Phosphorus Nutrition and Growth of Cotton Plants Inoculated With Growth-Promoting Bacteria Under Low Phosphate Availability
- 1Colombian Corporation for Agricultural Research (AGROSAVIA)-Tibaitatá, Mosquera, Colombia
- 2Colombian Corporation for Agricultural Research (AGROSAVIA)-Nataima, El Espinal, Colombia
The low availability of phosphorus (P) in the soil drastically limits the world productivity of crops such as cotton. In order to contribute sustainably to the solution of this problem, the current study aimed to evaluate the capacity of phosphate-solubilising bacteria to improve plant growth and its relationship with physiological parameters, as well as the shoot P content in cotton plants in a soil with low P availability amended with rock phosphate. The results showed that, of the six plant growth-promoting bacteria strains evaluated under greenhouse conditions, the Rhizobium strain B02 significantly promoted growth, shoot P content and photosynthetic rate. This strain also improved the transpiration rate and the relative content of chlorophyll but without significant differences. Remarkably, Rhizobium sp. B02 had a more significant effect on plant growth compared to the P nutrition. Furthermore, the effect of its inoculation was more pronounced on the roots' growth compared to the shoot. Finally, application of Rhizobium strain B02 showed the capacity to optimize the use of low-solubility fertilizer as the rock phosphate. These findings could be associated with the metabolic activities of plant growth promotion exhibited by phosphate-solubilising strains, such as phosphate solubilisation, production of indole compounds and siderophores synthesis. In conclusion, this research provides evidence of the biotechnological potential of the Rhizobium genus as phosphate-solubilising bacteria with multiple plant growth-promoting activities capable of improving the plant growth and phosphate nutrition of non-leguminous crops such as cotton in soil with low P availability amended with rock phosphate.
Introduction
Phosphorus (P) is an essential element for various plant metabolic processes and contributes to the formation of cellular biomolecules such as adenosine triphosphate (ATP), nucleic acids and proteins (Heuer et al., 2017). The biological role of this macronutrient is significant, but its low availability (~0.1%) in the soil, estimated in 5.7 billion hectares of land worldwide, severely limits the yield of crops such as cotton, corn, rice, soybeans and wheat in tropical soils (Granada et al., 2018). In addition to this, P is derived from finite resources such as P-rich rock in the form of phosphate, which has been 83% exploited in its world reserves (Magallon-Servín et al., 2019). Despite this problem, regular applications of phosphate fertilizers are required to maintain crop yields; nevertheless, its application is increasing rapidly—around 2.5% per year under intensified agriculture due to the growing demand for food (Bashan et al., 2013).
Unlike many countries with agricultural potential, Colombia has not shown substantial progress in the management of nutrients like P from the soil to the plant nor in optimizing the efficiency of the use of fertilizers (Dhir, 2017). For example, cotton (Gossypium hirsutum L.) is a widely planted crop (~10,000 ha) in Colombia, mainly under soils of the order Vertisol and Inceptisol, but its production is being affected by the high costs of the excessive use of phosphate fertilizers (Martínez-Reina and Hernández, 2015). One of the causes associated with this problem is that soils where cotton has been planted in the last 20 years present excessive accumulation of P fixed to soil minerals (Vargas et al., 2019). Cotton is one of the most critical and sensitive fiber-producing crops to P, as this macronutrientit plays an indispensable role during the early developmental phase, production of biomass and final yield of the cotton crop (Amin et al., 2017). Specifically, P deficiency in cotton plants causes slow shoot development and flower buds, dark green leaves, flower bud necrosis, and yellowing of older leaves (Li et al., 2020). Therefore, new strategies to improve the efficiency of the use of phosphate fertilizers and reduce their application, while maintaining high cotton crop yields, have become one of the greatest challenges in Colombia.
As the efficiency of the use of P is composed of two major components, P uptake and utilization efficiency, some researchers have already reported that an effective strategy is the integration of the increase of the availability of P in the soil through microbial action and the improvement of genotypes with high-absorption efficiency of P (Mai et al., 2018; Iqbal et al., 2019). Currently, studies in plant growth-promoting bacteria (PGPB) have become a focus of soil science research for plant nutrition and agriculture (Rai et al., 2020). Within the PGPB, there is a bacterial group with the ability to affect the dynamics of phosphorus in the soil and increase its availability that is denominated as phosphate-solubilising bacteria (PSB), and the genera Pseudomonas, Bacillus, Azotobacter, and Rhizobium are the most reported with this metabolic capacity (Alori et al., 2017). Among those bacterial genera, the role of Rhizobium strains on P cycling in plant nutrition is the least explored. To provide P to plants, PSB alter the sorption balance of P in the soil and solubilises and mineralises P in unavailable inorganic and organic forms (Richardson and Simpson, 2011). In some studies, PSB have shown biofertilising potential, allowing the reduction of 33–75% of the dose of soluble phosphate fertilizers in different crops (Sahandi et al., 2019; Rosa et al., 2020). Likewise, PSB have also shown the ability to optimize the efficiency of low P solubility fertilizers such as rock phosphate and compost (Estrada-Bonilla et al., 2021). However, there are limited reports on the potential of PSB in cotton cultivation, since the major focus of development has been on nitrogen nutrition and phytostimulation (Pereg and McMillan, 2015; Diaz et al., 2019).
Curiously, several results of studies on PSB have shown discrepancies between the methodologies used and the results obtained in vitro and in vivo assays (Kishore et al., 2015; Pii et al., 2015; Granada et al., 2018). The main inconsistencies that have been found are focused on effective selection of PSB and the appropriate environmental conditions to evaluate if PSB contribute to plant nutrition and plant growth from the laboratory to the field. Two of the most common criteria proposed by various researchers (Collavino et al., 2010; Bashan et al., 2013) to evaluate and show the impact of PSB are as follows: (i) a high capacity to increase soluble P from sources with a very low degree of solubility, e.g., rock phosphate, hydroxyapatite, metal-phosphorous compounds and organic phosphate esters, associated with physicochemical parameters (organic matter and pH, among others) of the soil, and (ii) a plant growth-promoting effect by metabolic activities in soils deficient in available P. Based on these two criteria, as well as the P fertilization challenge in cotton crops and the scarce information about PSB inoculation in the same context, the objective of this research was to study how PSB inoculation influences plant growth, P nutrition and physiological parameters of cotton plants on soil with limited P available and amended with an insoluble source like rock phosphate.
Materials and Methods
Bacterial Strains and Preparation of Inoculants
In this study, we used the PGPB strains SP20, N8, N9, G56, G58, and B02, which were provided by the microorganism collection of Colombian Corporation for Agricultural Research (AGROSAVIA), Colombia. Strains G56, G58, and B02 were isolated from nodules of Vigna unguiculata (Mendoza and Bonilla, 2014), while strains N8 and N9 were isolated from the rhizosphere of silvopastoral systems composed of Pennisetum clandestinum, Plantago major, and Lolium perenne (unpublished data). Regarding SP20 strain, it has been reported to have various phenotypes required for colonization of plant root surface and endurance in the rhizosphere and was isolated from the cotton rhizosphere (Amaya-Gómez et al., 2020). These strains were selected because in previous experiments they proved capable of promoting the growth of cotton seedlings under in vitro conditions (Supplementary Figure 1). For the preparation of the inocula, the strains were grown on yeast-mannitol agar plates (Vincent, 1970) under standard conditions, i.e., 30°C for 24 h of incubation. Each bacterial inoculum (~109 CFU mL−1) was produced aerobically under standard conditions on a rotary shaker (150 rpm) in yeast-mannitol broth.
In vivo Screening of Plant Growth Promotion With Phosphorus-Deficient Soil
Greenhouse experiments were carried out at the research center Nataima of AGROSAVIA in Espinal, Tolima, Colombia (4°11′28.39″ N latitude and 74°57′38.69″ W longitude). The soil type used in the study is sieved airdried Inceptisol soil (Soil Science Division Staff, 2017) typical of the region where the cotton crop is cultivated. In pots, 1 kg of unsterile soil with low P content was placed (pH: 6.12, organic matter: 1.69 mg kg−1, P: 12.28 mg kg−1, effective cation exchange coefficient: 6.90 cmol kg−1, Ca: 4.79 cmol kg−1, Mg: 1.74 cmol kg−1, K: 0.23 cmol kg−1, Na: < 0.14 cmol kg−1). Four cotton seeds (Gossypium hirsutum L.) of the M-123 variety were sown per pot. A completely randomized experimental design was carried out with eight treatments, three replicates and two independent experiments.
The treatments were as follows: (i) complete fertilization without bacterial inoculation [urea, diammonium phosphate (DAP) and potassium chloride (KCl)], (ii) control treatment with fertilization using urea and KCl replacing DAP with rock phosphate (RP) (9 mg kg−1 P2O5 total) without bacterial inoculation, and (iii–viii) fertilization with urea, RP and KCl, and inoculation with each of the PGPB strains evaluated. Based on soil analysis and conventional fertilization rates recommended in the cotton crop [urea (75 kg ha−1), DAP (50 kg ha−1) and KCl (50 kg ha−1)], 5 mL pot−1 of the following concentrations were applied: 6.3 g L−1 of urea, 16 g L−1 of DAP, and 5 g L−1 of KCl. Regarding RP, 0.152 g pot−1 was applied, which is equivalent to the DAP doses. Inoculation and fertilization, according to the treatments, were carried out 8 and 13 days after sowing, respectively.
Inoculation was carried out by applying 5 mL of each inoculum or sterile YM broth (uninoculated treatment) to the rhizosphere. After 30 days at 18–38°C and ~55% humidity, the relative chlorophyll content measurement was carried out employing a SPAD-502 chlorophyll meter (Konica Minolta, Tokyo, Japan) when the plants had reached the main growth stage (the fifth leaf unfolded) (Munger et al., 1998). Likewise, gas exchange measurements were carried out as follows: net photosynthesis (Pn) and transpiration rate (E), between 09:00 and 11:00 h with the LI-6400 XT infrared gas analyser (Lincoln, NE. USA). The evaluation parameters were established at 400 μmol s−1 for the flow rate, with a concentration of 400 μmol of CO2 and a photonic flux density of 1,200 μmol of light photons m−2 s−1 (Chastain et al., 2016). Then, the length of the root and the shoot were measured. Plant tissues were oven-dried separately at 60°C for 48 h to measure their dry weights. Shoot tissues were ground to powder and wet oxidized in a solution of nitric acid and 30% hydrogen peroxide (4:2, v-v). Digests were analyzed for P concentration by the molybdenum blue method (Murphy and Riley, 1962).
Genome Sequencing
The total genomic DNA of the selected PGPB with the highest plant growth-promoting effect was extracted using the QIAamp DNA Mini Kit (Qiagen, Canada). The concentration of the DNA was measured using a Qubit Fluorometer (Thermofisher Scientific, United Stated) through the Quant-iT dsDNA HS Assay Kit following the manufacturer's instructions. Genome library preparation was done with the Nextera XT (Illumina) and the sequencing was conducted on a MiSeq (Illumina) with 250 bp pair end reads at the Microbial Genomics Laboratory of the Molecular Genetics and Antimicrobial Resistance Unit at Universidad El Bosque, Bogotá, Colombia. Read quality was evaluated using FastQC; low quality reads were removed using Trimmomatic v0.32. High quality reads were assembled into contigs using IDBA-UD. Taxonomic mapping was then performed using MyTaxa. From these results, evolutionary associations with nearby complete genomes stored in NCBI were inferred by calculating the Average Nucleotide Identity (ANI) value using FastANI (Santos-Torres et al., 2021).
Screening of Plant Growth-Promoting Activities
The in vitro characterization consisted of biochemical tests to determine if the selected strain exhibited plant growth-promoting traits related to the improvement of P nutrition. For the following assays, a bacterial suspension (~109 CFU mL−1) in sterile saline solution (0.89% w v−1), washed twice and centrifuged (5,220 xg for 10 min) from an inoculum produced under the standard conditions mentioned above, was used. First, the ability of the strains to increase the availability of P was evaluated in both P solubilisation and mineralisation. In solubilisation, two types of insoluble sources of P at 5 g L−1 were used: tricalcium phosphate and RP. For this, experiments were performed in 250 mL Erlenmeyer flasks filled with 75 mL of NBRIP medium supplemented with each P source (Nautiyal, 1999). Bacteria were inoculated by adding 7.5 mL aliquots of the bacterial suspension. The medium inoculated with sterile water was used as a control treatment. After the culture was incubated for 5 days for tricalcium phosphate and 12 days for RP at 30 ± 1°C at 200 rpm on a rotary shaker, the liquid medium was centrifuged at 16,000 xg for 10 min. The culture supernatant was then used to evaluate the P released into the solution using the molybdate blue colorimetric method (Fiske and Subbarow, 1925). The P quantities were calculated from a standard curve of soluble phosphate (KH2PO4). For mineralisation, qualitative detection of phytase production was carried out by growing the cultures (50 μL) from bacterial suspension in a solid NBRIP medium supplemented with 1% (w v−1) sodium phytic acid (C6H18O24 × NaH2O, Sigma-Aldrich, Germany) for 15 days, as described by Kerovuo et al. (1998).
As a second feature, the synthesis of indole compounds with and without the addition of tryptophan was estimated using the colorimetric assay based on the modified Salkowski reagent (Glickmann and Dessaux, 1995). Bacteria were inoculated by adding 7.5 mL aliquots of the bacterial suspension in 250 mL Erlenmeyer flasks filled with 75 mL of tryptic soy broth (TSB) supplemented with 100 mM of tryptophan. After the culture was incubated for 3 days at 200 rpm, the liquid medium was centrifuged at 16,000 xg for 10 min, and supernatants obtained were mixed with the Salkowski reagent in a 4:1 ratio for 20 min under dark conditions. Indole compounds were spectrophotometrically determined at 535 nm. Finally as third trait, the production of siderophores was qualitatively detected by the method of Schwyn and Neilands (1987). For this, 50 μL bacterial suspension was inoculated in plates containing agar Chrome Azurol S (CAS). These plates were incubated at 30°C for 5 days and observed for orange color formation around each colony. Each test was performed in a completely randomized design, with three replicates and two independent experiments.
Data Analysis
The data were subjected to one-way ANOVA and multivariate analysis of variance (MANOVA) to examine the significance of differences and variability at the 95% confidence level (p < 0.05), respectively, using the SPSS 22.0 programme (SPSS Inc., Chicago, IL, USA). Moreover, treatment means were compared by applying the Duncan's test. The correlation between the variables evaluated in in vivo screening was conducted through a principal component analysis (PCA) with a biplot using the software PAST 3.03 (Hammer et al., 2001). The graphics were performed using the GraphPad Prism 8 software (Graphpad, San Diego, CA).
Results
Greenhouse Study
As expected, the application of the DAP fertilizer had a more positive impact on all the parameters measured in cotton plants than RP in the absence of PGPB inoculation. In contrast, when analyzing all treatments, we observed significant increases of 36 and 21% in shoot dry weight with DAP and B02+RP compared to the treatment RP uninoculated (Figure 1A). In the shoot length, improvements of 13 and 7% were found when DAP and B02+RP were applied compared to the control (Figure 1B). In root development, uninoculated treatment plants with RP exhibited a biomass of 1.86 g plant−1. In contrast, DAP and B02+RP treatments produced higher biomass with values of 3.36 and 3.25 g plant−1, representing significant increases of 81 and 75%, respectively (Figure 1C). The application of B02+RP significantly promoted the root length by 34% with a value of 24.73 cm plant−1 (Figure 1D). Meanwhile, DAP promoted length by 24% (23.02 cm plant−1) compared to the control (18.44 cm plant −1). The other biological treatments did not differ significantly from the control. In P nutrition, a beneficial effect was observed when B02, SP20, and N8 were inoculated. For example, the shoot P content in control treatment increased by 12 and 9% with DAP and B02+RP (Figure 1E). Regarding the physiological parameters, the photosynthetic rate increased by 15 and 14% in the SP20+RP and DAP treatments (Figure 2A). For the chlorophyll measurement, a 9% increase was observed with B02+RP and DAP (Figure 2B). In addition, increases of 12, 8, and 6% were evidenced with N8+RP, DAP, and SP20+RP in transpiration rate (Figure 2C).
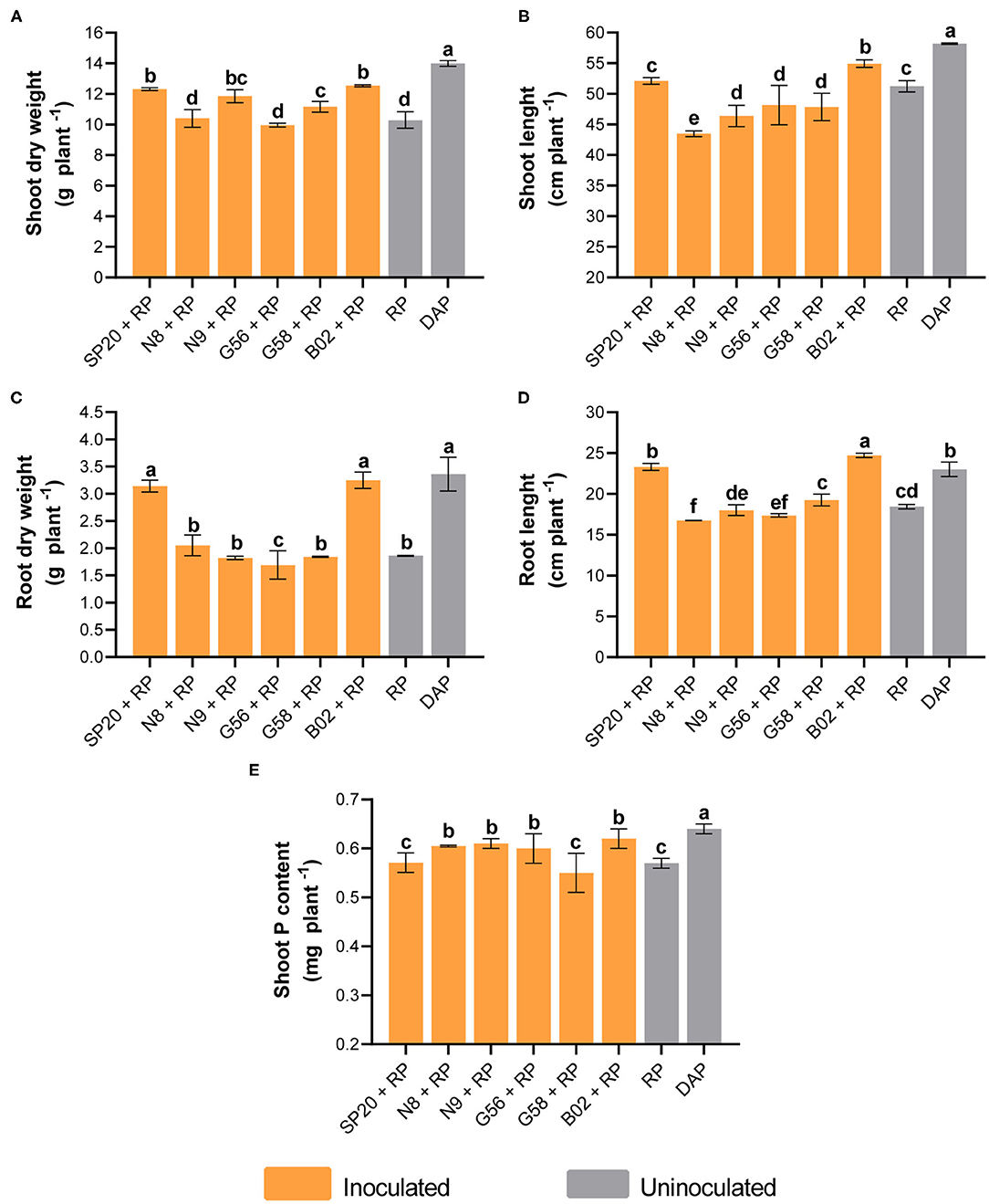
Figure 1. Influence of the PGPB inoculation of SP20, N8, N9, G56, G58, and B02 strains with RP on plant growth and shoot P content of cotton plants. Shoot dry weight (A), shoot length (B), root dry weight (C), root length (D), and shoot P content (E). The means and standard deviations are the results of three replicates per treatment, and the results are representative of two independent experiments. Different letters indicate significant differences based on Duncan's test. DAP, diammonium phosphate; RP, rock phosphate.
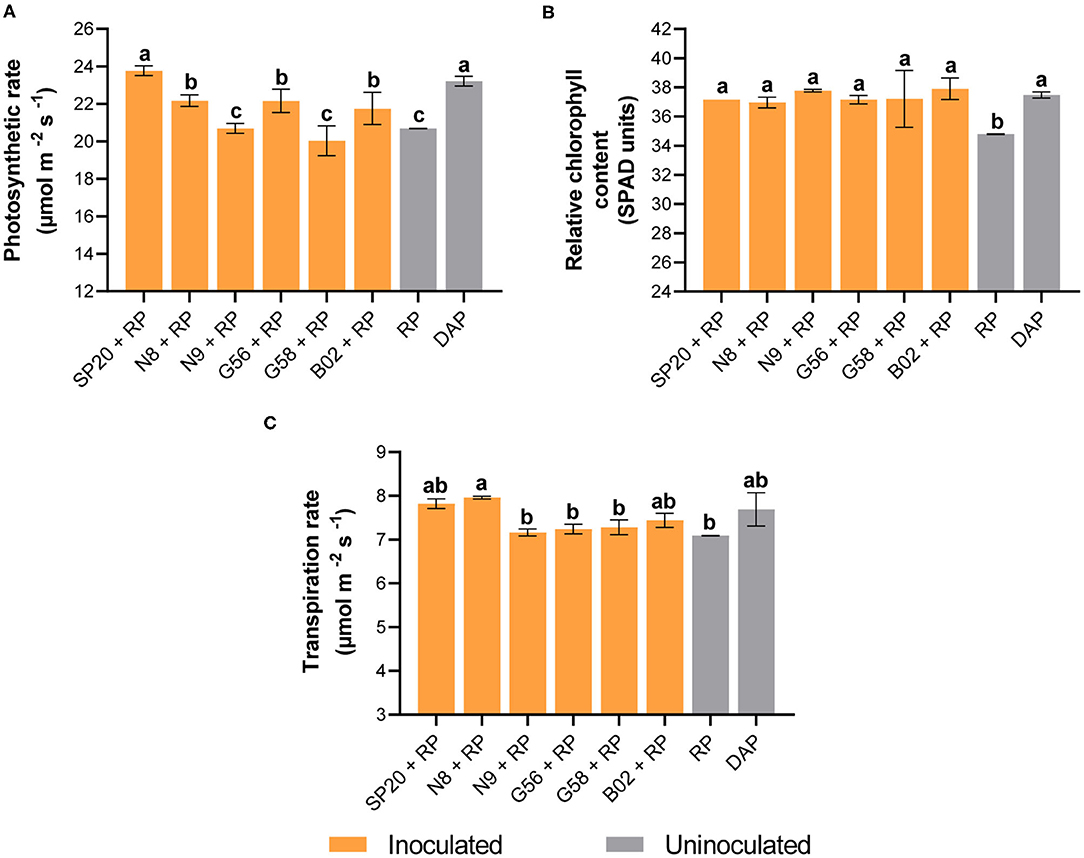
Figure 2. Effect of the PGPB inoculation of SP20, N8, N9, G56, G58, and B02 strains with RP on physiological parameters of cotton plants. Photosynthetic rate (A), relative chlorophyll content (B), and transpiration rate (C). The means and standard deviations are the results of three replicates per treatment, and the results are representative of two independent experiments. Different letters indicate significant differences based on Duncan's test. DAP, diammonium phosphate; RP, rock phosphate.
PCA analysis (Figure 3) allowed us to evidence the effect of PGPB application in a more integrated way between the eight response variables initially raised. The first two components of PCA, i.e., PC1 and PC2, explained ~64% of the experiment variation. PC1 accounted for 48.55% and was associated positively with all parameters evaluated. PC2 accounted for 15.34% and showed a positive correlation with photosynthetic rate, P content and transpiration rate and negatively with the other parameters. The angle included between the arrows pointing at two variables determined the correlation between the parameters as follows: sharp angles defined positive correlations, squared angles defined a null correlation, and obtuse angles defined negative correlations (de Almeida Carvalho-Estrada et al., 2020). Based on this, we observed a positive relationship between the three growth parameters (shoot dry weight, root length, and shoot length) and between the photosynthetic rate and P content. In addition, the position over the two dimensions on the graph indicates how the variables clustered and revealed that all treatments were grouped into two major clusters. Cluster 1 was composed of uninoculated treatment amended with DAP, B02+RP, and SP20+RP, while Cluster 2 was composed of the other strains with RP and the uninoculated treatment amended with RP. The relative position of clusters with respect to the arrows for each parameter suggests that the treatments in Cluster 1 exerted the greatest beneficial influence on plant growth, which corroborates what was evidenced when analyzing each parameter in the one-way ANOVA. Remarkably, the MANOVA analysis (Table 1) confirmed that there were significant differences between the treatments of inoculation with B02 and SP20 compared to the uninoculated treatment with RP, but that there were no significant differences with the treatment with DAP application. Among the strains B02 and SP20, it was observed that B02 caused the greatest plant growth-promoting effect in five parameters of the eight evaluated. Hence, our data indicate that B02 was the strain with the highest increase of soil P lability and was selected for the following trials. Based on the P-limiting conditions of the experiment and the improvement in the physiological parameters and P content, this strain is considered PSB.
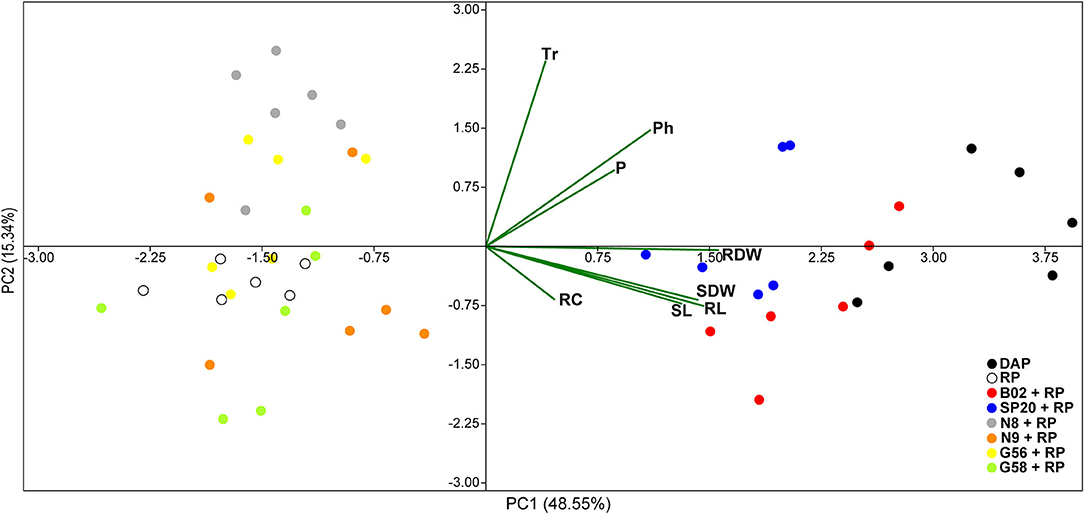
Figure 3. Principal component analysis (PCA) plot correlating the plant growth, shoot P content, and physiological parameters of cotton plants. RDW, root dry weight; SDW, shoot dry weight; RL, root length; SL, shoot length; P, shoot P content; Tr, transpiration rate; Ph, photosynthetic rate; RC, relative chlorophyll content; DAP, diammonium phosphate; RP, rock phosphate.
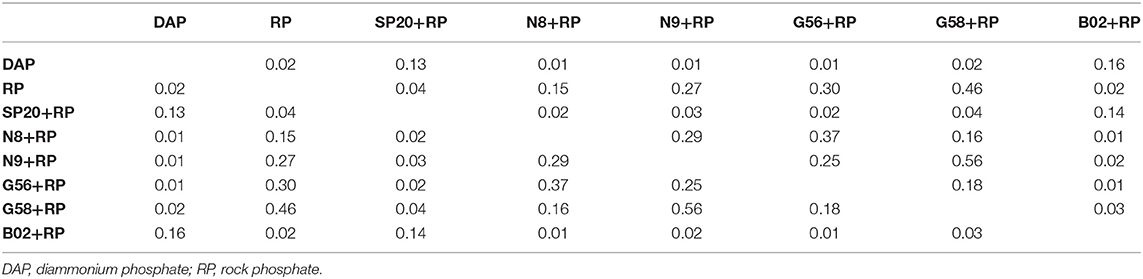
Table 1. P-values of the MANOVA analysis at α = 0.05 between treatments evaluated under greenhouse conditions.
Phylogeny and Characterization of Plant Growth-Promoting Features of Selected PSB
Strain B02 has a genome size of 4.99 Mb and belongs to the Rhizobium genus (p-value: 0.024). Nevertheless, it probably does not belong to any species stored in the NCBI, since the closest species were Agrobacterium pusense GCF013285525 (formerly Rhizobium pusense) and Rhizobium oryzihabitans GCF010669145 with an ANI values of 87 and 85%. The genome is available at the NCBI genome database under the de accession number SAMN16969919 in the bioproject number PRJNA627728.
We observed that the B02 strain showed several plant growth-promoting activities (Table 2). This strain was active in P solubilisation using tricalcium phosphate and RP as P sources. We found approximately 10 times more P soluble in tricalcium phosphate than RP. The observed values were 0.41 mg mL−1 for tricalcium phosphate and 0.5 mg mL−1 for RP. Regarding organic P mineralisation, we did not observe halos around the colonies as a positive indicator of sodium phytate hydrolysis; therefore, B02 strain showed that they are not capable of producing phytases. In the production of indole compounds, the strain exhibited this activity. Interestingly, we observed the B02 strain also produce indole compounds without tryptophan addition, though the highest amount (more than double) was measured with tryptophan addition. Finally, the strain showed the capacity for producing siderophores through orange color formation around each colony.
Discussion
There is a global demand for developing sustainable crop production, where adequate use of phosphate represents a challenge in the agricultural sector worldwide (Bindraban et al., 2020). Due to the environmental concerns associated with improving the efficient use of phosphate sources such as DAP fertilizer with high solubility and RP with low solubility, the study and appropriate selection of PGPB with biofertiliser potential acquire more and more relevance (Wan et al., 2020). In this research, evidence of the plant growth-promoting effects of PSB inoculation using RP as P source on cotton was shown in P-deficient soils.
Previously, the plant growth-promoting capacity of our strains was evaluated under controlled in vitro conditions on cotton plants (Supplementary Figure 1). The six strains showed the ability to significantly (p < 0.05) promote cotton seedling length between 10 and 71%. The above showed the positive interaction between plant and microorganism. Then, the greenhouse experiment was carried out in a nutritionally deficient soil with the application of an insoluble source like RP. The results showed that bacterial inoculation caused significantly different responses in the plants. Of the six strains evaluated under greenhouse conditions, inoculation of Rhizobium sp. B02 exerted a significant influence (p < 0.05) on plant morphometric parameters (biomass and length), shoot P content and photosynthetic rate of cotton. In the other parameters, i.e., transpiration rate and relative chlorophyll content, increases were observed but without significant differences. As the sampling time is a determining condition to assertively select bacterial strains and is one of the most criticized aspects (Bashan et al., 2020), we measured these findings in the growth stage of three true leaves (30 days), during which cotton plants require more nutrients, especially P, and respond positively to their availability (Grant et al., 2001; Iqbal et al., 2020).
Interestingly, when comparing the results of both biomass and length obtained in the treatments with bacterial inoculation plus RP with respect to the control (RP uninoculated), we inferred that the positive influence was more pronounced on the development of the root compared to shoot. Furthermore, based on the PCA obtained between plant parameters, we deduced that the inoculation of B02 and SP20 had a higher effect on growth parameters in contrast to parameters related to P nutrition and plant physiology. Likewise, the inoculation of Rhizobium sp. B02 with RP application generated a similar response without significant differences to the application of DAP fertilizer uninoculated in cotton plants, which allows us to infer that the individual inoculation of this strain could partially replace the DAP application.
Several studies have reported on the use of Rhizobium sp. as a successful strategy to improve the growth of legumes, such as bean, lentil, pea, chickpea and soybean (Wang et al., 2019). Interestingly, over the last few years, many studies have reported the presence of Rhizobium in the rhizosphere, endosphere and phyllosphere of non-leguminous crops (Díez-Méndez and Menéndez, 2021). However, the number of publications on the inoculation of Rhizobium sp. on cotton is very scarce. For example, Hafeez et al. (2004) showed that the inoculation of rhizobia improves seedling emergence, nutrient absorption (K+ and Ca+2) and cotton growth. To our knowledge, no reports demonstrate whether the single use of Rhizobium sp. beneficially influences cotton development in phosphorus-deficient soils.
A determining condition in this study was the soil characteristics. The soil used had low available P (12.28 mg kg−1). According to Ramírez and Kloepper (2010), this condition is necessary to observe plants respond to an increase in soluble P generated by microbial action. Another parameter of the soil, which is often forgotten in PSB studies, is the P sorption index, which represents the retention capacity of P in soil. Previous findings have shown that the effectiveness of PSB is controlled by the sorption capacity of P in the soil, demonstrating an inverse relationship (Osorio and Habte, 2015). In our study, the soil used belongs to the order of the inceptisols, which are characterized by having an average P sorption capacity (andisols > inceptisols > entisols) (Brenner et al., 2019). This condition allows the released P ions to be moderately absorbed by the soil components, reducing their availability to the plant, which does not show the full effectiveness of the biological activity of the strains. However, under this average level of P sorption, a beneficial effect of Rhizobium strain B02 on cotton plants growth was observed. Additionally, the soil was not sterilized and was obtained from fields where cotton is regularly planted. The objective of using these conditions was to demonstrate the effect of plant growth promotion of the PSB by simulating real commercial crop conditions (Romero-Perdomo et al., 2017).
In the biochemical tests carried out for the PSB selected, we show that Rhizobium sp. B02 have multiple plant growth-promoting features. Our results suggest that B02 strain is capable of solubilising tricalcium phosphate and RP. Tricalcium phosphate was used because calcium is immobilized in soils with a pH between 6 and 8 (Estrada et al., 2013), like the soil used in this study. In addition, we added RP, an insoluble source of P, to activate and evaluate the metabolic features of the strain in relation to P. These results suggest that the strain solubilised the insoluble P associated with both soil and insoluble fertilizer (RP), which improves cotton plant P absorption. Therefore, it is possible to suggest that Rhizobium sp. B02 increased the efficiency of the RP fertilizer. Regarding the synthesis of siderophores, B02 showed positive results for their production. Siderophores are mainly associated with the sequestration of iron in the soil, which may increase P availability (Pii et al., 2015). Furthermore, we evaluated the synthesis of indole compounds, an activity that stimulates various processes at the root level of the plant (Moreno-Galván et al., 2020). We observed that the strain exhibits this activity, and that tryptophan improves their production. Interestingly, B02 produce indole compounds in the absence of tryptophan, revealing different biosynthetic pathways for their production (Patten and Glick, 1996). Moreover, according to Amaya-Gómez et al. (2020), Rhizobium strain B02 is able to move, survive various concentrations of H2O2, form biofilms and metabolize different carbon substrates as phenotypes required for colonization of plant root surfaces and endurance in the rhizosphere. In that study, the strain B02 was identified by partial 16S rRNA gene sequencing.
A possible explanation for these findings could be based on a theoretical relationship between the in vitro and in vivo results. Based on Ramírez and Kloepper (2010) and Romero-Perdomo et al. (2017), the indole compounds produced by the strains stimulate the length of the cotton root, allowing greater exploration of the soil and higher absorption of nutrients. As P is limited, the phosphate solubilisation and the siderophores production of the strains increase its availability. Additionally, the enhancement of the transpiration rate could also have contributed to the improvement of the shoot P content in the inoculated plants, providing additional influences on the movement of P toward the roots. This process is mediated by the P function at the aquaporin level, since the phosphorylation process regulates the activity and the number of aquaporins that are directly related to the hydraulic conductivity and water absorption in roots (Zhou et al., 2013). The synergy of these plant growth-promoting activities could also have a beneficial influence on the chlorophyll content and photosynthetic capacity of cotton plants. Hence, plants could absorb more light energy to drive photosynthesis, which is related to the significant increase and the positive correlation evidenced in this parameter. This is consistent with previous reports in other plants (Sahandi et al., 2019; Wu et al., 2019). Furthermore, several findings have shown that the P supply generated by the increase in the photosynthetic rate directly influences the accumulation of dry matter, increasing its dry weight (Liu et al., 2020). To prove the biological contribution of these metabolic activities, research with mutant strains will be required (Rilling et al., 2019). The present description of the plant growth-promoting abilities of Rhizobium strain B02 corroborates that Rhizobium sp. is well-known for being a P solubiliser, a synthesis of indole compounds and a producer of siderophores (García-Fraile et al., 2012; Estrada-Bonilla et al., 2021).
Additionally, we did not perform a statistical correlation between in vitro results and in vivo results in the plant. Previous reports have shown that the number of growth-promoting features is not directly proportional to the effect on the plant and that the measurement of the capacity of each mechanism, such as P solubilising and mineralising under in vitro conditions, does not occur similarly in soil (Raymond et al., 2020). According to Patel et al. (2010), the soil provides very different conditions than a solid or liquid medium under controlled conditions. Moreover, the soil has different physical properties (adsorption, absorption, etc.) that can affect the behavior of microorganisms. A finding that corroborates this suggestion was found by Collavino et al. (2010). They found low statistical correlations between solubilisation halos and soluble P in both solid and liquid NBRIP medium with the P content in plant tissue.
Based on these findings, we show that PSB inoculation improves the growth, the P content of plant tissue and the physiological parameters in plants. For this, selection criteria such as strains that release soluble P from sources with a very low degree of solubility associated with environment conditions and strains that promote plant growth in soils deficient in available P are suggested. Although over the years, the taxonomy of the genus Agrobacterium and Rhizobium has undergone various revisions due to the transfer of species, the taxonomic analysis obtained with the sequencing of the genome of the B02 strain indicates a possible new species of the genus Rhizobium (Mousavi et al., 2015; Delamuta et al., 2020). Therefore, polyphasic taxonomic studies are necessary to carry out, such as housekeeping genes recA and atpD, to confirm the position of the B02 strain as distinct from the recognized Rhizobium species (Flores-Félix et al., 2020). Rhizobium, among many other PGPB, are the most studied microorganisms—mainly for their ability to form an effective symbiosis with leguminous crops in order to transform atmospheric nitrogen into assimilable nitrogen—the presented results show the biotechnological potential of this genus as a PSB in cotton plants. Likewise, this work provides evidence on the potential of PSB inoculation to increase the efficient nutritional use of P for developing more sustainable cotton production.
Conclusion
In this research, the inoculation of Rhizobium sp. B02 together with the application of RP in cotton plants, improves growth, shoot P content, photosynthetic rate, transpiration rate and relative chlorophyll content in soil with low P availability. Furthermore, this strain showed potential as phosphate-solubilising bacteria to increase the efficient use of RP. These findings might be associated with the ability of the strains to solubilise both tricalcium phosphate and rock phosphate, to synthesize indole compounds, and to produce siderophores. This work is a first approximation to optimize the efficient use of P in cotton cultivation through the application of PSB and low-solubility sources such as RP.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found here: https://www.ncbi.nlm.nih.gov/genbank/, SAMN16969919.
Author Contributions
FR-P and RB: conceptualization. GE-B and RB: funding acquisition and supervision. FR-P, IB, and JM-L: methodology. FR-P and GE-B: analysis and interpretation of the data. FR-P, IB, JM-L, GE-B, and RB: writing—original draft. FR-P, GE-B, and RB: writing—review & editing. All authors have made a direct and intellectual contribution to the manuscript.
Funding
This work was associated with two projects: (i) Phosphate solubiliser biofertiliser for rotation crops and (ii) a liquid biofertiliser based on Azotobacter chroococcum and a mixed biofertiliser based on Azotobacter chroococcum and phosphate-solubilising microorganisms for cotton, which were supported by AGROSAVIA and the Ministry of Agriculture and Rural Development from Colombia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
All authors are very grateful to the members of the Sustainable Agriculture Systems Group, especially to Mauricio Barón for his support during the greenhouse evaluations, to Cristian Vargas for the collaboration in the genomic description and to Lady Molano for carrying out the administrative management of this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsufs.2020.618425/full#supplementary-material
References
Alori, E. T., Glick, B. R., and Babalola, O. O. (2017). Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 8:971. doi: 10.3389/fmicb.2017.00971
Amaya-Gómez, C. V., Porcel, M., Mesa-Garriga, L., and Gómez-Álvarez, M. I. (2020). A framework for the selection of plant growth promoting rhizobacteria based on bacterial competence mechanisms. Appl. Environ. Microbiol. 86:e00760. doi: 10.1128/AEM.00760-20
Amin, A., Nasim, W., Mubeen, M., Nadeem, M., Ali, L., Hammad, H. M., et al. (2017). Optimizing the phosphorus use in cotton by using CSM-CROPGRO-cotton model for semi-arid climate of Vehari-Punjab, Pakistan. Environ. Sci. Pollut. Res. 24, 5811–5823. doi: 10.1007/s11356-016-8311-8
Bashan, Y., Kamnev, A. A., and de-Bashan, L. E. (2013). Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol. Fert. Soils 49, 465–479. doi: 10.1007/s00374-012-0737-7
Bashan, Y., Prabhu, S. R., de-Bashan, L. E., and Kloepper, J. W. (2020). Disclosure of exact protocols of fermentation, identity of microorganisms within consortia, formation of advanced consortia with microbe-based products. Biol. Fert. Soils 56, 443–445. doi: 10.1007/s00374-020-01464-x
Bindraban, P. S., Dimkpa, C. O., and Pandey, R. (2020). Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fert. Soils 56, 299–317. doi: 10.1007/s00374-019-01430-2
Brenner, J., Porter, W., Phillips, J. R., Childs, J., Yang, X., and Mayes, M. A. (2019). Phosphorus sorption on tropical soils with relevance to earth system model needs. Soil Res. 57, 17–27. doi: 10.1071/SR18197
Chastain, D. R., Snider, J. L., Choinski, J. S., Collins, G. D., Perry, C. D., and Whitaker, J. (2016). Leaf ontogeny strongly influences photosynthetic tolerance to drought and high temperature in Gossypium hirsutum. J. Plant Physiol. 199, 18–28. doi: 10.1016/j.jplph.2016.05.003
Collavino, M. M., Sansberro, P. A., Mroginski, L. A., and Aguilar, O. M. (2010). Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol. Fert. Soils 46, 727–738. doi: 10.1007/s00374-010-0480-x
de Almeida Carvalho-Estrada, P., Fernandes, J., da Silva, É. B., Tizioto, P., de Fátima Paziani, S., Duarte, A. P., et al. (2020). Effects of hybrid, kernel maturity, and storage period on the bacterial community in high-moisture and rehydrated corn grain silages. Syst. Appl. Microbiol. 43:126131. doi: 10.1016/j.syapm.2020.126131
Delamuta, J. R. M., Scherer, A. J., Ribeiro, R. A., and Hungria, M. (2020). Genetic diversity of Agrobacterium species isolated from nodules of common bean and soybean in Brazil, Mexico, Ecuador and Mozambique, and description of the new species Agrobacterium fabacearum sp. nov. Int. J. Syst. Evol. 70, 4233–4244. doi: 10.1099/ijsem.0.004278
Dhir, B. (2017). “Biofertilizers and biopesticides: eco-friendly biological agents,” in Advances in Environmental Biotechnology, eds R. Kumar, A. K. Sharma, and S. S. Sarabjeet (Singapore: Springer), 167–188. doi: 10.1007/978-981-10-4041-2_10
Diaz, P. A. E., Baron, N. C., and Rigobelo, E. C. (2019). Bacillus spp. as plant growth-promoting bacteria in cotton under greenhouse conditions. Aust. J. Crop Sci. 13:2003. doi: 10.21475/ajcs.19.13.12.p2003
Díez-Méndez, A., and Menéndez, E. (2021). “Rhizobium presence and functions in microbiomes of non-leguminous plants,” in Symbiotic Soil Microorganisms: Biology and Applications, eds N. Shrivastava, S. Mahajan, and A. Varma (Switzerland: Springer), 241–266. doi: 10.1007/978-3-030-51916-2_16
Estrada, G. A., Baldani, V. L., de Oliveira, D. M., de Oliveira, D. M., Urquiaga, S., and Baldani, J. I. (2013). Selection of phosphate-solubilizing diazotrophic Herbaspirillum and Burkholderia strains and their effect on rice crop yield and nutrient uptake. Plant Soil 369, 115–129. doi: 10.1007/s11104-012-1550-7
Estrada-Bonilla, G. A., Durrer, A., and Cardoso, E. J. (2021). Use of compost and phosphate-solubilizing bacteria affect sugarcane mineral nutrition, phosphorus availability, and the soil bacterial community. Appl. Soil Ecol. 157:103760. doi: 10.1016/j.apsoil.2020.103760
Fiske, C. H., and Subbarow, Y. (1925). The colorimetric determination of phosphorus. J. Biol. Chem. 66, 375–400.
Flores-Félix, J. D., Menéndez, E., Peix, A., García-Fraile, P., and Velázquez, E. (2020). History and current taxonomic status of genus Agrobacterium. Syst. Appl. Microbiol. 43:126046. doi: 10.1016/j.syapm.2019.126046
García-Fraile, P., Carro, L., Robledo, M., Ramírez-Bahena, M. H., Flores-Félix, J. D., Fernández, M. T., et al. (2012). Rhizobium promotes non-legumes growth and quality in several production steps: towards a biofertilization of edible raw vegetables healthy for humans. PLoS ONE 7:e38122. doi: 10.1371/journal.pone.0038122
Glickmann, E., and Dessaux, Y. (1995). A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 61, 793–796. doi: 10.1128/AEM.61.2.793-796.1995
Granada, C. E., Passaglia, L. M., de Souza, E. M., and Sperotto, R. A. (2018). Is phosphate solubilization the forgotten child of plant growth-promoting rhizobacteria? Front. Microbiol. 9:2054. doi: 10.3389/fmicb.2018.02054
Grant, C. A., Flaten, D. N., Tomasiewicz, D. J., and Sheppard, S. (2001). The importance of early season phosphorus nutrition. Can. J. Plant Sci. 81, 211–224. doi: 10.4141/P00-093
Hafeez, F. Y., Safdar, M. E., Chaudhry, A. U., and Malik, K. A. (2004). Rhizobial inoculation improves seedling emergence, nutrient uptake and growth of cotton. Aust. J. Exp. Agr. 44, 617–622. doi: 10.1071/EA03074
Hammer, Ø., Harper, D. A., and Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 1–9.
Heuer, S., Gaxiola, R., Schilling, R., Herrera-Estrella, L., López-Arredondo, D., Wissuwa, M., et al. (2017). Improving phosphorus use efficiency: a complex trait with emerging opportunities. Plant J. 90, 868–885. doi: 10.1111/tpj.13423
Iqbal, A., Gui, H., Zhang, H., Wang, X., Pang, N., Dong, Q., et al. (2019). Genotypic variation in cotton genotypes for phosphorus-use efficiency. Agronomy 9:689. doi: 10.3390/agronomy9110689
Iqbal, B., Kong, F., Ullah, I., Ali, S., Li, H., Wang, J., et al. (2020). Phosphorus application improves the cotton yield by enhancing reproductive organ biomass and nutrient accumulation in two cotton cultivars with different phosphorus sensitivity. Agronomy 10:153. doi: 10.3390/agronomy10020153
Kerovuo, J., Lauraeus, M., Nurminen, P., Kalkkinen, N., and Apajalahti, J. (1998). Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Appl. Environ. Microbiol. 64, 2079–2085. doi: 10.1128/AEM.64.6.2079-2085.1998
Kishore, N., Pind, P. K., and Reddy, S. R. (2015). “Phosphate-solubilizing microorganisms: a critical review,” in Plant Biology and Biotechnology, eds B. Bahadur, M. Venkat-Rajam, L. Sahijram, and K. Krishnamurthy (New Delhi: Springer), 307–333.
Li, H., Wang, J., Ali, S., Iqbal, B., Zhang, H., Wang, S., et al. (2020). Agronomic traits at the seedling stage, yield, and fiber quality in two cotton (Gossypium hirsutum L.) cultivars in response to phosphorus deficiency. J. Soil Sci. Plant Nutr. 66, 308–316. doi: 10.1080/00380768.2019.1709543
Liu, J., Liu, X., Zhang, Q., Li, S., Sun, Y., Lu, W., et al. (2020). Response of alfalfa growth to arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria under different phosphorus application levels. AMB Express 10, 1–13. doi: 10.1186/s13568-020-01137-w
Magallon-Servín, P., Antoun, H., Taktek, S., Bashan, Y., and de-Bashan, L. E. (2019). The maize mycorrhizosphere as a source for isolation of arbuscular mycorrhizae-compatible phosphate rock-solubilizing bacteria. Plant Soil 451, 169–186. doi: 10.1007/s11104-019-04226-3
Mai, W., Xue, X., Feng, G., Yang, R., and Tian, C. (2018). Can optimization of phosphorus input lead to high productivity and high phosphorus use efficiency of cotton through maximization of root/mycorrhizal efficiency in phosphorus acquisition? Field Crops Res. 216, 100–108. doi: 10.1016/j.fcr.2017.11.017
Martínez-Reina, A. M., and Hernández, M. L. (2015). Competitiveness of Colombian cotton in relation to the main producing countries through the focus of production costs. Ciencia y Tecnología Agropecuaria 16, 189–215. doi: 10.21930/rcta.vol16_num2_art:368
Mendoza, J. A., and Bonilla, R. (2014). Infectividad y efectividad de rizobios aislados de suelos de la Costa Caribe colombiana en Vigna unguiculata. Rev. Colomb. Biotecnol. 16, 84–89. doi: 10.15446/rev.colomb.biote.v16n2.47246
Moreno-Galván, A., Romero-Perdomo, F. A., Estrada-Bonilla, G., Meneses, C. H. S. G., and Bonilla, R. (2020). Dry-Caribbean Bacillus spp. strains ameliorate drought stress in maize by a strain-specific antioxidant response modulation. Microorganisms 8:823. doi: 10.3390/microorganisms8060823
Mousavi, S. A., Willems, A., Nesme, X., de Lajudie, P., and Lindström, K. (2015). Revised phylogeny of Rhizobiaceae: proposal of the delineation of Pararhizobium gen. nov., and 13 new species combinations. Syst. Appl. Microbiol. 38, 84–90. doi: 10.1016/j.syapm.2014.12.003
Munger, P., Bleiholder, H., Hack, H., Hess, M., Stauss, R., Van den Boom, T., et al. (1998). Phenological growth stages of the cotton plant (Gossypium hirsutum L.): codification and description according to the BBCH Scale 1. J. Agron. Crop Sci. 180, 143–149. doi: 10.1111/j.1439-037X.1998.tb00384.x
Murphy, J., and Riley, J. P. (1962). A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 27, 31–36. doi: 10.1016/S0003-2670(00)88444-5
Nautiyal, C. S. (1999). An efficient microbiological growth medium for screening phosphate solubilising microorganisms. FEMS Microbiol. Lett. 170, 265–270. doi: 10.1111/j.1574-6968.1999.tb13383.x
Osorio, N. W., and Habte, M. (2015). Effect of a phosphate-solubilizing fungus and an arbuscular mycorrhizal fungus on leucaena seedlings in tropical soils with contrasting phosphate sorption capacity. Plant Soil 389, 375–385. doi: 10.1007/s11104-014-2357-5
Patel, K. J., Singh, A. K., Nareshkumar, G., and Archana, G. (2010). Organic-acid-producing, phytate-mineralizing rhizobacteria and their effect on growth of pigeon pea (Cajanus cajan). Appl. Soil. Ecol. 44, 252–261. doi: 10.1016/j.apsoil.2010.01.002
Patten, C. L., and Glick, B. R. (1996). Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 42, 207–220. doi: 10.1139/m96-032
Pereg, L., and McMillan, M. (2015). Scoping the potential uses of beneficial microorganisms for increasing productivity in cotton cropping systems. Soil Biol. Biochem. 80, 349–358. doi: 10.1016/j.soilbio.2014.10.020
Pii, Y., Mimmo, T., Tomasi, N., Cesco, S., and Crecchio, C. (2015). Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fert. Soils 51, 403–415. doi: 10.1007/s00374-015-0996-1
Rai, P. K., Singh, M., Anand, K., Saurabh, S., Kaur, T., Kour, D., et al. (2020). “Role and potential applications of plant growth-promoting rhizobacteria for sustainable agriculture,” in New and Future Developments in Microbial Biotechnology and Bioengineering: Trends of Microbial Biotechnology for Sustainable Agriculture and Biomedicine Systems: Perspectives Perspectives for Human Health, eds A. A. Rastegari, A. N. Yadav, and N. Yadav (New York, NY: Elsevier), 49–60. doi: 10.1016/B978-0-12-820526-6.00004-X
Ramírez, C. A., and Kloepper, J. W. (2010). Plant growth promotion by Bacillus amyloliquefaciens FZB45 depends on inoculum rate and P-related soil properties. Biol. Fert. Soils 46, 835–844. doi: 10.1007/s00374-010-0488-2
Raymond, N. S., Gómez-Muñoz, B., van der Bom, F. J., Nybroe, O., Stoumann Jensen, L., Müller-Stöver, D. S., et al. (2020). Phosphate-solubilising microorganisms for improved crop productivity: a critical assessment. New Phytol. doi: 10.1111/nph.16924
Richardson, A. E., and Simpson, R. J. (2011). Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 156, 989–996. doi: 10.1104/pp.111.175448
Rilling, J. I., Acuna, J. J., Nannipieri, P., Cassan, F., Maruyama, F., and Jorquera, M. A. (2019). Current opinion and perspectives on the methods for tracking and monitoring plant growth–promoting bacteria. Soil Biol. Biochem. 130, 205–219. doi: 10.1016/j.soilbio.2018.12.012
Romero-Perdomo, F., Abril, J., Camelo, M., Moreno-Galván, A., Pastrana, I., Rojas-Tapias, D., et al. (2017). Azotobacter chroococcum as a potentially useful bacterial biofertilizer for cotton (Gossypium hirsutum): effect in reducing N fertilization. Rev. Argent. Microbiol. 49, 377–383. doi: 10.1016/j.ram.2017.04.006
Rosa, P. A. L., Mortinho, E. S., Jalal, A., Galindo, F. S., Buzetti, S., Fernandes, G. C., et al. (2020). Inoculation with growth-promoting bacteria associated with the reduction of phosphate fertilization in sugarcane. Front. Environ. Sci. 8:32. doi: 10.3389/fenvs.2020.00032
Sahandi, M. S., Mehrafarin, A., Badi, H. N., Khalighi-Sigaroodi, F., and Sharifi, M. (2019). Improving growth, phytochemical, and antioxidant characteristics of peppermint by phosphate-solubilizing bacteria along with reducing phosphorus fertilizer use. Ind. Crops Prod. 141:111777. doi: 10.1016/j.indcrop.2019.111777
Santos-Torres, M., Romero-Perdomo, F., Mendoza-Labrador, J., Gutiérrez, A. Y., Vargas, C., Castro, E., et al. (2021). Genomic and phenotypic analysis of rock phosphate-solubilizing rhizobacteria. Rhizosphere. 17:100290 doi: 10.1016/j.rhisph.2020.100290
Schwyn, B., and Neilands, J. (1987). Universal chemical assays for the detection and determination of siderophores. Anal. Biochem. 160, 47–56. doi: 10.1016/0003-2697(87)90612-9
Soil Science Division Staff (2017). Soil Survey Manual: USDA Handbook No. 18. Washington, DC: Government Printing Office.
Vargas, G., León, N., and Hernández, Y. (2019). “Agricultural socio-economic effects in colombia due to degradation of soils,” in Sustainable Management of Soil and Environment, eds R. S. Meena, S. Kumar, J. S. Bohra, and M. L. Jat (Singapore: Springer), 289–337. doi: 10.1007/978-981-13-8832-3_9
Wan, W., Qin, Y., Wu, H., Zuo, W., He, H., Tan, J., et al. (2020). Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front. Microbiol. 11:752. doi: 10.3389/fmicb.2020.00752
Wang, E. T., Chen, W. F., Tian, C. F., Young, J. P. W., and Chen, W. X. (2019). “Symbiosis between rhizobia and legumes,” in Ecology and Evolution of Rhizobia, eds E. T. Wang, W. F. Chen, C. F. Tian, J. P. W. Young, and W. X. Chen (Singapore: Springer), 3–19. doi: 10.1007/978-981-32-9555-1
Wu, F., Li, J., Chen, Y., Zhang, L., Zhang, Y., and Wang, S. (2019). Effects of phosphate solubilizing bacteria on the growth, photosynthesis, and nutrient uptake of Camellia oleifera Abel. Forests 10:348. doi: 10.3390/f10040348
Zhou, Y. C., Fan, J. W., Harris, W., Zhong, H. P., Zhang, W. Y., and Cheng, X. L. (2013). Relationships between C3 plant foliar carbon isotope composition and element contents of grassland species at high altitudes on the Qinghai-Tibet Plateau, China. PLoS ONE 8:e60794. doi: 10.1371/journal.pone.0060794
Keywords: phosphate-solubilising bacteria, PGPB, rock phosphate, indole compounds, siderophores
Citation: Romero-Perdomo F, Beltrán I, Mendoza-Labrador J, Estrada-Bonilla G and Bonilla R (2021) Phosphorus Nutrition and Growth of Cotton Plants Inoculated With Growth-Promoting Bacteria Under Low Phosphate Availability. Front. Sustain. Food Syst. 4:618425. doi: 10.3389/fsufs.2020.618425
Received: 16 October 2020; Accepted: 07 December 2020;
Published: 13 January 2021.
Edited by:
Everlon Cid Rigobelo, Universidade Estadual Paulista, BrazilReviewed by:
Praveen Rahi, National Centre for Cell Science, IndiaSumera Yasmin, National Institute for Biotechnology and Genetic Engineering (Pakistan), Pakistan
Copyright © 2021 Romero-Perdomo, Beltrán, Mendoza-Labrador, Estrada-Bonilla and Bonilla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: German Estrada-Bonilla, gaestrada@agrosavia.co; germanestra@gmail.com
 Felipe Romero-Perdomo
Felipe Romero-Perdomo Isidro Beltrán2
Isidro Beltrán2  Jonathan Mendoza-Labrador
Jonathan Mendoza-Labrador German Estrada-Bonilla
German Estrada-Bonilla