number of cyprinids were caught during the spawning season and processed as preserved food. At the same time, some carp were kept alive and released into confined, human regulated waters where they spawned naturally and their offspring grew by feeding on available resources. In autumn, water was drained from the ponds and the fish harvested…” Researchers hypothesized three stages of aquaculture development: (1) fishing in carp spawning areas in shallow marshes, (2) creation of hatcheries in the marshes by digging canals and controlling spawning and juveniles harvested, and (3) rice-fish culture and pond aquaculture.
There is documentation of integration of aquaculture into the networks of ponds and irrigation systems of China. Clay models of irrigation systems recovered from graves throughout southern China show that by the Han Dynasty (2,300–1,700 B.P.) fishponds were being employed widely for water storage (Bray, 1984; Li, 1994). An intact rice-field model was found having over 18 varieties of aquatic plants and animals which are used today that included lotus flowers, seeds and leaves, water chestnuts, soft-shelled turtles (Trionyx sinensis), grass carp (Ctenopharyngodon idella), and goldfish (Carassius auratus; Li, 1992).
Areas of southern China had high population densities with culturally advanced cities which reached their peak in the Song (Sung) Dynasty (960–1,276). New rice varieties were introduced from Vietnam. In the floodplains of China soils were excavated to construct elevated areas for homesteads and raising crops. Excavated areas became fishponds. Demands for fish and other aquatic foods increased and the practice of holding and growing fish became widespread as wild stocks became less abundant (Wu, 1985). Kwai Sin Chak Shik, a book written during the Sung Dynasty in 1,243 describes how carp fry were transported in bamboo baskets to ponds. Fry were collected in rivers and reared in ponds as recorded in A Complete Book of Agriculture written in 1639 (Balon, 1995).
The treatise published by the statesman Fan Li some 2,500 years B.P. describes common carp farming in sufficient detail to provide incontrovertible evidence that advanced fish culture developed in antiquity (Li, 1994). The monograph details the design and layout of fishponds, carp breeding, fry and fingerling rearing techniques. Accounts of the integration of fish culture with aquatic plants and vegetables exist in written records dating from 2,200 to 2,100 B.P. (Yang, 1994). Ruddle and Zhong (1988) describe the Zhejiang Huzhou mulberry-silkworm-fish-crop-animal farming ecosystem in China which is estimated to be more than 2,500 years old. Unfortunately these elegant aquaculture ecosystems have been declining since the 1990's (Edwards, 2009). Balon (1995) stated that domestication of common carp can be traced to the Romans and questioned if a separate center of domestication in ancient China existed. Similarly, Wohlfarth (1984) stated “there is evidence that in China the carp was never truly domesticated, but stocked at most, in a semi-domesticated condition with other fishes.” However, Nakajima et al. (2019) provide compelling recent archeological evidence that a separate center of carp domestication in ancient China existed.
Australia−8,000 Years+
More than 30,000 years ago a volcano known as Budj Bim (Mount Eccles) produced a lava flow (Tyrendarra lava flow) whose flow to the sea changed the watershed drainage patterns in western Victoria, Australia. Over centuries the landscape evolved into large, rich wetlands (Builth, 2006). For thousands of years the Gunditjmara people of Southwest Australia lived in this region in well-populated, permanent settlements (Lourandos, 1987).
McNiven (2015) studied the ancient aquaculture system of the Gunditjmara people as it evolved over 800 years. Gunditjmara engineered and managed water systems in the wetlands to channel and capture short finned eels (Anguilla australis) to ensure year round food supplies in an ecosystem over 75 km2 around Lake Condah (Coutts et al., 1978; McNiven and Bell, 2010). Gunditjmara dug ponds and linked the wetlands by channels to direct water and juvenile eels into ponds and weirs in low-lying wetlands. Woven baskets were placed in the weirs to harvest mature eels (McNiven and Bell, 2010). Sophisticated earthworks were engineered to live store seasonal abundances and ensure adequate fish supplies throughout the year.
Egypt−4,000 Years+
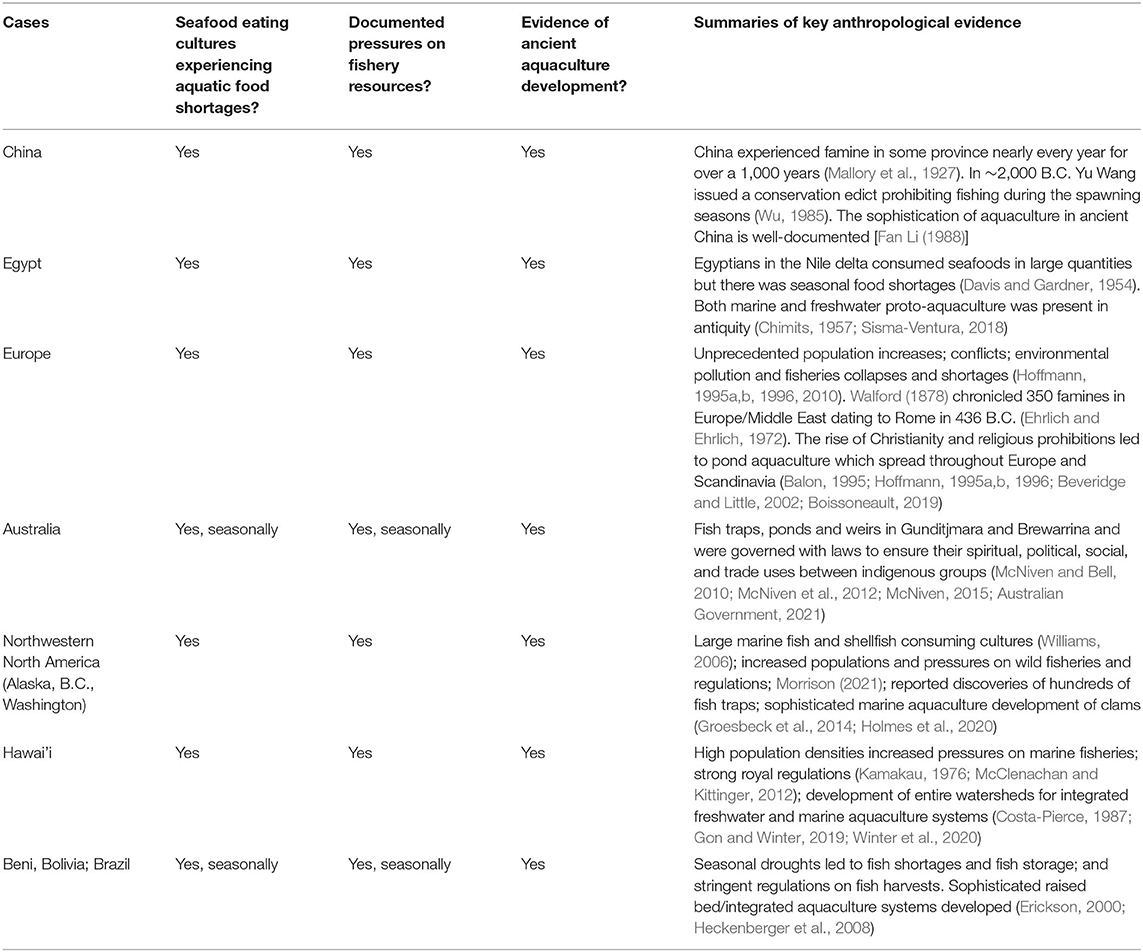
In ancient Egypt fish had sacred as well as prosaic roles in society. They were associated with the cyclical life-giving forces of the Nile and the New Kingdom Egyptian view of the world. Tilapia (Oreochromis niloticus) linked to the goddess Hathor and the concept of rebirth (Desroches-Noblecourt, 1954). Brewer and Friedman (1989) detail peculiar beliefs and taboos among the priesthood associated with fish. Although rod and line fishing is believed to have been common among all classes in ancient Egypt, fishing activities of the nobility were limited to fishing from their artificially constructed garden ponds. Their interest in fishing stemmed more from religious rituals associated with death and rebirth and less with pleasure or sustenance (Desroches-Noblecourt, 1954; Brewer and Friedman, 1989). Fishery innovations were present not only in the elites, however, but throughout Egyptian society (Chimits, 1957). Early travelers to Egypt confirmed that fish were of tremendous importance in the Egyptian diet. The Roman traveler Diodorus Scullus is quoted as saying that “…the Nile contains every variety of fish and in numbers beyond belief: for it supplies the native not only with fish freshly caught but also yields an unfailing multitude for salting.” Herodotus, who traveled to Egypt some 2,500 years ago reported that “…all Egyptians in the Nile Delta possess a net with which, during the day, they fish…”
In his account of tilapia in ancient Egypt, Chimits (1957) reproduces a 4,000-year-old bas relief from the tomb of Thebaine showing a nobleman sitting in his garden fishing using a double line with two hooks, his wife seated behind him unhooking fish (Figure 1). He appears to be fishing from an artificial, drainable, fish tank (Davis and Gardner, 1954). The relief is remarkable for also showing lotus growing on the top of the tank (for shade, ancient “aquaponics”), and papayas irrigated by pond water in a field being picked by servants (ancient “integrated aquaculture”). Tilapia were transferred from the Nile to not the ponds of nobility but also of commoners (Chimits, 1957) where they certainly would have spawned.
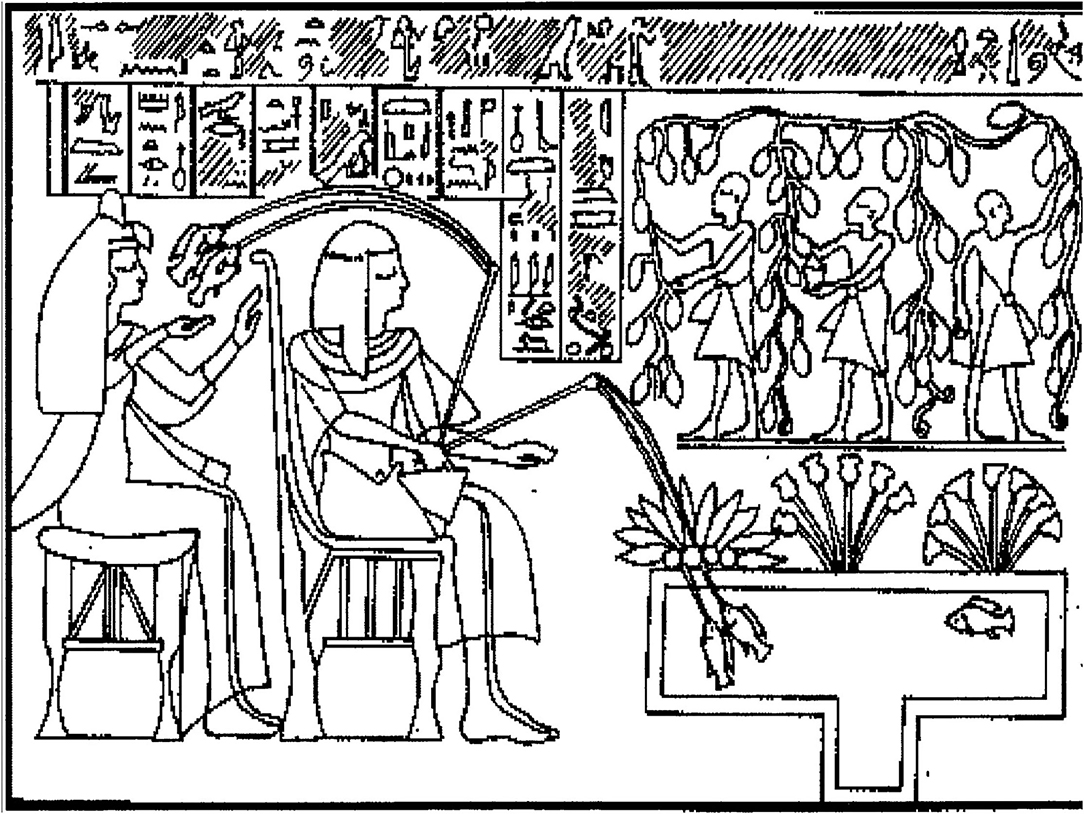
Figure 1. Bas relief on the Tomb of Thebaine, Nile Delta, Egypt circa 2000 B.C. Note the central drainage canal, floating lotus plants, and juxtaposition of tank to fruit trees. These components are used in modern aquaculture to harvest fish, provide shade, water quality control by aquaponics plants, and shelter for fish, and direct wastewaters for use by terrestrial agriculture in an integrated aquaculture system. From Chimits (1957) redrawn from author photographs (B.A. Costa-Pierce).
Canada, USA−3,500 Years+
“We hypothesize that some kind of shellfish management was indeed widespread in many traditional societies, as reflected in the disparate archaeological and ethnographic information compiled in this paper.” (Lepofsky et al., 2015)
In the Pacific Northwest of North America (modern Alaska, USA, British Columbia, Canada and Washington state, USA), vast fisheries with fish traps helped to sustain Indigenous communities for at least 12,000 years (Selkirk, 2021). Morrison (2021) reported a network of 300 or more fish traps installed by the K'omoks that date more than 1,300 years ago. Clam mariculture in engineered rock-walled intertidal terraces and sophisticated shellfish cultivation techniques were practiced (Augustine and Dearden, 2014; Deur et al., 2015; Lepofsky et al., 2015; Moss and Wellman, 2017). Smith et al. (2019) radiocarbon dated nine clam gardens on Northern Quadra Island, British Columbia, Canada and determined some clam gardens in their study area were over 3,500 years old.
First nations peoples along this coast constructed and managed clam gardens of littleneck clams (Leukoma staminea) and butter clams (Saxidomus giganteus; Williams, 2006; Groesbeck et al., 2014; Lepofsky et al., 2015; Jackley et al., 2016). Indigenous people created and maintained these systems by modifying the marine benthic substrate resulting in some systems that were at least four times more productive and resilient than non-clam gardens (Lepofsky et al., 2015; Holmes et al., 2020). Beyond increased the increased productivity, clam gardens created enhanced systems that promoted biodiversity of other marine species and mammals (Deur et al., 2015). Enhanced Indigenous research in the future could indicate if this overall ecosystem enhancement was the overall goal of these innovations. Recent research on clam gardens in British Columbia also showed that the unique clam garden design provided increased climate resilience by buffering water temperatures and carbonate fluctuations. Traditional practices of returning clam shells to the beach helped buffer against acidic coastal waters from upwellings (Lepofsky et al., 2015).
Europe−2,000 Years+
Romans in the southern part of the Empire preferred sea fish (Varro 116-27 B.C., 1912). Freshwater ponds were considered inferior and “plebeian” (Balon, 1995). Romans in the south grew oysters on artificial structures (Balon, 1995). Fish storage in brackish waters dates to Medieval times when lagoons and coastal ponds were first established to retain fish swept in by the tides including seabass (Dicentrarchus labrax), seabreams (Sparidae), and mullets (Mugillidae; Stead, 2019). The “vallicoltura” coastal aquaculture system was practiced widely on the Adriatic and Tyrrhenian coasts by the Etruscans (Beveridge and Little, 2002). Cicero's chef, Sergius Orata, built saltwater ponds and stored fish, a practice that may have originated in Agrigentum, Sicily (Zeuner, 1963).
The natal region of Cyprinus carpio carpio is the Black Sea drainages of the Balkan Peninsula in southeastern Europe including the Danube River below Pannonia, an ancient province of Rome bounded on the north and east by the Danube River (Hoffmann, 1994, 2005; Balon, 1995). Wild carp fisheries were practiced in prehistoric times by the many tribes living in this region (Hoffmann, 2010). Wild populations of common carp were known to the Romans living in riparian settlements along the floodplains of the Danube River which contained the Roman provinces of Camuntum, Peiso Piso, Gerulata, Brigetio, Celamantia where large populations of spawning common carp aggregated every spring (Balon, 1995). Roman population growth, attempts by political authorities to assert power and the evolution of state control, and wars played varying roles in the distribution of carp out of this natal region northwest into Europe.
Common carp (Cyprinus carpio carpio) aquaculture was well-developed in Roman lands from at least 100 B.C. to 500 A.D. then evolved after the collapse of the Roman Empire and the establishment of Christianity into carp aquaculture in monastery ponds (Beveridge and Little, 2002). Thereafter aquaculture of common carp in ponds spread widely into northwestern Europe in late Medieval to Early Modern times.
Balon (1995) attributes the wider distribution of common carp to Roman military advances and a mass migration of people north to the Danube River where they confronted the “formidable forces of Celts and Germans on the opposite shores.” Sitwell (1981 in Balon, 1995) state, “In the second century, the comparatively short stretch of river between Vienna and Budapest about 240 km (150 miles) long required no less than four legions to guard it. By contrast all Roman Britain in the second century required only three legions. Roman North Africa possessions managed with a single one.” Balon (1995) estimates 20,000 fighters were joined by wives, mistresses, children, slaves, and tradesmen to total 100,000 people along this short stretch of river. Fortresses and Roman towns were established. These people needed food and carp were abundant and easy to obtain protein resource in an area that bordered the largest floodplain (the Piedmont zone) of the Danube River, the westernmost spawning grounds of common carp (Balon, 1995).
Large road networks were constructed by the Romans at this time. As people gained a taste for carp they transported the fish. States Balon (1995), “The earliest record is by the secretary to king Theodorus (475–526 A.D.) of Ravenna, Cassiodorus (490–585 A.D.) who was ordered to transport carp from the Danube to Italy.” Live fish transport evolved as common carp can survive wide aquatic environmental conditions that most fish cannot especially adverse temperatures, oxygen, water qualities, and starvation in small containers (Balon, 1974). Zeuner (1963) quotes Pennant in a 1776 article in British Zoology who stated the remarkable observation that common carp placed in a “net well-wrapped in wet moss and hung up in a cellar will remain alive, providing the moss is kept wet.”
The demand for fish increased dramatically in Europe as Christianity became dominant in the 5th and 6th centuries and taboos on eating terrestrial “flesh” were enforced. The only meats that could be eaten on fasting days were cold-blooded animals such as fish, crustaceans, and shellfish. People were allowed to substitute fish for meat for about 130 days (35%) of the year. Medical advice promoted eating freshwater over salted fish (Hoffmann, 2005). Punishment for violations sometimes included the death penalty.
Transport evolved but was not enough to keep settlements and monasteries with adequate supplies of fish as disruptions occurred due to conflicts, weather, etc. which made fishing difficult or impossible. Monks, nuns, and priests who had to follow fasting regulations obediently had difficulty finding fish (Leonhardt, 1906). Storage of fish evolved from these scarcities. Artificial ponds and reservoirs were created called “piscinae” that became popular with Roman elites and monasteries. Charlemagne (768–814 A.D.) the first Holy Roman Emperor was known to store fish and maintain ponds. Some elites spent fortunes on their piscinae. Balon (1995) states that “Consul Lucullus (75 B.C.), whose reputation as a gourmet is well-known, dug through a hill near Naples to bring water to his ponds, which were reputedly more costly than his villa.”
The first monasteries were founded in the early 6th century (Monte Cassino monastery in 529 A.D.). Common carp aquaculture grew as monasteries spread northwest and south into Europe from these Roman roots (Dubravius, 1547 in Balon, 1995; Beveridge and Little, 2002). As monasteries gained land and farms during Medieval and Late Medieval times aquaculture pond designs became more sophisticated (Hoffmann and Winiwarter, 2010; Figure 2).
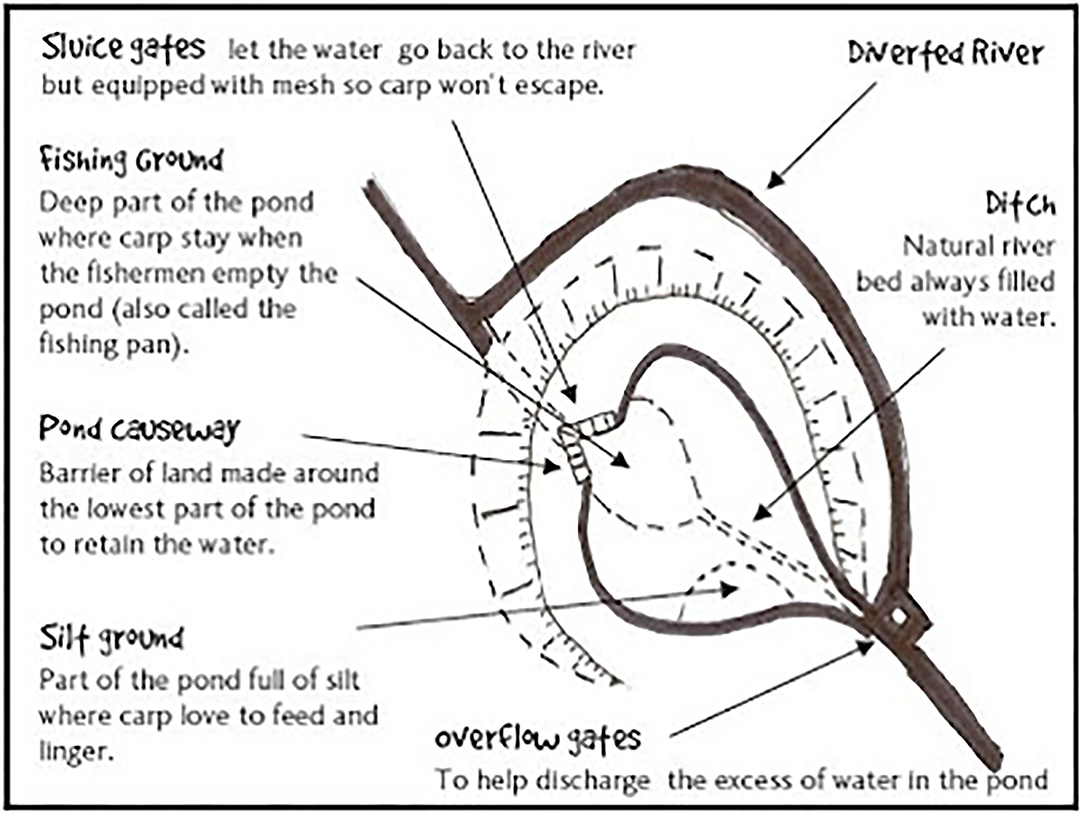
Figure 2. Sophisticated aquaculture engineering of a monastery pond in Medieval Europe reconstructed from archeological investigations. Taken from Moisand (2008).
Hoffmann (1994, 2005) believe domestication of common carp was obtained in Roman times as spawning was reported to occur in Roman piscinae and also in monastery ponds. Aquaculture flourished in monasteries as the domination of Christianity grew in Europe, and centers of aquaculture expertise became well-known (Figure 3). Aquaculture expanded beyond northwestern Europe to the British Isles and Scandinavia (Bonow et al., 2016) (Figure 4). European pond aquaculture expanded in early modern times into the English colonies of North America as the rapid depletion of anadromous fish populations occurred there (Robert, 2008).
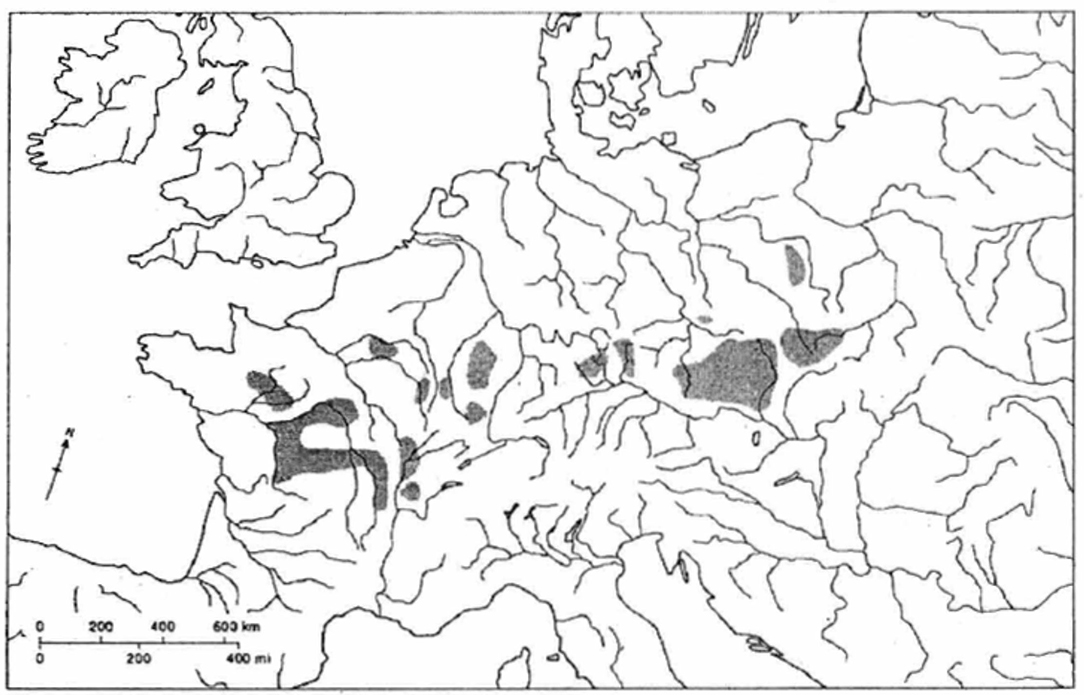
Figure 3. Major regions of carp aquaculture by the end of the Medieval. From Hoffmann (2002) and Hoffmann and Winiwarter (2010). Reproduced with permission from the author (R. C. Hoffmann).
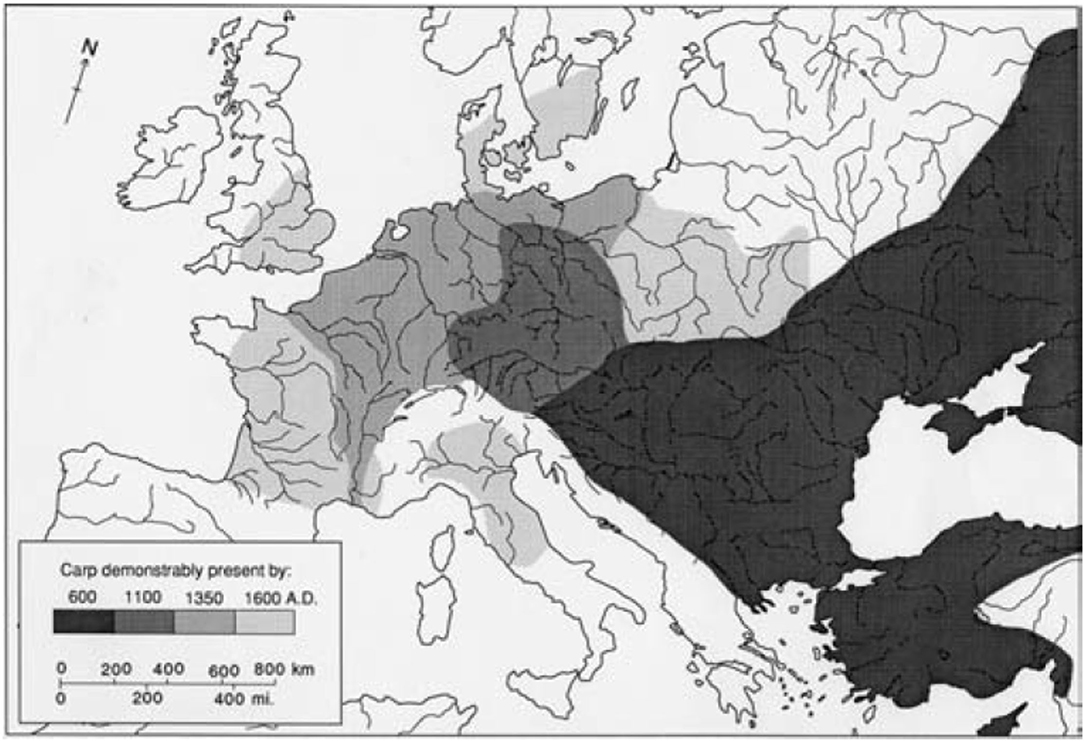
Figure 4. Expansion of carp aquaculture over 1,000 years from natal river regions of southeastern Europe northwest to reach the British Isles and Scandinavia. From Hoffmann (2005). Reproduced with permission from the author (R. C. Hoffmann).
South America−2,000 Years+
“Before it became the New World, the Western Hemisphere was vastly more populous and sophisticated than has been thought—an altogether more salubrious place to live at the time than, say, Europe.” Mann (2005).
The agricultural technology called “chinampas” was well-known throughout ancient Latin and Northern South America in antiquity (Figure 5). “Chinampa” is a Nahuatl word spoken since ancient times by the Aztecs and translates roughly to “net of branches”. Chinampas have been thought to have developed in the Valley of Mexico in and around the Texcoco and Xochimilco lakes (Coe, 1964). Chinampas were built by cutting trees and piling up tree branches and debris in mounds, then heaping nutrient rich sediments dug from canals, causeways, lakes and wetlands onto the tree debris to make raised agricultural beds about 100–200 m long and 5–20 m wide. Chinampas were designed to be surrounded by excavated canals. Water containing nutrients would be drawn up into the agriculture beds by a dense network of crop roots (manioc, beans, squash, and sweet potatoes) and agroforestry species (palm, nut, and fruit trees). Nutrient rich organic muds would be taken regularly from canals and ponds and piled on top to maintain structural integrity and fertility (Aghajanian, 2007). Large compost pits of dark earth have been found indicating intensive crop production on the raised beds (Carneiro, 1984; Glaser and Woods, 2004).
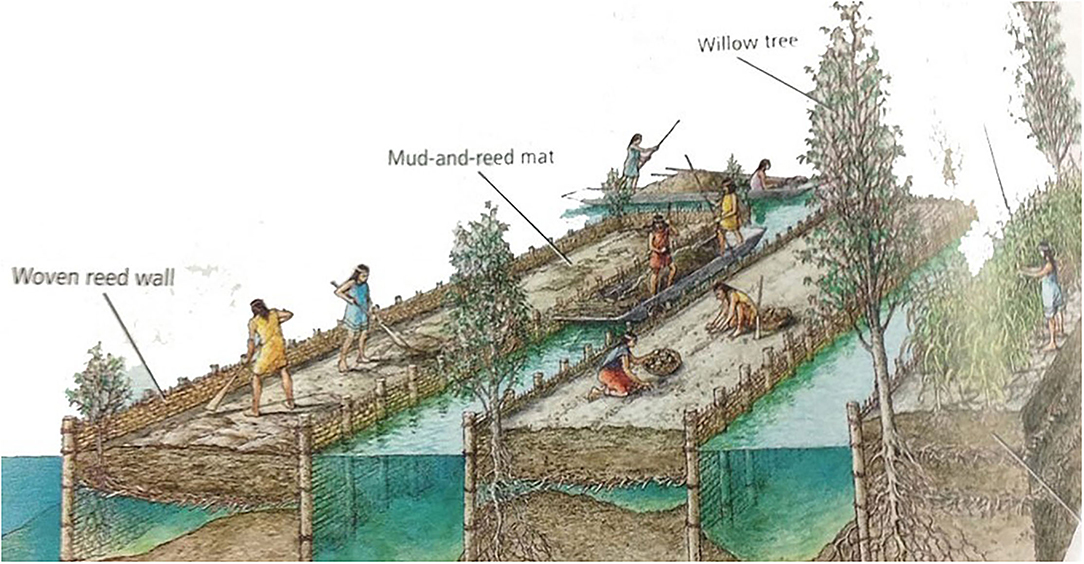
Figure 5. An artist's conceptual illustration of chinampas, a wetland agriculture-aquaculture system that was common from the valley of Mexico City to the western Amazon regions of South America. Development of the systems stretch back as far as 6,000 years B.P. From Aghajanian (2007).
Erickson (2000, 2001) and Heckenberger et al. (2008) reported that 2,000 years B.P. extensive settlements were present in the western Amazon region of Bolivia (Pando, Beni) and Brazil (Acre, Rondônia) and that these cultures were sustained by a vast network of chinampas. The western Amazon region is a vast area of low-lying savannahs that for most of the year are dry and water scarce. During the rainy season however, floodwaters cover much of the low-lying lands turning the ecosystem into shallow wetlands and ponds. Erickson (2000, 2001) studied the Baure in Bolivia where Indigenous nations developed and managed sophisticated wetland/pond/canal aquaculture systems that covered at least 525 km2 (Figure 6). These integrated agriculture-aquaculture ecosystems likely extended into what is today tropical forests of the western Amazon all the way to the Llanos regions of modern Columbia and Venezuela. Roosevelt et al. (1996) found such systems throughout the “várzea” as the Amazonian floodplain is known.

Figure 6. An aerial LIDAR image of a portion of the Beni region of Bolivia that reveals the extent of the chinampas (raised agricultural fields) and the vast irrigation canals that today is hidden by a dense forest canopy. The Beni is about 78,000 km2 of raised agricultural fields (chinampas) integrated with fishponds/canals (Erickson, 2001; Mann, 2005, 2008; Heckenberger et al., 2008). Photograph by Clark Erickson, University of Pennsylvania. Reproduced with permission from the author (Clark Erickson).
Mann (2008) stated that “sizable, regionally organized populations” extended across this vast region of South America. Research by Heckenberger et al. (2008) showed a distribution of population centers across about 20,000 km2 having an estimated a pre-colonial population of 50,000. Debates about population sizes have fueled decades of anthropological debates that have called the “Amazon archaeology wars” (Erickson, 1994, 2000). Research has questioned if the dense Amazonian forests, vast savannahs and wetlands of the western Amazon are primordial or are secondary growth ecosystems modified extensively by anthropogenic agriculture-fishing and aquaculture before European colonialism. Are the modern landscapes, ecological forms and functions of this area much more recent and exist now as secondary growth that occurred only after the massive depopulation of these regions 400–500 years B.P. due to European colonialism and diseases?
Western Amazonian settlements were not cities in the modern context but were “highly self-organized anthropogenic landscape(s) of late prehistoric towns, villages, and hamlets, with well-planned road networks” (Heckenberger et al., 2008). Permanent plazas were political ritual centers. Heckenberger et al. (2008) theorized that ancient civilizations “in broadly forested regions, such as temperate Europe, eastern North America, and the Amazon basin, are generally more dispersed and less centralized than classical (oasis) civilizations in Egypt, Mesopotamia, and Indus River areas or, in the South American case, coastal desert or arid highland river valleys.” Erickson (cited in Mann, 2008) stated these cultures were radically different than the Aztecs, Incas, and Mayas as they transformed permanently regional ecosystems, creating “a richly patterned and humanized landscape” that is “one of the most remarkable human achievements on the continent.”
Fish traps and weirs were present throughout the ancient world. Aquaculture likely arose from capture and holding wild fish and other aquatic species. There is extensive archeological and anthropological evidence that the interactive land-water farming systems of the western Amazon were more than a floodplain trap fishery. They were capture-based aquaculture systems that produced tremendous amounts of aquatic proteins for thousands of people (Erickson, 2000, 2001; Erickson and Brinkmeier, 2007; Blatrix et al., 2018). Blatrix et al. (2018) studied the earthwork systems in Bolivia and compared them to floodplain fisheries in Africa. In contrast to Africa, Blatrix et al. (2018) found large, designed ponds and canal diversions that served not only as traps but engineered aquaculture systems that could store fish alive for long periods of time. They stated that while both “weir-fishing and pond-fishing are both practiced in African floodplains today…in combining the two, this pre-Columbian system appears unique in the world.” Erickson (2000) estimates that 100,000–400,000 fish/ha in the canals and yields of 1 metric ton/ha/year for shallow ponds in these seasonal systems. He also points out that large middens of edible snails (Pomacea gigas) have been found which likely were managed/cultured in the canals and ponds. These snails are prolific, growing rapidly at high densities and could have produced hundreds of tons of additional aquatic foods. Erickson (2000) cite Swing et al. (1987) who found Pomacea gigas at a density of about 24/m3 in Bolivian wetlands. Snail middens have been found throughout the Beni and nearby regions of Bolivia and Brazil. Erickson (2000, 2001) calls the wetland complex of Baures in Bolivia “a form of intensive aquaculture” as chinampas, settlement mounds, raised causeways and extensive wetland management, with canals, dams, ponds, and fish weirs and traps were found throughout the landscape. It is unknown if domestication of aquatic species occurred. Erickson states, “Rather than domesticate the species that they exploited, the people of Baure domesticated the landscape.”
Hawaii−700 Years+
“The whole distance to the village of Whyeete is taken up with innumerable artificial fishponds extending a mile inland from shore, in these the fish taken by nets in the sea are put, and though most of the ponds are fresh water, yet the fish seem to thrive and fatten… The ponds are several 100 in number and are the resort of ducks and other water fowl.” T. Bloxam, British naturalist on H. M. S. Blonde, describing Waikiki in 1825 (Handy and Handy, 1972).
The ancient aquaculture systems of Hawai'i are unique in that they connect an isolated island society with sophisticated ocean harvesting and integrated marine aquaculture to an entire watershed management/food production system (the ahupua'a; Costa-Pierce, 1987; Gon and Winter, 2019; Winter et al., 2020; Figure 7). Hawaiian marine aquaculture systems are remarkable in terms of their diversity, distinctive management, and extent of development, especially given the small size of Hawai'i. Although the Hawaiian systems are relatively recent (about 1,500–2,000 years old) in comparison to others reviewed here, the evolution of ocean fishing to trapping/storage and onwards to aquaculture farming systems parallels the evolution of aquaculture in Indigenous societies worldwide.
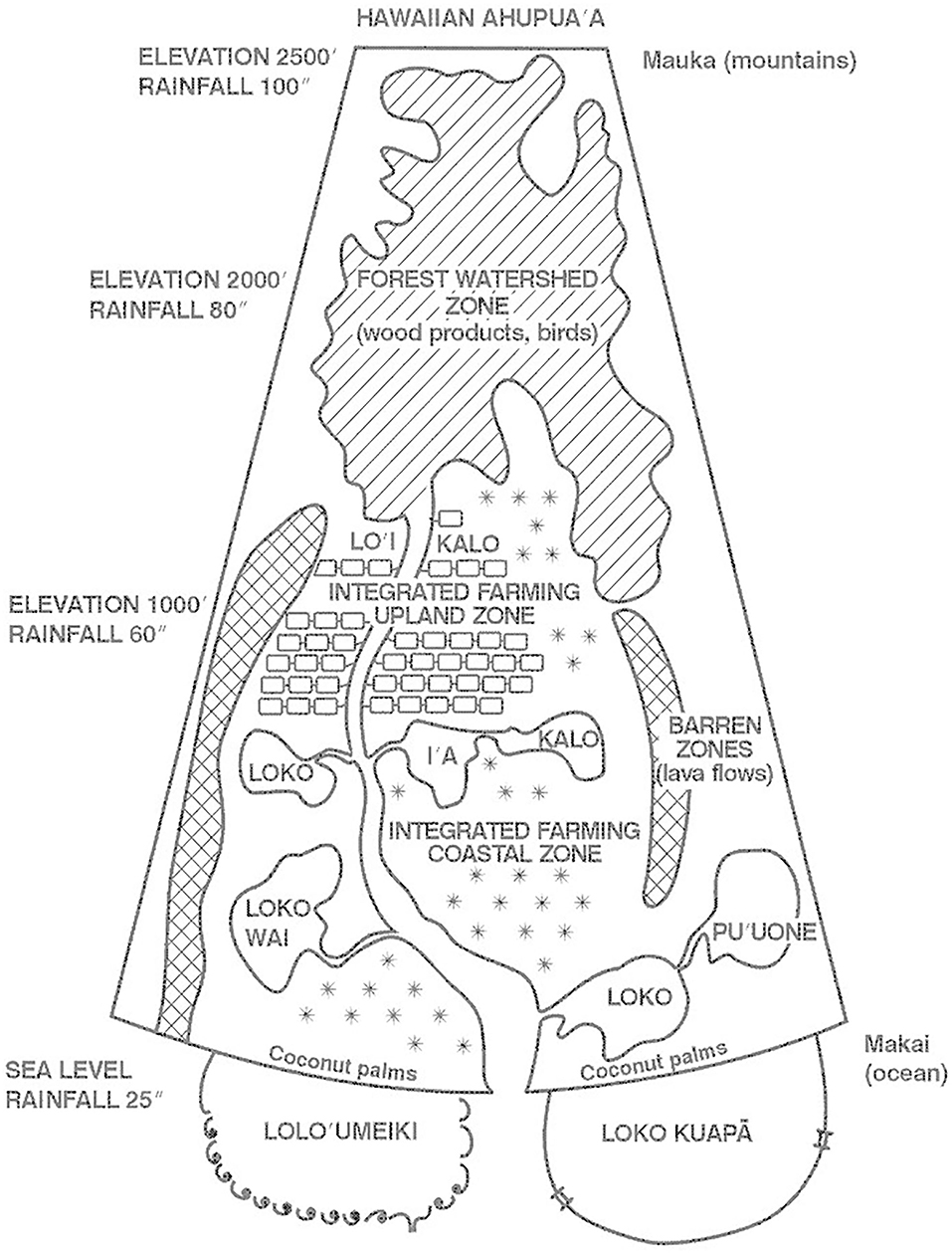
Figure 7. The ahupua'a aquaculture ecosystems stretched from inland highland integrated taro-fishponds, to lakes, reservoirs and ponds in the wetland lowlands to sophisticated marine aquaculture systems built out into nearshore oceans. They sustained a high population density of Hawaiians until European contact (Kikuchi, 1973, 1976; Costa-Pierce, 1987; Gon and Winter, 2019; Winter et al., 2020). Reproduced with permission from the author (B.A. Costa-Pierce).
There is evidence that Hawaiian fisheries had sustaining yields of more than 12 MT/km2 (120 kg/ha) prior to the arrival of Europeans (McClenachan and Kittinger, 2012). Fisheries were characterized by adaptive management whose design had a unique royal control and enforcement of marine common properties. Hawaiians had strict regulations on marine fisheries as the documented high population density in antiquity added pressure on marine fishery ecosystems (Summers, 1964; Kamakau, 1976). Kaiser and Roumasset (2014) discussed the tumultuous social-ecological transitions from the ancient Hawaiian konohiki management/regulatory system of the ahupua'a ecosystems that occurred when taken over into the United States.
Other Ancient Centers of Aquaculture
There are other notable centers of aquaculture in antiquity that deserve mention and further research. Integrated agriculture/aquaculture systems were present in Cambodia 1,000 years B.P. Cage culture of fish may have developed first in Cambodia then spread to Indonesia (Beveridge, 1996). Freshwater integrated aquaculture in Cambodia was likely influenced to a large extent by connections to China (Edwards et al., 1997). Milkfish aquaculture in coastal “tambaks” in Indonesia dates from 1,200 to 1,400 AD (Schuster, 1952).
Indigenous Nations Moving Forward
There is a new and growing recognition of the historical and cultural significance of Indigenous aquaculture worldwide by international and non-governmental organizations, scientists, and most importantly, by Indigenous nations themselves. For Indigenous peoples their ancient advances, as exemplified by their past successes, can be a source of pride and help ameliorate the cultural damage of colonialization. The wisdom and spiritual connections to their aquaculture ancestors and their network connections throughout the world to other Indigenous aquaculture communities is being incorporated into education programs and lessons for future generations.
Recovery of the traditional knowledge and wisdom of Indigenous people who designed and managed aquaculture ecosystems is important not only culturally and spiritually to them but also for the world as they demonstrate alternative, community-based models of applied, ecological aquaculture leadership, knowledge, and science. Indeed, these communities are ancestors of all global aquaculture practitioners alive today and into the future. The Indigenous knowledge systems of aquaculture are part of not only their birthrights but of all humanity (Ogar et al., 2020).
Recovery of the past wisdom of aquaculture through partnerships globally between Indigenous communities and anthropologists/archeologists needs to be prioritized (e.g., the “applied archaeology” of Erickson, 1994). McNiven (2003) emphasized that investigations of the spiritual complexity of Indigenous “saltwater people” in Australia assisted in explaining enigmatic marine stone arrangements found in Queensland. There is recent recognition that the Indigenous aquaculture wisdom of the past needs not only to be preserved and celebrated but also can lead development of an alternative future for aquaculture globally.
FAO (2019) designated the mulberry-silkworm-fishpond and rice-fish aquaculture ecosystems as Globally Important Agricultural Heritage Systems (GIAHS; Figure 8). In Victoria, southwest Australia, the short-finned eel aquaculture ecosystem of the Gunditjmara people was one of the first places included on Australia's National Heritage List in 2004. The site was then designated as a UNESCO World Heritage Site, The Budj Bim National Heritage Landscape (Builth, 2014; McNiven, 2017). In 2005 another site, the Brewarrina homeland of the Barkindji people, was designated as the Brewarrina Aboriginal Fish Traps National Heritage Place (Australian Government, 2021). At this site fish traps, ponds and weirs stretch some 0.5 km along the Darling (Baaka, Barka) river in New South Wales. These Australian sites are much more than museums of the past but continue as examples of ecological aquaculture and the FAO ecosystem approach to aquaculture as the Gunditjmara continue their ancestral aquatic management to today (McNiven and Bell, 2010). The Gunditj Mirring Traditional Owners Aboriginal Corporation was “established to continue our connection to Gunditjmara country and to progress our rights and interests in our cultural identity, social justice, native title, cultural heritage and land justice for our Gunditjmara country” (Gunditjmirrig Traditional Owners Aboriginal Corporation, 2015).

Figure 8. The Zhejiang Huzhou Mulberry-Silkworm-Fish Pond Aquaculture Ecosystem in China is estimated to be more than 2,500 years old existing to today. It has been designated by the FAO (2019) as a “Globally Important Agricultural Heritage System.” Photo taken from: http://www.xinhuanet.com.
There are larger opportunities. Community-based, hyper-local aquaculture ecosystems serve to demonstrate new aquatic food production systems rooted in the ancient past but that continue to evolve today. These systems can ameliorate climate change and help preserve the Earth's remaining biodiversity. An estimated 80% of the world's remaining biodiversity is located in the Indigenous nations worldwide (Sobrevila, 2008). Indigenous communities can not only reclaim their past wisdom but also advance an alternative path to intensive, industrial aquaculture plus lead locally and globally the ecosystem approach to aquaculture advanced by the FAO (2010). A transformation of food production systems is needed to meet the challenges of simultaneously adhering to the planetary dimensions, food security and advancing human health and wellness (Bengoa, 2001; Gordon et al., 2017; Chang et al., 2018; Willett et al., 2019; Kuempel et al., 2021).
Indigenous cultures of Bolivian Amazon were once densely populated in well-organized settlements. They designed, developed and managed a savannah ecosystem experiencing seasonal floods and seasonal droughts. The land had poor soils and lacked drainage. Using ecological knowledge they nurtured an integrated, interactive land-water farming ecosystem combining chinampas agriculture, fisheries and aquaculture to support their societies. States Erickson (1994), “The ancient inhabitants of the area created an agricultural landscape to solve these problems and make the area highly productive.” Realizing the productive potential this integrated farming ecosystem and its applicability to many areas with similar conditions throughout the lowland tropics as an alternative to cutting down the rainforest, participatory research and community-based programs over the last 20 years have been developed. Erickson (1994) called these “applied archaeology” and joined with partners from international to Indigenous (Inter-American Foundation, the Parroquia of San Ignacio, the Bolivian Institute of Archaeology, Biological Station of the Department of the Beni, and the University of Pennsylvania Museum of Anthropology and Archaeology) to reconstruct the ecosystem designs for the ancient chinampas/integrated aquaculture systems of their ancestors.
Erickson and Brinkmeier (2007) describes work with the Community of Bermeo, Department of the Beni, Bolivia with Indigenous families. Design and reconstruction works were based on traditional knowledge and participatory research informed by archaeological excavations and mapping. The reconstructed modern systems have produced impressive harvests and success spread to the communities of Bermeo and Villa Esperanza who donated lands, with the Inter-American Foundation providing funds to pay community members a daily wage to build and maintain the systems. Community members recorded data to document if production can be sustainable. Results to date are that over a long period of continuous cropping high yields can be maintained and that the systems are labor-efficient with little maintenance necessary to keep them at high production (Erickson and Brinkmeier, 2007).
The Hawaiian fishpond/ahupua'a ecosystem restoration movement involves many segments of society to recover and advance Indigenous knowledge in aquaculture. Alternative models for local sustainability are being led by Kua‘aina Ulu ‘Auamo, the Edith Kanakaole Foundation, Kamehameha Schools, among others. In Washington and Alaska, USA, the Swinomish nation is recovering its ancient Indigenous aquaculture knowledge to implement its first modern-day clam garden as part of their comprehensive plan to strengthen solutions through Indigenous knowledge for food, climate, cultural, and environmental benefits (Morrison, 2020). Members of the Eyak Athabaskan Alaska Indigenous community are developing seaweed aquaculture as an alternative/supplement to the herring fishery in the Copper River Delta. In Australia, the North Australian Indigenous Land and Sea Management Alliance Ltd. has been created. In New Zealand, nearly 21,000 ha have been set aside for aquaculture by the Māori community.
Internationally, at the 2020 IUCN World Conservation Congress in Marseille, France, the Global Indigenous Network for Aquaculture (GINA) was established (Global Indigenous Network for Aquaculture, 2020). In 2019, the Pacific Indigenous Aquaculture Collaborative Hub created by Alaska, Hawai'i and Washington NOAA Sea Grant programs gathered over 150 participants from throughout the Pacific basin for an Indigenous aquaculture meeting in Hawai'i. One outcome was formation of the Pacific Sea Garden Collective (2022) which has developed a spectacular, interactive website of Indigenous fisheries and aquaculture innovations throughout the Pacific basin as “a collective of Indigenous knowledge holders, community practitioners, university researchers, and artists working together to foster learning about sea gardens drawing from traditional and scientific knowledge with the vision of supporting their resurgence as adaptive strategies today.”
Restoration aquaculture has been defined recently by The Nature Conservancy (2021) as occurring “when commercial or subsistence aquaculture provides direct ecological benefits to the environment, with the potential to generate net positive environmental outcomes.” There is growing evidence that Indigenous peoples practiced restoration aquaculture in ancient times. For example, the shellfish mariculture practices of the Kwakwaka'wakw people on the Northwest Coast of North America enhanced rocky reef habitats that increased the biodiversity of numerous species, including octopus, sea cucumber, whelks, chiton, and red turban snails, improving marine ecosystem biodiversity (Deur et al., 2015; Smith et al., 2019). Restoration aquaculture has the opportunity to recognize and embrace further the wisdom and technological advances of Indigenous peoples and support knowledge production to develop modern restorative practices that advance the environment together with equality, justice, social and cultural benefits, including greater community health and wellness.
Conclusion and Recommendations
Seven case studies of the cultural/environmental history of aquaculture were reviewed. Case studies covered an extensive part of human history in diverse parts of the world (China, Australia, Egypt, Europe, Latin/South America, Canada/USA, Hawai'i). Evidence was found that: (1) in all cultures aquatic foods were among the most important, traditional protein foods, (2) regular, long term and/or seasonal resource scarcities of wild aquatic or marine resources existed with documented resource regulations resulting and demands increasing, and (3) aquaculture development evolved from fishery knowledge, scarcities and demands to storage/banking of wild catches (Table 2). Evolution of sophisticated aquaculture farming systems may be a natural evolutionary part of societies whose population densities and increased resource demands exceeded the carrying capacities of wild aquatic ecosystems to support them. This review supports a structural theory of aquaculture anthropology that when the demands of aquatic/seafood-eating peoples exceeded available supplies from wild fisheries to provide for them, they developed aquaculture.
Two shortcomings of this review were: (1) is its singular authorship and (2) selected case studies reviewed here limited to those available in English. Singular authorship resulted in the unintended exclusion of ancient wisdom in traditional knowledge systems that exists in the oral traditions of Indigenous Nations worldwide not only in written documents as reviewed herein (Gewin, 2021). Reid et al. (2020) has pointed to more inclusive methods of “two-eyed seeing” that engage Indigenous peoples in a pro-active, participatory manner to lead transformations of the fisheries profession. As a review paper there will be rightful criticisms of both the content and also of this lack of inclusion. It is my sincere wish that this paper will lead to more active attention to the need for Indigenous aquaculture to be examined not only as part of our shared historical legacies but also globally on its own merits as a viable, alternative path for the future of aquaculture.
The anthropology and archeology of aquaculture in Indigenous societies worldwide is little developed outside of the case studies reviewed here in English. There are a very small number of scientists working together with Indigenous aquaculture societies. McNiven (2017) called for an “Indigenous archaeology” which he “described variously as a sea change, a quiet revolution, and paradigm shift.” This relates directly to another limitation of this study since only the available literature in English was used. Balon (1995) also pointed to this problem of bias when analyzing eastern European, Balkan, and Russian literature on aquaculture in antiquity. Such bias extends to other known ancient centers of Indigenous aquaculture and languages with a special note here of Mexico and Central America, Japan, Korea, Cambodia, Indonesia, Melanesia, and Micronesia. In addition, most of this review concerned “fish.” There was no extensive study of the ancient Indigenous aquaculture developments in molluscs and other invertebrates, seaweeds, or marine and aquatic plants which are known to be important parts of the culture of aquaculture throughout human history. Macroalgae serve as traditional foods in China, Japan, Korea, and Indonesia as well as other Asian countries and coastal communities in Europe, Canada, and the USA (Delaney et al., 2016). In Japan, scallop systems date to the Jumon period 14,000 years B.P. (Kosaka, 2016). It is apparent from these limitations that Western, colonial and Indigenous communities can use new ways of thinking to rediscover, reengage, and reclaim the sites, traditions, and forgotten dimensions of their aquaculture heritages.
Perino et al. (2019) give a feasible pathway for the restoration of complex ecosystems through a “rewilding” pathway that is feasible financially. Modern aquaculture concepts in “restoration aquaculture” (The Nature Conservancy, 2021) point a way for new communities of practice for community-based aquaculture informed by Indigenous anthropology and archaeology to heal and advance both colonially-impacted (Euro-American, Australian) and intact Indigenous Nations worldwide. More aquaculture alternatives to export-driven, large-scale upscaling are waiting to be found using livelihood approaches (St. Gelais et al., 2022) and making financial instruments available to Indigenous aquaculture communities for restoration, nutrient credits and trading, and community-conserved biodiversity hotspots (such as aquaculture in MPAs, Le Gouvello et al., 2022). These would allow aquaculture developers worldwide to support aquaculture development by traditional knowledge keepers in Indigenous Nations, help ameliorate climate and biodiversity crises, and reorient economies to more su stainable approaches. Tangible actions with Indigenous Nations such as participatory governance for “radical transformation” of aquaculture (Costa-Pierce, 2021) are required where respect and adherence to treaty rights and making long-term investments to build trusted relationships must go hand-in-hand with conservation and non-Indigenous entities working in aquaculture.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author Contributions
BC-P designed the study and wrote the manuscript.
Funding
BC-P received funding from the Fulbright Commission, Reykjavik, Iceland for a Fulbright Specialist Award at the University of Akureyri, Iceland; Ögmundur Knútsson and Rannveig Björnsdóttir served as outstanding hosts. Additional funding was received from the Ecological Aquaculture Foundation, LLC, the United States Department of Energy Macroalgae Research Inspiring Novel Energy Resources (MARINER) program award #DEAR0000917, and the University of New England (UNE).
Conflict of Interest
BC-P was employed by Ecological Aquaculture Foundation LLC.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This paper was written while on the territories of the Hawaiian Nation (Ho‘oulu Lāhui), the Wabanaki Confederacy, and the Sámi Nation. I acknowledge with gratitude the Hawaiian elders (Kapuna) and their families (Ohana) who taught me at a young age the true meaning and purpose of aquaculture, namely, that aquaculture was culture. Kupuna are expected to speak out and help make decisions on important issues not only for the family and the community but also for Nature. I have gratefully carried their wisdom with me throughout my 40+ years in aquaculture R&D globally. I acknowledge with great respect all of the Indigenous peoples and scientists who work with them as equals to recover the Indigenous wisdom and sophistication of aquaculture and advance it, as reviewed in this paper. The ancient sites and Indigenous roots of aquaculture are treasures and serve as an alternative models of integrated food production that can heal damaged societies and save this Planet. They serve as inspirations to the next generation of aquaculture pioneers. As Gon and Winter (2019) stated, they are a renaissance that could save the World. I would like to thank Clark Erickson of the University of Pennsylvania and Richard C. Hoffmann for their permissions to use their figures, to cite their works extensively in this paper, and for their scholarship and inspiration in the Indigenous anthropology and archeology of aquaculture. I acknowledge Kristina Snuttan Sundell of the Swedish Mariculture Research Center (SWEMARC) for her gracious hosting of a sabbatical at the Kristineberg Station, Sven Lovén Center for Marine Infrastructure, University of Gothenburg, Sweden where this manuscript was conceived and partially written. I would like to acknowledge University of the Arctic (UArctic) for their leadership in including Indigenous leaders in all of UArctic programming.
References
Aghajanian, A. (2007). Chinampas: Their Role in Aztec Empire-Building and Expansion. Los Angeles, CA: Indo-European Publishing.
Atlas, W. I., Ban, N. C., Moore, J. W., Tuohy, A. M., Greening, S., et al. (2020). Indigenous systems of management for culturally and ecologically resilient Pacific salmon (Oncorhynchus spp.) fisheries. BioSci. 2020:biaa144. doi: 10.1093/biosci/biaa144
Augustine, S., and Dearden, P. (2014). Changing paradigms in marine and coastal conservation: a case study of clam gardens in the Southern Gulf Islands, Canada. Canadian Geogr. 58:305–314. doi: 10.1111/cag.12084
Australian Government (2021). Brewarrina Aboriginal Fish Traps National Heritage Place. Available online at: https://www.awe.gov.au/parks-heritage/heritage/places/national/brewarrina (accessed December 23, 2021).
Balon, E. K. (1974). Domestication of the carp Cyprinus carpio L. Toronto, ON: Royal Ontario Mus. Life Sci. Misc. Pub. doi: 10.5962/bhl.title.60738
Balon, E. K. (1995). Origin and domestication of the wild carp, Cyprinus carpio: from Roman gourmets to he swimming flowers. Aquaculture 129, 3–48. doi: 10.1016/0044-8486(94)00227-F
Bengoa, J. M. (2001). Food transitions in the 20th−21st century. Pub. Health. Nut. 4.6a, 1425–1427. doi: 10.1079/PHN2001232
Berkes, F., and Folke, C. (1998). Linking Social and Ecological Systems: Management Practices and Social Mechanisms for Building Resilience. New York, NY: Cambridge University Press.
Beveridge, M. C. M., and Little, D. (2002). “The history of aquaculture in traditional societies,” in Ecological Aquaculture: The Evolution of the Blue Revolution, ed B.A. Costa-Pierce (Oxford: Blackwell Science), 3–29.
Blatrix, R., Roux, B., Béarez, P., Prestes-Carneiro, G., Amaya, M., et al. (2018). The unique functioning of a pre-Columbian Amazonian floodplain fishery Sci. Rpts. 8:59981 doi: 10.1038/s41598-018-24454-4
Boissoneault, L. (2019). The medieval practices that reshaped Europe's fish. More than 700 years ago, demand for sturgeon, salmon, and other fish was so high that kings had to start regulating fishing. The Atlantic. Available online at: https://www.theatlantic.com/science/archive/2019/05/medieval-people-were-already-ruining-fish/589837/ (accessed December 20, 2021).
Bonow, M., Olsén, H., and Svanberg, I. (2016). Historical Aquaculture in Northern Europe. Stockholm: Elanders. Available online at: https://www.diva-portal.org/smash/get/diva2:1051141/FULLTEXT01.pdf (accessed May 10, 2022).
Bray, F. (1984). “Biology and Biological Technology” in Part II: Agriculture. Science and Civilisation in China. Volume VI, ed J. Needham (Cambridge: Cambridge University Press).
Brewer, D. J., and Friedman, R. F. (1989). Fish and Fishing in Ancient Egypt. Warminster,: Aris & Phillips.
Builth, H (2014). Ancient Aboriginal Aquaculture Rediscovered. The Archaeology of an Australian Cultural Landscape. Moldova: LAP Lambert Academic Publishing.
Builth, H. (2006). “Gunditjmara environmental management: the development of a fisher-gatherer-hunter society in temperate Australia,” in Beyond Affluent Foragers, eds J. Kim, C. Grier, and J. Uchiyama (Oxford: Oxbow Books), 4–23. doi: 10.2307/j.ctt1w0df3b.5
Carneiro, R. (1984). “The cultivation of manioc among the Kuikuru Indians of the Upper Xingu,” in Adaptive Responses of Native Amazonians, eds R. Hames and W. Vickers (New York, NY: Academic Press), 65–111. doi: 10.1016/B978-0-12-321250-4.50007-5
Chang, X., et al. (2018). Understanding dietary and staple food transitions in China from multiple scales. PLoS ONE 13.4:e0195775. doi: 10.1371/journal.pone.0195775
Clarkson, C., et al. (2017). Human occupation of northern Australia by 65,000 years ago. Nature 547, 306–310. doi: 10.1038/nature22968
Coe, M. D. (1964). The chinampas of Mexico. Sci. Am. 211, 90–98. doi: 10.1038/scientificamerican0764-90
Costa-Pierce, B. A. (1987). Aquaculture in ancient Hawaii. BioSci. 37, 320–331. doi: 10.2307/1310688
Costa-Pierce, B. A. (2003). Use of ecosystems science in ecological aquaculture. Bull. Aquacul. Assoc. Canada 103–2, 32–40.
Costa-Pierce, B. A. (2010). Sustainable ecological aquaculture systems: the need for a new social contract for aquaculture development. Mar. Tech. Soc. Jor. 44, 1–25. doi: 10.4031/MTSJ.44.3.3
Costa-Pierce, B. A. (2021). Radical Aquaculture: transformational social-ecological systems that advance Sustainable Development Goals (SDGs). World Aqua. 52, 18–32.
Costa-Pierce, B. A., and Chopin, T. (2021). The hype, fantasies and realities of aquaculture development globally and in its new geographies. World Aqua. 52, 23–35.
Costa-Pierce, B. A., and Effendi, P. (1988). Sewage fish cages of Kota Cianjur. Naga. ICLARM Quarterly 11, 7–9.
Costello, C., Cao, L., Gelcich, S., Cisneros-Mata, M. Á., Free, C. M., et al. (2020). The future of food from the sea. Nature 588, 95–100. doi: 10.1038/s41586-020-2616-y
Coutts, P. J., et al. (1978). Aboriginal engineers of the western district, Victoria. Rec. Vic. Archaeol. Surv. 7, 1–47.
Davidson, I. (2013). Peopling the last new worlds: the first colonisation of Sahul and the Americas. Quatern. Int. 285, 1–29. doi: 10.1016/j.quaint.2012.09.023
Delaney, A., Frangoudes, K., and Ii, S. A. (2016). “Society and seaweed: understanding the past and present,” in Seaweed in Health and Disease Prevention, eds J. Fleurence and I. Levine (London: Academic Press), 7–40. doi: 10.1016/B978-0-12-802772-1.00002-6
Desroches-Noblecourt, C. (1954). Poissons, tabous et transfromations du mort. Nouvelles considerations sur les pelerinages aux villes saintes. Kemi 13, 33–42.
Deur, D., Dick Kwaxsistalla, A., Recalma-Clutesi, K., and Turner, N. J. (2015). Kwakwaka′wakw “clam gardens”: motive and agency in traditional northwest coast mariculture. Hum. Ecol. 43, 201–212. doi: 10.1007/s10745-015-9743-3
Edwards, P. (2009). “Traditional Asian aquaculture,” in New Technologies in Aquaculture, eds G. Burnell and G. Allen, pages 1029–1063. (Sawston: Woodhead Publishing). doi: 10.1533/9781845696474.6.1029 Available online at: http://hdl.handle.net/1834/18129
Edwards, P., Little, D. C., and Yakupitiyage, A. (1997). A comparison of traditional and modified inland artisanal aquaculture systems. Aquacul. Res. 28, 777–788. doi: 10.1111/j.1365-2109.1997.tb01002.x
Ehrlich, P. R., and Ehrlich, A. H. (1972). Population, Resources and Environment. San Francisco, CA: W. H. Freeman.
Erickson, C. L. (1994). “Raised fields for sustainable agriculture in the Bolivian Amazon,” in 1994 Festival of American Folklife: Culture and Development in Latin America and the Caribbean (Washington, DC: Smithsonian Institution). Available online at: https://www.sas.upenn.edu/~cerickso/applied3.html (accessed May 9, 2022).
Erickson, C. L. (2000). An artificial landscape-scale fishery in the Bolivian Amazon. Nature 408, 190–193. doi: 10.1038/35041555
Erickson, C. L. (2001). Precolumbian fish farming in the Amazon. Expedition 43:7. Available online at: https://www.academia.edu/45591057/Precolumbian_fish_farming_in_the_Amazon
Erickson, C. L., and Brinkmeier, D. (2007). Pre-Columbian fishermen of the Bolivian Amazon: Indigenous Technology and the Transformation of the South American Landscape. Chicago, IL: Harris Educational Loan Program; Field Museum of Natural History. (accessed May 9, 2022).
Erlandson, J. M., and Braje, T. J. (2015). Coasting out of Africa: the potential of mangrove forests and marine habitats to facilitate human coastal expansion via the Southern Dispersal Route. Quatern. Int. 382, 31–41. doi: 10.1016/j.quaint.2015.03.046
European Commission (2018). A Short History, Aquaculture. Available online at: https://ec.europa.eu/fisheries/cfp/aquaculture/aquaculture_methods/history_en (accessed November 29, 2021).
Fan Li. (1988) The Chinese Fish Culture Classic. Reprinted as Contribution 489. Solomons, MD: Chesapeake Biological Laboratory (473 B.C.).
FAO (2016). The State of World Fisheries and Aquaculture 2016: Contributing to Food Security and Nutrition for All. Rome, FAO.
FAO. (2019). GIAHS: Globally Important Agricultural Heritage Systems. Rome: FAO. Available online at: www.fao.org/giahs/giahsaroundtheworld/en/ (accessed May 2, 2022).
Finfgeld, D. (2003). Metasynthesis: the state of the art – so far. Qual. Health Res., 13, 893–904. doi: 10.1177/1049732303253462
Frankfort-Nachmias, C., Nachmias, D., and DeWaard, J. (2015). Research Methods in the Social Sciences. New York, NY: Worth Publishers.
Fujita, M., et al. (2016). Advanced maritime adaptation in the western Pacific coastal region extends back to 35,000–30,000 years before present. Proc. Natl. Acad. Sci. U. S. A. 113, 11184–11189. doi: 10.1073/pnas.1607857113
Gentry, R. R., Froehlich, H. E., Grimm, D., Kareiva, P., Parke, M., et al. (2017). Mapping the global potential for marine aquaculture. Nat. Ecol. Evol. 1:1317–1324. doi: 10.1038/s41559-017-0257-9
Gewin, V. (2021). How to include indigenous researchers and their knowledge. Nature 589, 315–317. doi: 10.1038/d41586-021-00022-1
Glaser, B., and Woods, W. (2004). Amazonian Dark Earths: Explorations in Space and Time. Berlin: Springer. doi: 10.1007/978-3-662-05683-7
Global Indigenous Network for Aquaculture (2020). International Union for the Conservation of Nature (IUCN) Declaration at the World Conservation Congress 2020. Available online at: https://www.iucncongress2020.org/motion/055 (accessed December 10, 2021).
Gon, I. I. I., S., and Winter, K. (2019). A Hawaiian renaissance that could save the World. Am. Sci. 107, 232–239. doi: 10.1511/2019.107.4.232
Gordon, L. J., Bignet, V., Crona, B., Henriksson, P., Van Holt, T., et al. (2017). Rewiring food systems to enhance human health and biosphere stewardship. Env. Res. Ltrs. 2017:100201. doi: 10.1088/1748-9326/aa81dc
Gray, J. P. (1998). Ethnographic atlas codebook. World Cultures 10, 86–136. https://web.econ.ku.dk/bentzen/Codebook4EthnoATlas.pdf
Groesbeck, A. S., Rowell, K., Lepofsky, D., and Salomon, A. K. (2014). Ancient clam gardens increased shellfish production: adaptive strategies from the past can inform food security today. PLoS ONE 9:e91235. doi: 10.1371/journal.pone.0091235
Gunditjmirrig Traditional Owners Aboriginal Corporation (2015). Sustainable Development of the Budj Bim Cultural Landscape. Availablde online at: https://www.gunditjmirring.com/budjbimsdp (accessed December 15, 2021).
Handy, E. S. C., and Handy, E. G. (1972). Native Planters in Old Hawaii. Honolulu: Bishop Museum Press.
Heckenberger, M. J., Russell, C., Fausto, C., Toney, J. R., Schmidt, M. J., et al. (2008). Pre-Columbian urbanism, anthropogenic landscapes, and the future of the Amazon. Science. 321:1159769. doi: 10.1126/science.1159769
Hoegh-Guldberg, O., Caldeira, K., Chopin, T., Gaines, S., Haugan, P., et al (2019). The Ocean as a Solution to Climate Change: Five Opportunities for Action. Washington DC: World Resources Institute, iv + 111. Available online at: http://www.oceanpanel.org/climate (accessed May 5, 2022).
Hoffmann, R. C. (1994). “Remains and verbal evidence of carp (Cyprinus carpio) in medieval Europe,” in Fish exploitation in the past. Proceedings of the 7th meeting of the I.C.A.Z. Fish Remains Working Group. ed W. Van Neer (Tervuren: Muse'e Royal de l'Afrique Central), 139–150.
Hoffmann, R. C. (1995a). An Environmental History of Medieval Europe. Cambridge: Cambridge University Press.
Hoffmann, R. C. (1995b). Environmental changes and culture of common carp in Medieval Europe. Guelph Ich. Rev. 3, 57–85.
Hoffmann, R. C. (1996). Economic development and aquatic ecosystems in Medieval Europe. The Am. Hist. Rev. 101, 631–669. doi: 10.2307/2169418
Hoffmann, R. C. (2002). “Carp, cods and connections: new fisheries in the medieval European economy and environment,” in Animals in Human Histories: The Mirror of Nature and Culture. ed M.J. Henninger-Voss (Rochester, NY: University of Rochester Press), 3–55.
Hoffmann, R. C. (2005). A brief history of aquatic resource use in medieval Europe. Helgol Mar Res. 59, 22–30. doi: 10.1007/s10152-004-0203-5
Hoffmann, R. C. (2010). “Elemental resources and aquatic ecosystems: medieval Europeans and their rivers,” in A History of Water. Series II Volume 2: Rivers and Society: From Early Civilizations to Modern Times. eds T. Tvedt and R. Coopey (London: I. B. Tauris), 165–202.
Hoffmann, R. C., and Winiwarter, V. (2010). Making land and water meet: the cycling of nutrients between fields and ponds in pre-modern Europe. Ag. History 84, 352–380. doi: 10.1215/00021482-84.3.352
Holmes, K., Cox, K., Cline, A. R., Hatch, M. B. A., Black, M. J., Salomon, A. K., et al. (2020). Ancient ecology: the Quadra Island clam gardens. Fisheries 45, 151–156. doi: 10.1002/fsh.10374
Horn, J., Rosenband, L., and Smith, M. (2010). Reconceptualizing the Industrial Revolution. Cambridge, MA: MIT Press. doi: 10.7551/mitpress/8585.001.0001
Hu, Y., Shang, H., Tong, H., Nehlich, O., Liu, W., et al. (2009). Stable isotope dietary analysis of the Tianyuan 1 early modern human. Proc. Nat. Acad. Sci. U. S. A. 106, 10971doi: 10.1073/pnas.0904826106
Jackley, J., Gardner, L., Djunaedi, A. F., and Salomon, A. K. (2016). Ancient clam gardens, traditional management portfolios, and the resilience of coupled human-ocean systems. Eco. Soc. 21:20. doi: 10.5751/ES-08747-210420
Kaifu, Y. (2015). Emergence and Diversity of Modern Human Behavior in Paleolithic Asia. College Station, TX: Texas A&M University, 345–361.
Kaifu, Y., Kuo, T.-H., Kubota, Y., and Jan, S. (2020). Palaeolithic voyage for invisible islands beyond the horizon. Sci. Rep. 10:19785 s41598-020-76831-7. doi: 10.1038/s41598-020-76831-7
Kaiser, B. A., and Roumasset, J. A. (2014). Transitional forces in a resource based economy: phases of economic and institutional development I Hawai'i. Re. Econ. Inst. 5:44. doi: 10.5202/rei.v5i2.118
Kikuchi, W. K. (1973). Hawaiian aquacultural systems. (Ph.D. Dissertation), University of Arizona, Tucson, AZ, United States.
Kikuchi, W. K. (1976). Prehistoric Hawaiian fishponds. Science 193, 295–299. doi: 10.1126/science.193.4250.295
Klinger, D. H., Turnipseed, M., Anderson, J. L., Asche, F., Crowder, L. B., et al. (2013). Moving beyond the fished or farmed dichotomy. Mar. Pol. 38, 369–374. doi: 10.1016/j.marpol.2012.06.015
Kosaka, Y. (2016). “Scallop fisheries and aquaculture in Japan,” in Scallops: Biology, Ecology, Aquaculture and Fisheries, eds S. E. Shumway and G. J. Parsons (Amsterdam: Elsevier), 891–936. doi: 10.1016/B978-0-444-62710-0.00021-3
Krause, G., Brugere, C., Diedrich, A., Ebeling, M. W., Ferse, S. C., et al. (2015). A revolution without people? Closing the people-policy gap in aquaculture development. Aquaculture 447, 44–55. doi: 10.1016/j.aquaculture.2015.02.009
Kuempel, C., Froehlich, H. E., and Halpern, B. S. (2021). An informed thought experiment exploring the potential for a paradigm shift in aquatic food production. Oc. Coast. Mgt. 206:105574. doi: 10.1016/j.ocecoaman.2021.105574
Le Gouvello, R., Brugere, C., and Simard, F. (2022). Aquaculture and Nature-based Solutions. Identifying Synergies Between Sustainable Development of Coastal Communities, Aquaculture, and Marine and Coastal Conservation. Gland: IUCN. doi: 10.2305/IUCN.CH.2022.02.en
Leonhardt, E. (1906). Der Karpfen. Geschichte, Naturgeschichteund wirtschaftlicheBedeutungunseres wichtigsten Zuchtfisches. (Neudamm: J. Neumann).
Lepofsky, D., Smith, N. F., Cardinal, N., Harper, J., Morris, J. M., et al. (2015). Ancient shellfish mariculture on the northwest coast of North America. Am. Antiq. 80, 236–259. doi: 10.7183/0002-7316.80.2.236
Li, K. M. (1992). “Rice –fish farming systems in China: past, present and future,” in Rice-Fish Research and Development in Asia, eds C. R. de la Cruz, C. Lightfoot, B. A. Costa Pierce, V. R. Carangal and M. P. Bimbao (Manila: ICLARM), 17–26.
Li, S. F. (1994). “Introduction: freshwater fish culture,” in Freshwater Fish Culture in China: Principles and Practice, eds S. Li and J. Mathias (Amsterdam: Elsevier), 1–25.
Lourandos, H. (1987). “Swamp managers of southwestern Victoria,” in Australians to 1788, eds D.J. Mulvaney and J.P. White (Fairfax, Syme and Weldon), 292–307.
Lovatelli, A., and Holthus, P. F. (2008). Capture-Based Aquaculture, Global Overview. FAO Fisheries Technical Paper. No. 508. Rome: FAO.
Mallory, W. H., Vinacke, W. H., and King-Hall, S. (1927). China: land of famine. J. Royal Inst. Int. Affairs 6, 185–187. doi: 10.2307/3014847
Mann, C. (2008). Ancient earthmovers of the Amazon. Science 321, 1148–1152. doi: 10.1126/science.321.5893.1148
Mann,. C. (2005). 1491 New Revelations of the Americas Before Columbus. New York, NY: Alfred A. Knopf.
McClenachan, L., and Kittinger, J. H. (2012). Multicentury trends and the sustainability of coral reef fisheries in Hawai‘i and Florida. Fish Fisheries 14, 239–255. doi: 10.1111/j.1467-2979.2012.00465.x
McNiven, I., et al. (2015). Phased redevelopment of an ancient Gunditjmara fish trap over the past 800 years: Muldoons Trap Complex, Lake Condah, southwestern Victoria. Aust. Archaeol. 81, 44–58. doi: 10.1080/03122417.2015.11682064
McNiven, I. (2017). Theoretical challenges of indigenous archeology: setting an agenda. Am. Antiq. 81, 27–41. doi: 10.7183/0002-7316.81.1.27
McNiven, I. J. (2003). Saltwater people: spiritscapes, maritime rituals and the archaeology of Australian indigenous seascapes. World Arch. 35, 329–349. doi: 10.1080/0043824042000185757
McNiven, I. J., and Bell, D. (2010). Fishers and farmers: historicising the Gunditjmara freshwater fishery, western Victoria. Trobe J. 85, 83–105. Available online at: https://www.academia.edu/42933833/Fishers_and_farmers_historicising_the_Gunditjmara_freshwater_fishery_western_Victoria
McNiven, I. J., Crouch, J., Richards, T., Dolby, N., Jacobsen, J., and Gunditj Mirring Traditional Owners Aboriginal Corporation (2012). Dating Aboriginal stone-walled fishtraps at Lake Condah, southeast Australia. J. Arch. Sci. 39:268e286. doi: 10.1016/j.jas.2011.09.007
Moisand, J. (2008). Old babbling carp – part 1. Available online at: http://carpiopedia.pbworks.com/w/page/15277489/Article%20Old%20Babbling%20Carp%20-%20Part%201 (accessed December 19, 2021).
Morrison, J. (2020). An Ancient People With a Modern Climate Plan. Washington Post. Available online at: https://www.washingtonpost.com/climate-solutions/2020/11/24/native-americans-climate-change-swinomish (accessed December 23, 2021).
Morrison, R. (2021). Canada's Underwater Wooden Stakes Mystery Is FINALLY Solved: Thousands of Sticks Off Vancouver Island Are Up to 1,300 Years Old and One of the Most Sophisticated Indigenous Fishing Traps Ever Found, Experts Say. Daily Mail Online. Available online at: https://www.dailymail.co.uk/sciencetech/article-10095591/Archaeology-Canadas-underwater-wooden-stakes-mystery-FINALLY-solved.html (accessed December_20, 2021).
Moss, M. L., and Wellman, H. P. (2017). The Magoun clam garden near Sitka, Alaska: Niche construction theory meets traditional ecological knowledge, but what about the risks of shellfish toxicity? Alaska J. Anthrop. 15, 7–24.
Murdock, G. P., and White, D. R. (1969). Standard cross-cultural sample. Ethnology 9, 329–369. doi: 10.2307/3772907
Nahuelhual, L., Defeo, O., Vergara, X., Blanco, G., Mar?n, S. L., and Bozeeda, F. (2019). Is there a blue transition underway? Fish Fish. 20, 584–595. doi: 10.1111/faf.12354
Nakajima, T., Hudson, M. J., Uchiyama, J., Makibayashi, K., and Juzhong Zhang, K. (2019). Common carp aquaculture in Neolithic China dates back 8,000 years. Nat. Ecol. Evol. 3, 1415–1418. doi: 10.1038/s41559-019-0974-3
Nash, C. E. (2011). The History of Aquaculture. Hoboken, NJ: Wiley-Blackwell. doi: 10.1002/9780470958971
Naylor, R. L., et al. (2021). A 20-year retrospective review of global aquaculture. Nature 591:551. doi: 10.1038/s41586-021-03308-6
Nelson, B. (2017). Elaborate stone structures in the Taiwanese ocean are actually ancient fish traps. Available online at: SEAFOODNEWS.COM (accessed November 29, 2021).
O'Connell, J. F., et al. (2018). When did Homo sapiens first reach Southeast Asia and Sahul? Proc. Natl. Acad. Sci. U. S. A. 115, 8482–8490. doi: 10.1073/pnas.1808385115
O'Connor, S., Ono, R., and Clarkson, C. (2011). Pelagic fishing at 42,000 years before the present and the maritime skills of modern humans. Science 334, 1117–1121. doi: 10.1126/science.1207703
Ogar, E., Pecl, G., and Mustonen. (2020). Science must embrace traditional and indigenous knowledge to solve our biodiversity crisis. One Earth 3, 162–165. doi: 10.1016/j.oneear.2020.07.006
Pacific Sea Garden Collective (2022). Sea Gardens Across the Pacific: Reawakening Ancestral Mariculture Innovations. Version 1. Washington Sea Grant at the University of Washington. Washington, DC: University of Washington.
Perino, A., Pereira, H. M., Navarro, L. M., Fernandez, N., Bullock, J. M., et al. (2019). Rewilding complex ecosystems. Science 364:eaav5570. doi: 10.1126/science.aav5570
Rana, K. J. (1998). “Recent developments in aquaculture statistics. Fishery and aquaculture statistics in Asia,” in Proceedings of the FAO/SEAFDEC Regional Workshop on Fishery Statistics. 19-21 August 1997. Vol. 11, 242–254. Rome: FAO.
Reid, A., Eckert, L., Lane, J. F., Young, N., Hinch, S., et al. (2020). Two-Eyed Seeing: an indigenous framework to transform fisheries research and management. Fish Fisheries 2020:faf.12516. doi: 10.1111/faf.12516
Robert, S. E. (2008). “Esteeme a Little of Fish:” fish, fishponds, and farming in eighteenth-Century New England and the Mid-Atlantic. Ag. History 82, 143–163. doi: 10.1215/00021482-82.2.143
Roosevelt, A. C., da Costa, M. L., Machado, C. L., Michab, M., Mercier, N., et al. (1996). Paleoindian cave dwellers in the Amazon: the peopling of the Americas. Science 272:373. doi: 10.1126/science.272.5260.373
Ruddle, K., and Zhong, G. (1988). Integrated Agriculture-Aquaculture in South China. The Dike-Pond System of the Zhujiang Delta. Cambridge: Cambridge University Press.
Schuster, W. H. (1952). Fish Culture in Brackish-Water Ponds of Java. Indo-Pacific Fisheries Council Special Publication No 1. Rome: FAO.
Selkirk, S. (2021). Tens of Thousands of Wooden Stakes Poking Up From British Columbia's Shoreline Have Smashed a Long-Held Stereotype of Canada's First Nation People. Available online at: https://www.bbc.com/travel/article/20211013-an-underwater-mystery-on-canadas-coast (accessed December 20, 2021).
Sharma, K. P., and Rana, R. (1986). “Seasonal ponds - a potential source of fish seed in Rajasthan, India,” in The First Asian Fisheries Forum, eds J. L Maclean, L. B. Diizon and L. V. Hosillos (Manila: Asian Fisheries Society), 87–88.
Sisma-Ventura, G. (2018). Tooth oxygen isotopes reveal Late Bronze Age origin of Mediterranean fish aquaculture and trade. Nat. Sci. Reps. 8:14086. doi: 10.1038/s41598-018-32468-1
Smith, N. F., Lepofsky, D., Toniello, G., Holmes, K., Wilson, L., et al. (2019). 3,500 years of shellfish mariculture on the Northwest Coast of North America. PLoS ONE 14:211194. doi: 10.1371/journal.pone.0211194
Sobrevila, C. (2008). The Role of Indigenous Peoples in Biodiversity Conservation: The Natural But Often Forgotten Partners, World Bank Working Paper 44300. Available online at: http://documents.worldbank.org/curated/en/995271468177530126/The-role-of~indigenous-peoples-in-biodiversity-conservation-the-natural-but-often-forgotten-partners (accessed May 10, 2022).
St. Gelais, A. T., Fredriksson, D. W., Dewhurst, T., Miller-Hope, Z. S., Costa-Pierce, B. A., and Johndrow, K. (2022). Engineering a low-cost kelp aquaculture system for community-scale seaweed farming at nearshore exposed sites via userfocused, social ecological design process. Front. Sus. Food Sys. 2022:848035. doi: 10.3389/fsufs.2022.848035
Stead, S. M. (2019). Using systems thinking and open innovation to strengthen aquaculture policy for the United Nations Sustainable Development Goals. J. Fish. Biol. 94, 837–844. doi: 10.1111/jfb.13970
Steneck, R. S., and Pauly, D. (2019). Fishing through the anthropocene. Cur. Biol. 29, R942–R995. doi: 10.1016/j.cub.2019.07.081
Swing, C. K., Isley, W., and Lutz, M. W. (1987). Notas sobre la ecologõia reproductiva del caracol. Pomacea gigas. Ecologõia en Bolivia 10, 19–31.
The Nature Conservancy (2021). Global Principles of Restorative Aquaculture. Arlington, VA: The Nature Conservancy. Available online at: https://www.nature.org/content/dam/tnc/nature/en/documents/TNC_PrinciplesofRestorativeAquaculture.pdf (accessed November 20, 2021).
Walford, C. (1878). The famines of the World: past and present. Royal Stat. Soc. 41, 433–526. doi: 10.2307/2338978
Waters, W., et al. (2016). The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351:aad2622. doi: 10.1126/science.aad2622
Willett, J., Rockström, B., Loken, M., Springmann, T., Lang, S., et al. (2019). Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet Commission 393, 447–492. doi: 10.1016/S0140-6736(18)31788-4
Williams, J. (2006). Clam Gardens: Aboriginal Mariculture on Canada's West Coast. Vancouver: New Star Books.
Winter, K. B., Lincoln, N. K., Berkes, F., Alegado, R. A., Kurashima, N., et al. (2020). Ecomimicry in indigenous resource management: optimizing ecosystem services to achieve resource abundance, with examples from Hawai?i. Ecol. Soc. 25:26. doi: 10.5751/ES-11539-250226
Wohlfarth, G. W. (1984). “Common carp,” in Evolution of Domesticated Animals, ed I. L. Mason (London: Longman), 375–380.
Wu, B. L. (1985). “Traditional management of coastal systems in China,” in The Traditional Knowledge and Management of Coastal Systems in Asia and the Pacific, eds K. Ruddle and R. E. Johannes (Jakarta: UNESCO), 181–190.
Yang, H. (1994). “Integrated fish farming,” in Freshwater Fish Culture in China: Principles and Practice, eds S. Li and J. Mathias (Amsterdam: Elsevier), 219–270.
Zajicek, P., Corbin, J., Belle, S., and Rheault, R. (2021). Refuting marine aquaculture myths, unfounded criticisms, and assumptions. Rev. Fish. Sci. Aquacul. 2021:1980767. doi: 10.1080/23308249.2021.1980767
Keywords: structuralism, Indigenous aquaculture, Indigenous anthropology, case studies, content analysis
Citation: Costa-Pierce BA (2022) The Anthropology of Aquaculture. Front. Sustain. Food Syst. 6:843743. doi: 10.3389/fsufs.2022.843743
Received: 26 December 2021; Accepted: 09 May 2022;
Published: 09 June 2022.
Edited by:
Carla Pinheiro, New University of Lisbon, PortugalReviewed by:
Brooks A. Kaiser, University of Southern Denmark, DenmarkHalley Elizabeth Froehlich, University of California, Santa Barbara, United States
Copyright © 2022 Costa-Pierce. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Barry Antonio Costa-Pierce, bcp@oceanfoods.org
 Barry Antonio Costa-Pierce1,2,3*
Barry Antonio Costa-Pierce1,2,3*