Female reproductive health impacts of Long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review
- 1Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, MA, United States
- 2Patient-Led Research Collaborative, Washington, DC, United States
- 3Department of Epidemiology & Biostatistics, School of Medicine, University of California, San Francisco, San Francisco, CA, United States
Long COVID disproportionately affects premenopausal women, but relatively few studies have examined Long COVID's impact on female reproductive health. We conduct a review of the literature documenting the female reproductive health impacts of Long COVID which may include disruptions to the menstrual cycle, gonadal function, ovarian sufficiency, menopause, and fertility, as well as symptom exacerbation around menstruation. Given limited research, we also review the reproductive health impacts of overlapping and associated illnesses including myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), postural orthostatic tachycardia syndrome (POTS), connective tissue disorders like Ehlers-Danlos syndrome (EDS), and endometriosis, as these illnesses may help to elucidate reproductive health conditions in Long COVID. These associated illnesses, whose patients are 70%–80% women, have increased rates of dysmenorrhea, amenorrhea, oligomenorrhea, dyspareunia, endometriosis, infertility, vulvodynia, intermenstrual bleeding, ovarian cysts, uterine fibroids and bleeding, pelvic congestion syndrome, gynecological surgeries, and adverse pregnancy complications such as preeclampsia, maternal mortality, and premature birth. Additionally, in Long COVID and associated illnesses, symptoms can be impacted by the menstrual cycle, pregnancy, and menopause. We propose priorities for future research and reproductive healthcare in Long COVID based on a review of the literature. These include screening Long COVID patients for comorbid and associated conditions; studying the impacts of the menstrual cycle, pregnancy, and menopause on symptoms and illness progression; uncovering the role of sex differences and sex hormones in Long COVID and associated illnesses; and addressing historical research and healthcare inequities that have contributed to detrimental knowledge gaps for this patient population.
1. Introduction
Long COVID (LC) is a disabling illness that can develop in anyone after a SARS-CoV-2 infection, independent of severity of COVID-19 disease. LC is defined as experiencing symptoms within three months from the initial infection that last at least two months (1). Symptoms can include fatigue, cognitive dysfunction, post-exertional malaise (PEM), headaches, insomnia, and muscle aches (2, 3). LC pathophysiology includes immune dysregulation and autoimmunity, pathogen persistence/reactivation, neurological abnormalities and neuroinflammation, tissue and organ damage, hypoperfusion and autonomic dysfunction, fibrin amyloid microclots, and microbiome dysregulation (3–7).
Evidence suggests that LC affects twice as many women as men (8) and may disproportionately impact transgender people (9). Premenopausal women have an elevated risk for LC (8), suggesting that sex hormones may play a key role in LC development (10). Reproductive health (RH) conditions are common pathologies within LC, but they remain significantly understudied.
This review examines evidence of female RH symptoms among LC patients. Given limited research, we also review RH impacts of associated illnesses including myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), postural orthostatic tachycardia syndrome (POTS), connective tissue disorders like Ehlers-Danlos syndrome (EDS), and endometriosis. Like LC, these illnesses predominantly impact women (11–13) and may help elucidate reproductive health in LC. This review highlights the importance of studying RH in LC, especially as RH conditions in general have been historically under-researched (14–16). It also proposes research priorities to advance knowledge of RH in LC and to improve LC patient outcomes.
2. Female reproductive health in Long COVID
Few studies have investigated the impact of LC on female RH, and emerging research suggests that LC can impact the menstrual cycle, ovarian health, and fertility.
2.1. Long COVID and the menstrual cycle
Research has found that premenopausal women with LC may commonly experience worsening of premenstrual symptoms and/or exacerbation of LC symptoms linked to menstrual cycle changes. In one cross-sectional study (n = 1,792), over one-third of menstruating LC patients reported an exacerbation of symptoms the week before or during menses (2). In another cross-sectional study (n = 460), 62% of LC patients experienced symptom worsening on days prior to menses (17).
LC patients report experiencing menstrual cycle irregularities, including changes to the length of the cycle, duration, and intensity of the menses. In a multi-country patient-led survey of 1,792 LC patient respondents with a menstrual cycle, 33.8% reported menstrual issues, which included abnormally irregular cycles (26%) and heavy periods (19.7%) (2). Additionally, 4.5% of 1,123 cisgender women aged 49 or older reported post-menopausal bleeding (2). Another survey study found that LC patients (n = 748) report higher rates of menstrual cycle changes (OR 1.34, 95% CI 1.15–1.57) compared to the general population, both with (n = 2,299) and without (n = 15,156) a history of COVID-19 (OR 0.99, 95% CI 0.91–1.09) (18). A longitudinal prospective cohort study with no control arm found that 16% of women and nonbinary people experienced menstrual cycle changes 28 to 222 days after SARS-CoV-2 infection (19). These include irregular or infrequent menstruation and increased premenstrual syndrome symptoms. A retrospective case-control study comparing the effects of COVID-19 (n = 1,066) and vaccination (n = 4,989) on menstrual health found that a history of COVID-19, but not vaccination, was associated with an increased risk of changes in menstrual cycle duration, bleeding between periods, increased menstrual flow, and missed periods (20).
Limitations of most of these studies include lack of healthy control or comparison groups, not screening participants for associated illnesses or comparison with these illnesses, and lack of consideration in the study design of factors that may impact menstrual cycles, such as vaccination (21).
2.2. Long COVID, fertility, and ovarian health
Case reports suggest that COVID-19 infection may be associated with long-term decline of ovarian health, including premature ovarian insufficiency (POI), a loss of normal ovary function before age 40. Based on transvaginal ultrasound examination and follicle-stimulating hormone (FSH) testing, POI was reported in a 34-year-old patient who had been experiencing irregular menstrual cycles and abnormally low bleeding since COVID-19 infection 12 months prior (22). Seven months after COVID-19, another 34-year-old LC patient was diagnosed with POI due to elevated gonadotropin levels and irregular menstrual cycles with oligomenorrhea (23). The patient's menstrual cycle was regular and hormone levels were normal two months before having COVID-19 (23). Eight months post-COVID-19, a 27-year old patient, who had been experiencing amenorrhea since infection, was diagnosed with POI based on high levels of gonadotropin hormones (FSH and LH) and menopausal levels of estradiol (24). Patients in these case reports were not screened for LC associated illnesses (e.g., ME/CFS or POTS), hampering the understanding of the pathophysiology of POI in LC. Given that only case reports exist, there are no estimates of the post-COVID-19 incidence of POI or its relation with associated illnesses, and whether it is more prevalent among people with LC.
Follicular fluid composition may be altered months after COVID-19 infection, which could affect oocyte quality and overall fertility (25). Levels of the cytokine IL-1 and vascular endothelial growth factors (VEGF) — key peptides in angiogenesis and vascular permeability and thus, oocyte development — were lower in the follicular fluid of people undergoing assisted reproductive treatment 2 to 9 months (average 4.5 months, n = 46) post-COVID-19 compared to controls who were SARS-CoV-2-negative or never had COVID-19 symptoms (n = 34) (26).
Premature menopause, associated with long-term health risks including increased morbidity and mortality (27), was reported by 3% of a sample of 938 cisgender women with LC in their 40s (2) who had persistent symptoms for 7 months post-COVID-19. This finding is higher than pre-pandemic estimates of premature menopause among cisgender women in their 40s [e.g., 1% in the US (28)].
2.3. Long COVID and pregnancy
Few studies have investigated LC in pregnant people. A cross sectional survey in Ecuador found that pregnant (n = 16) and non-pregnant (n = 231) women with LC experienced the same symptoms, with the three most commonly reported symptoms for both groups being fatigue, hair loss, and difficulty concentrating (29). A US prospective cohort study of pregnant people found that 25% had LC symptoms eight or more weeks after testing positive for SARS-CoV-2 (30). However, studies have not yet examined how LC affects pregnancy.
A small but important control-matched prospective cohort study in Brazil (n = 88) followed pregnant women after testing positive for COVID-19 (n = 84), finding that 75.9% developed LC (31). This study also found that patients given glucocorticoids to treat COVID-19 during pregnancy were at higher risk (RR 6.92, 95% CI 1.70–28.07) of persistent fatigue (31), a key and debilitating LC symptom.
3. Female reproductive health in illnesses comorbid or associated with Long COVID: A brief overview of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), postural orthostatic tachycardia syndrome (POTS), Ehlers-Danlos syndrome (EDS), and endometriosis
Given minimal research on RH conditions in LC, we draw upon the limited, often cross-sectional research on RH in ME/CFS and POTS — illnesses frequently triggered by infection that many people with LC develop (4, 5, 32–35). Additionally, we examine research on connective tissue disorders, namely EDS, and endometriosis, illnesses with significant RH implications that are comorbid with ME/CFS and POTS and may be associated with LC. See Figure 1 for a summary of the female reproductive conditions we review in Long COVID, ME/CFS, POTS, and EDS.
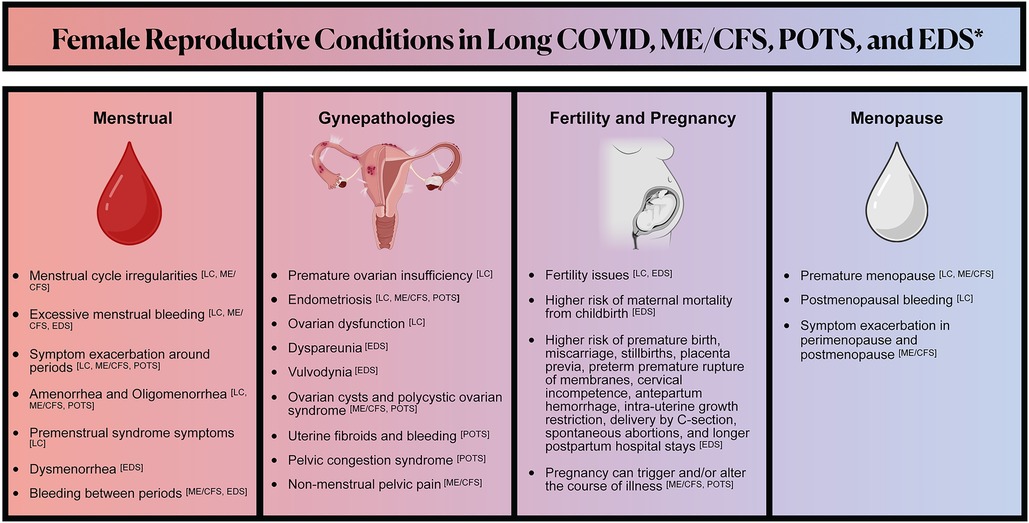
Figure 1. Illustrates the reproductive symptoms and conditions that may be associated with Long COVID, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), postural orthostatic tachycardia syndrome (POTS), and Ehlers-Danlos Syndrome (EDS). Reproductive symptoms and conditions in illnesses associated with Long COVID are highlighted to help elucidate knowledge gaps in Long COVID reproductive health. *The lists of symptoms and conditions represented are non-exhaustive and reflect what is discussed in this literature review. There are relevant gaps in reproductive health research in all of the conditions represented.
3.1. Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and reproductive health
Approximately 45% of LC patients develop ME/CFS (33, 34), a multi-system neuroimmune illness characterized by: neurological, vascular, and cognitive symptoms; pain; extreme fatigue unrelieved by sleep; and an exacerbation of symptoms or “crash” after physical or cognitive exertion (PEM) (33, 36). Infection is the most common onset event for ME/CFS (37), accounting for up to 75% of cases (5).
Female sex is a significant and consistent risk factor for ME/CFS (38), and sex and endocrine events influence the course of the illness (38). Many women with ME/CFS report that menstrual cycles, pregnancy, and menopause exacerbate symptoms (37). Compared to healthy controls, women with ME/CFS have disproportionately reported irregular menstrual cycles (39), amenorrhea (39, 40), excessive menstrual bleeding (41), bleeding between periods (39, 41), non-menstrual pelvic pain (40, 41), endometriosis (40, 42), gynecological surgery (especially hysterectomy) (41), and a history of polycystic ovarian syndrome (PCOS) and ovarian cysts (39). Half to two thirds (53%–67%, n = 120 and n = 42) of female ME/CFS patients report increased symptoms before menstruation, though these survey-based studies lack healthy controls (37, 43). A longitudinal case-controlled study (n = 157) revealed that early onset menopause is a risk factor for ME/CFS; mean age of menopause in ME/CFS (37.6 y) was earlier than healthy controls (48.6 y) (41), possibly due in part to gynecological surgery (40). Menopause exacerbated symptoms in 38% (n = 150) of perimenopausal and postmenopausal women with ME/CFS in a cross-sectional survey study (37).
Pregnancy is reported as a trigger for ME/CFS in 3%–10% of cases (4, 37). A study (n = 77) with age, sex, and education matched controls found that women who had been pregnant in the previous year were over 31 times more likely to develop ME/CFS (37, 44). Evidence is mixed for symptomatology among ME/CFS patients who become pregnant: nearly equal subsets may see symptoms improve, stay the same, or worsen (37).
3.2. Postural orthostatic tachycardia syndrome (POTS) and reproductive health
POTS is a type of dysautonomia involving orthostatic tachycardia without orthostatic hypotension (35). Common symptoms include lightheadedness, tachycardia, presyncope, and headaches (35). Among LC patients, 28% and 30% (n = 42, n = 70) had POTS in two NASA lean test and active stand test studies (32, 45). Pre-pandemic prevalence of POTS is estimated at 0.2%–1% (46), with 41% of POTS (n = 1,933) patients citing an infectious trigger in a non-controlled survey study (35).
Menstruation can significantly impact POTS symptoms. Menstrual cycle hormones have been found to affect hemodynamics (cardiac output, stroke volume, and total peripheral resistance) in POTS patients but not in healthy controls, possibly by modulating the renin-angiotensin-aldosterone system (47). Elevated estrogen and progesterone in the mid-luteal phases may increase blood volume retention and are associated with increased levels of renal-adrenal hormones (47). A prospective, questionnaire-based study of POTS patients (n = 65) and healthy controls (n = 95) found that dizziness fluctuated in the menstrual cycle, with greatest lightheadedness during menses, a decrease in the follicular phase, lowest in the mid-luteal phase, and an increase in the late luteal phase (48).
Studies (n = 65 and n = 191) have found increased rates of dysfunctional uterine bleeding, secondary amenorrhea, uterine fibroids (25% vs. 10% of healthy controls), endometriosis, ovarian cysts (43% vs. 13% of healthy controls), and pelvic congestion syndrome in POTS patients (48, 49). A cross-sectional survey study of 3,652 POTS patients reporting at least one pregnancy found that 81% report symptoms worsening during pregnancy (50), while subsets of patients in other small studies report symptom improvement (51, 52). Similar to ME/CFS, 9% of POTS patients (n = 1,933) in a cross-sectional survey study report that pregnancy triggered their POTS (35).
3.3. Ehlers-Danlos syndrome (EDS), connective tissue disorders (CTDs), and reproductive health
Approximately 23% of LC patients may fit within a musculoskeletal and nervous system phenotype characterized in part by having a higher prevalence of connective tissue disorders (CTDs), according to an EHR study of over 34,000 LC patients (53). The US Centers for Disease Control and Prevention identified systemic involvement of connective tissue as a COVID-19-related condition (54). While the prevalence of CTDs in LC is not yet established, case studies suggest that some patients develop joint hypermobility post-COVID-19 (55).
CTDs, including EDS and its largest subtype hypermobile EDS (hEDS) (56), are relatively common in patients with ME/CFS and POTS (57, 58). hEDS symptoms include joint subluxations, stretchy skin, pain, and fatigue, as well as cardiovascular, gastrointestinal, neurological, and musculoskeletal manifestations, including spinal conditions (56). Exact prevalence varies across studies, but some have found that up to 31% of POTS patients (n = 91) (57) and 20% of ME/CFS patients have hEDS (n = 229) (58), while 50%-81% of ME/CFS patients are hypermobile (n = 229, and n = 63) (58, 59) — striking considering the estimated general population prevalence of EDS is 0.2%–3.4% and hypermobility is 12–28% (56). Diagnosis can take years (56) and hEDS has strict diagnostic criteria, so the overall prevalence of CTDs in these illnesses may be higher than these hEDS statistics suggest. While mechanisms of CTDs are under-researched, especially in relation to infection-associated chronic illness, some researchers suspect that mast cell activation (MCA) may be implicated in some CTDs because mast cell mediators like tryptase and histamine can damage collagen (60, 61). Notably, symptoms of MCA are prevalent in LC patients (62), and there are substantial rates of diagnostic comorbidity between mast cell activation syndrome (MCAS) and EDS (63), ME/CFS (64), and POTS (65).
Menstrual symptoms can be severe in EDS patients: 50%–76% (n = 26, n = 386, n = 1,352) report menorrhagia (heavy periods) (66–68); 72% (n = 386) report dysmenorrhea (menstrual cramps) (67), 40% (n = 1,352) severe dysmenorrhea (68); 16% (n = 1,352) experience related moderate to severe pain (68); and 31% (n = 1,352) have intermenstrual bleeding (68) (studies lack controls). Furthermore, 43%–64% (n = 386, and n = 1,146) of EDS patients report dyspareunia (painful intercourse) (67, 69), and 50% (n = 1,146) report vulvodynia (pain around the vagina) (69).
Serious pregnancy complications are prevalent in patients with EDS and CTDs, but less than half are informed about these risks (68). In a 16-year population-based retrospective study, pregnancies in EDS patients were associated with higher rates of adverse outcomes, including prematurity, cervical incompetence, antepartum hemorrhage, placenta previa, delivery by cesarean section, longer postpartum stays, maternal death, and having infants with intra-uterine growth restriction (70). In a survey study of 1,352 women with EDS without controls, 43.3% experienced infertility; 54.5% of those who had been pregnant experienced at least one miscarriage; and 29.6% of those who had given birth had at least one premature birth (68). Multiple studies have found that, compared with the general population, women with the three most common types of EDS have higher rates of spontaneous abortions, miscarriages, stillbirths, and preterm premature rupture of membranes (PPROM) (71).
3.4. Endometriosis
Endometriosis patients may have an increased risk of developing LC (aHR = 1.19, 95% CI 1.11–1.28) based on a population-based retrospective cohort-matched study using data from electronic health records of non-hospitalized LC patients; however, more research is needed to understand contributing factors (72). Endometriosis is a chronic, systemic disease where tissue similar to the lining of the uterus grows outside the uterus (73, 74). The disease affects 10% of reproductive-age girls and women (75), as well as premenarcheal girls (76) and postmenopausal women (77), nonbinary and transgender people, and, in rare cases, men (78–80). Endometriosis can be associated with a range of severe, disabling symptoms, including but not limited to pain with menstruation, penetration, bowel movement, and/or urination, chronic pain, infertility, fatigue (75), and with occurrences like preeclampsia and other adverse pregnancy outcomes (81, 82). Invasive surgical and histological diagnosis, which is widely considered the gold standard to diagnose (83), stigma, symptom normalization, and lack of practitioner awareness (75) drive average diagnostic delays of up to 11 years (84–87), which may reduce understanding of endometriosis prevalence in LC.
Approximately 36% of women with ME/CFS (n = 36) and 20% of women with POTS (n = 65) report endometriosis (42, 48, 88). Reduced natural killer cell cytotoxic function (89), macrophage alterations (90), lowered cortisol (91), elevated oxidative stress (92), and allergies (93) are implicated in endometriosis, as well as in ME/CFS (94–96), highlighting overlaps and the role of dysfunctional immune and endocrine systems in both diseases. Some of these mechanisms may also play a role in LC (2, 97–99).
Despite the prevalence of and disablement caused by endometriosis, its pathogenesis remains unknown (100). A lesser known and under-investigated hypothesis proposes that microbes may contribute to endometriosis etiopathology (i.e., “bacterial contamination hypothesis”) (101) via an inflammatory response and TLR4 (102–104) and TLR2 activation (104, 105). This research is notable in the context of studies on pathogen persistence and pathobionts in infection-associated chronic illnesses and how they may be implicated in multiple pathologies (106–108). Studies have found evidence of persistent SARS-CoV-2 infection, antigen reservoirs, and oral and intestinal dysbiosis in subsets of LC patients, which have been linked to gastrointestinal symptoms and an elevated risk of developing LC (5, 6, 109–112).
4. Discussion
Research on RH in LC is severely lacking, despite patient-reported symptoms and documented impacts in LC and associated conditions. Studies mentioned in this review across all illnesses have largely been underpowered cross-sectional studies, case reports, and lacking in healthy or other relevant control groups—in part due to historic underfunding of ME/CFS and associated illnesses (113). RH conditions in these illnesses have been under-researched, as has female RH more broadly (14–16), and more research funding should be allocated to study RH within LC and associated illnesses.
We urge researchers to investigate the below RH research priorities and to design studies that account for overlapping associated illnesses (Figure 2). Because risk factors for developing LC may include female sex, socioeconomic deprivation, and racial/ethnic minority identity (72), these questions would ideally be explored through large, control-matched prospective longitudinal cohort studies and clinical trials that have significant representation of cisgender women and gender diverse people in all reproductive life phases.
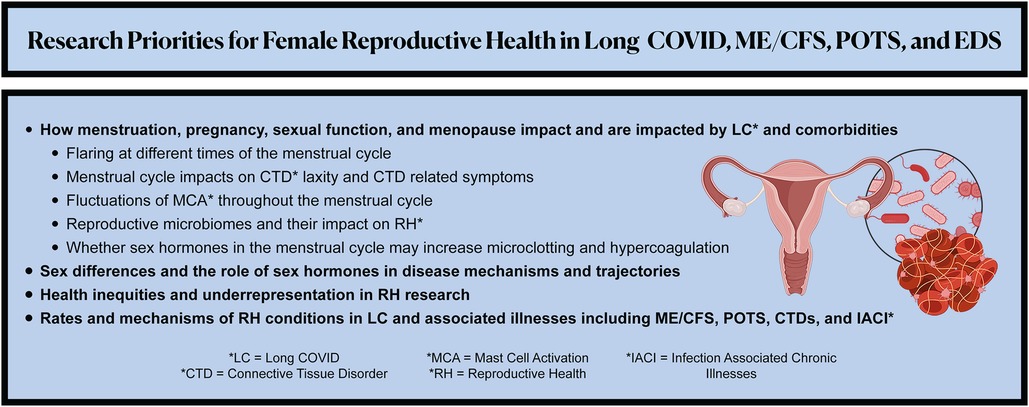
Figure 2. Summarizes the recommended patient-led research priorities to advance the understanding, management, and therapeutics of female reproductive health in Long COVID, myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), postural orthostatic tachycardia syndrome (POTS), Ehlers-Danlos Syndrome (EDS) and other associated illnesses.
Sex Differences. Female sex is a risk factor for associated illnesses: 70%–80% of patients with ME/CFS, POTS, and EDS are female (10–12). Thus, the roles of chromosomal, hormonal, and anatomical sex are vital to uncovering an understanding of how these illnesses predominate in female patients, worsen with the menstrual cycle and menopause, and could be impacted by hormonal therapies. Given that cisgender females and transgender people may have a higher prevalence of LC than cisgender males (9), LC research should examine LC sex dimorphism, sex differences in immune dysregulation and immune responses to infection, and the role of sex hormones in disease trajectories and RH etiopathology.
Menstrual Cycle. Research is urgently needed to understand how LC symptoms are impacted by the menstrual cycle and to identify therapeutics. It is critical to investigate how the menstrual cycle can drive symptom exacerbation, specifically:
(1) Mechanisms of flares at different times of the menstrual cycles (e.g., whether menstruation can induce PEM).
(2) Whether menstruation and female sex hormones (and possibly synthetic contraceptives), increase hypercoagulation in LC and ME/CFS. Fibrinaloid microclots were found in 100% of LC patients (n = 70) (114) and also found in ME/CFS patients (n = 25) (115). They are suspected of contributing to pathogenesis, hypoperfusion, and vascular dysfunction (7, 114). Interestingly, sex hormones including estrogen and progesterone have fibrinogen hypercoagulable properties (116). Thus, we encourage research on whether sex hormones impact hypercoagulation and microclotting in LC and ME/CFS.
(3) The link among ACE2 expression, viral persistence, and RH. ACE2 expression in the reproductive tract fluctuates throughout the menstrual cycle (117, 126), and viral persistence is one of the hypothesized mechanisms contributing to the pathophysiology of LC (6, 118).
(4) Whether menstrual cycle-related fluctuations in connective tissue laxity are exacerbated in LC patients with CTDs, as female joint and connective tissue laxity may fluctuate with hormones throughout the menstrual cycle, especially estradiol (119).
(5) Fluctuations of MCA throughout the menstrual cycle (120) and the role of chronic MCA, especially infection-induced chronic MCA, in RH conditions (e.g., MCA may be implicated in the pathophysiology and symptomatology of endometriosis (93, 121, 122) as well as in preeclampsia (123)).
(6) Vaginal and menstrual effluent microbiomes in LC and their impact on RH. Microbiome dysbiosis is a proposed etiopathology of LC (5, 6, 111), and microbiome signatures drive and are driven by gynecological conditions (124–129).
(7) How symptoms impact and are impacted by menopause, given evidence of early menopause (41) and exacerbated symptoms in perimenopausal and postmenopausal women (38).
Pregnancy. It is critical to study how pregnancy impacts and is impacted by LC, especially given that some ME/CFS and POTS patients report symptom improvement or remission during and after pregnancy, while others report symptom worsening or new illness onset with pregnancy (35, 37, 51). Additionally, as research evolves, clinicians working with LC patients who are pregnant or considering pregnancy should be aware of and discuss potential pregnancy-related risks, especially for patients with CTDs.
Screening for Long COVID Comorbidities and Associated Illnesses. Rehabilitation care and research on LC patients should include screening for RH symptoms and conditions (e.g., endometriosis), as well as overlapping illnesses like ME/CFS, POTS, CTDs, and others.
Additional Reproductive Health Impacts. To the best of our knowledge, there are no published studies investigating premenstrual dysphoric disorder (PMDD) and/or female sexual dysfunction following COVID-19 infection [compared to 60 studies on erectile dysfunction post-COVID-19 infection (130)]. It will also be critical to investigate whether pathologies associated with LC increase the risk of cancer in the reproductive tract through mechanisms such as infection-induced dysbiosis, chronic inflammation, and promotion of oncogenic pathways (106, 131).
Infection-Associated Chronic Illness Beyond Long COVID. Lack of discussion related to other infection-associated chronic illnesses and vaccine-onset illness is not due to their lack of relevance but rather absence of research on their RH impacts. Including these patient populations (e.g., chronic Lyme disease) in future research would be beneficial in understanding infection-associated chronic illnesses across antigen triggers.
Health Inequities in Research. The literature reviewed studied largely white populations in high-income countries where these illnesses may be systemically underdiagnosed among people from marginalized groups (132, 133). Clinical research has historically lacked appropriate racial/ethnic/gender representation due to barriers to participation and systemic and institutional racism in healthcare (15, 134). To promote health equity and counteract historic racism and bias in health research, studies must prioritize recruiting and retaining representative samples. Moreover, it will be important for studies to focus on RH experiences in the LC population living in low- and middle-income countries (LMIC), as the frequency and impact of menstrual disorders was higher among adults and adolescents in these countries even before the COVID-19 pandemic (14, 135).
Lack of research and insufficient research funding for RH conditions and infection-associated chronic illnesses contributes to clinical care shortcomings. Gender and racial/ethnic healthcare disparities amplify these shortcomings (136, 137), along with over-psychologicalization of pain and illness in female patients and patients of color (138, 139). Menstrual and sexual health continue to be stigmatized in healthcare (75, 140, 141), a setting where hyperfocus on reproduction in a historically male-dominated OBGYN field has overshadowed women's health and well-being outside of their ability to reproduce (140, 142). Patients with LC and related conditions report often feeling dismissed by clinicians (143), with many of the conditions in this review having extensive diagnostic delays and high rates of initial misdiagnosis (35, 56, 144, 145).
The number of patients with LC continues to rise as COVID-19 persists, yet a paucity of research exists on the female RH implications of LC. LC may be associated with disruptions to the menstrual cycle, gonadal function, ovarian insufficiency, premature menopause, and fertility problems. RH conditions connected to associated illnesses (e.g., ME/CFS, POTS, EDS) include dysmenorrhea, amenorrhea, oligomenorrhea, dyspareunia, endometriosis, infertility, vulvodynia, intermenstrual bleeding, ovarian cysts, uterine fibroids and bleeding, pelvic congestion syndrome, and adverse pregnancy complications, such as maternal mortality, preeclampsia, and premature birth. RH conditions negatively impact quality of life and hinder many women, girls, nonbinary, and trans people from fully participating in the economy and society. We recommend that researchers, patients, and clinicians collaboratively shape a better future for research on the RH impacts of LC and associated illnesses.
Author contributions
LSo, LM, LSa, BP, and EvS: contributed to the conception of the article. BP, EvS, and LSo led the drafting of the manuscript (listed in order of writing contribution), and LM, LSa, AH, and AC contributed to writing and editing sections. LM and BP: led project management. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank the following clinicians, scientists, and patients for providing feedback and review: Myra Batchelder, Dr. Lisa Owens, Dr. Caroline Mitchell. Thank you to Grace Sisemore Blacker for her help designing the figures. Additionally, we thank the Patient-Led Research Collaborative and the Long COVID, ME/CFS, POTS, EDS, endometriosis and infection-associated chronic illness patient and research communities for their knowledge and support of patient-driven research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a delphi consensus. Lancet Infect Dis. (2022) 22:e102–7. doi: 10.1016/S1473-3099(21)00703-9
2. Davis HE, Assaf GS, McCorkell L, Wei H, Low RJ, Re’em Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019
3. Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
4. Campen CM, Rowe PC, Visser FC. Orthostatic symptoms and reductions in cerebral blood flow in long-haul COVID-19 patients: similarities with myalgic encephalomyelitis/chronic fatigue syndrome. Medicina (Mex). (2022) 58:28. doi: 10.3390/medicina58010028
5. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. (2022) 28:911–23. doi: 10.1038/s41591-022-01810-6
6. Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. (2021) 12:698169. Available at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.698169 (Accessed November 18, 2022). doi: 10.3389/fmicb.2021.698169
7. Pretorius E, Vlok M, Venter C, Bezuidenhout JA, Laubscher GJ, Steenkamp J, et al. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. (2021) 20:172. doi: 10.1186/s12933-021-01359-7
8. Sigfrid L, Drake TM, Pauley E, Jesudason EC, Olliaro P, Lim WS, et al. Long COVID in adults discharged from UK hospitals after COVID-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. Lancet Reg Health—Eur. (2021) 8:100186. doi: 10.1016/j.lanepe.2021.100186
9. Long COVID—Household Pulse Survey—COVID-19. (2022). Available at: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (Accessed December 2, 2022).
10. Stewart S, Newson L, Briggs TA, Grammatopoulos D, Young L, Gill P. Long COVID risk—a signal to address sex hormones and women's Health. Lancet Reg Health Eur. (2021) 11:100242. doi: 10.1016/j.lanepe.2021.100242
11. Low PA, Sandroni P, Joyner M, Shen W-K. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol. (2009) 20:352–8. doi: 10.1111/j.1540-8167.2008.01407.x
12. Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, Taylor RR, et al. A community-based study of chronic fatigue syndrome. Arch Intern Med. (1999) 159:2129–37. doi: 10.1001/archinte.159.18.2129
13. Demmler JC, Atkinson MD, Reinhold EJ, Choy E, Lyons RA, Brophy ST. Diagnosed prevalence of ehlers-danlos syndrome and hypermobility spectrum disorder in Wales, UK: a national electronic cohort study and case–control comparison. BMJ Open. (2019) 9:e031365. doi: 10.1136/bmjopen-2019-031365
14. Harlow SD, Campbell OMR. Epidemiology of menstrual disorders in developing countries: a systematic review. BJOG Int J Obstet Gynaecol. (2004) 111:6–16. doi: 10.1111/j.1471-0528.2004.00012.x
15. Mirin AA. Gender disparity in the funding of diseases by the U.S. National institutes of health. J Womens Health. (2021) 30:956–63. doi: 10.1089/jwh.2020.8682
16. Weisman CS, Cassard SD. Health consequences of exclusion or underrepresentation of women in clinical studies (I). Washington (DC): National Academies Press (US) (1999. Available at: https://www.ncbi.nlm.nih.gov/books/NBK236583/ (Accessed December 12, 2022).
17. Newson L, Lewis R, O’Hara M. Long COVID and menopause—the important role of hormones in long COVID must be considered. Maturitas. (2021) 152:74. doi: 10.1016/j.maturitas.2021.08.026
18. Medina-Perucha L, López-Jiménez T, Holst AS, Jacques-Aviñó C, Munrós-Feliu J, Martínez-Bueno C, et al. Self-reported menstrual alterations during the COVID-19 syndemic in Spain: a cross-sectional study. Int J Womens Health. (2022) 14:529–44. doi: 10.2147/IJWH.S354655
19. Khan SM, Shilen A, Heslin KM, Ishimwe P, Allen AM, Jacobs ET, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. (2022) 226:270–3. doi: 10.1016/j.ajog.2021.09.016
20. Alvergne A, Kountourides G, Argentieri MA, Agyen L, Rogers N, Knight D, et al. A retrospective case-control study on menstrual cycle changes following COVID-19 vaccination and disease. iScience. (2023) 26:106401. doi: 10.1016/j.isci.2023.106401
21. Lee KMN, Junkins EJ, Luo C, Fatima UA, Cox ML, Clancy KBH. Investigating trends in those who experience menstrual bleeding changes after SARS-CoV-2 vaccination. Sci Adv. (2022) 8:eabm7201. doi: 10.1126/sciadv.abm7201
22. Madaan S, Talwar D, Jaiswal A, Kumar S, Acharya N, Acharya S, et al. Post-COVID-19 menstrual abnormalities and infertility: repercussions of the pandemic. J Educ Health Promot. (2022) 11:170. doi: 10.4103/jehp.jehp_1200_21
23. Wilkins J, Al-Inizi S. Premature ovarian insufficiency secondary to COVID-19 infection: an original case report. Int J Gynecol Obstet. (2021) 154:179–80. doi: 10.1002/ijgo.13719
24. Puca E, Puca E. Premature ovarian failure related to SARS-CoV-2 infection. J Med Cases. (2022) 13:155–8. doi: 10.14740/jmc3791
25. Revelli A, Piane LD, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. (2009) 7:40. doi: 10.1186/1477-7827-7-40
26. Herrero Y, Pascuali N, Velázquez C, Oubiña G, Hauk V, de Zúñiga I, et al. SARS-CoV-2 infection negatively affects ovarian function in ART patients. Biochim Biophys Acta —Mol Basis Dis. (2022) 1868:166295. doi: 10.1016/j.bbadis.2021.166295
27. Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas. (2010) 65:161–6. doi: 10.1016/j.maturitas.2009.08.003
28. Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi-ethnic population study of the menopause transition*. Hum Reprod. (2003) 18:199–206. doi: 10.1093/humrep/deg005
29. Vásconez-González J, Fernandez-Naranjo R, Izquierdo-Condoy JS, Delgado-Moreira K, Cordovez S, Tello-De-la-Torre A, et al. Comparative analysis of long-term self-reported COVID-19 symptoms among pregnant women. J Infect Public Health. (2023) 16:430–40. doi: 10.1016/j.jiph.2023.01.012
30. Afshar Y, Gaw SL, Flaherman VJ, Chambers BD, Krakow D, Berghella V, et al. Clinical presentation of coronavirus disease 2019 (COVID-19) in pregnant and recently pregnant people. Obstet Gynecol. (2020) 136:1117–25. doi: 10.1097/AOG.0000000000004178
31. Santos CAD, Fonseca Filho GG, Alves MM, Macedo EYL, Pontes MdA, Paula AP, et al. Maternal and neonatal outcomes associated with mild COVID-19 infection in an obstetric cohort in Brazil. Am J Trop Med Hyg. (2022) 107:1060–5. doi: 10.4269/ajtmh.22-0421
32. Vernon SD, Funk S, Bateman L, Stoddard GJ, Hammer S, Sullivan K, et al. Orthostatic challenge causes distinctive symptomatic, hemodynamic and cognitive responses in long COVID and myalgic encephalomyelitis/chronic fatigue syndrome. Front Med. (2022) 9:917019. doi: 10.3389/fmed.2022.917019
33. Kedor C, Freitag H, Meyer-Arndt L, Wittke K, Hanitsch LG, Zoller T, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. (2022) 13:5104. doi: 10.1038/s41467-022-32507-6
34. Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post–coronavirus disease. JACC Heart Fail. (2021) 9:927–37. doi: 10.1016/j.jchf.2021.10.002
35. Shaw BH, Stiles LE, Bourne K, Green EA, Shibao CA, Okamoto LE, et al. The face of postural tachycardia syndrome—insights from a large cross-sectional online community-based survey. J Intern Med. (2019) 286:438–48. doi: 10.1111/joim.12895
36. Carruthers BM, van de Sande MI, De Meirleir KL, Klimas NG, Broderick G, Mitchell T, et al. Myalgic encephalomyelitis: international consensus criteria. J Intern Med. (2011) 270:327–38. doi: 10.1111/j.1365-2796.2011.02428.x
37. Chu L, Valencia IJ, Garvert DW, Montoya JG. Onset patterns and course of myalgic encephalomyelitis/chronic fatigue syndrome. Front Pediatr. (2019) 7:12. doi: 10.3389/fped.2019.00012
38. Thomas N, Gurvich C, Huang K, Gooley PR, Armstrong CW. The underlying sex differences in neuroendocrine adaptations relevant to myalgic encephalomyelitis chronic fatigue syndrome. Front Neuroendocrinol. (2022) 66:100995. doi: 10.1016/j.yfrne.2022.100995
39. Harlow BL, Signorello LB, Hall JE, Dailey C, Komaroff AL. Reproductive correlates of chronic fatigue syndrome. Am J Med. (1998) 105:94S–9S. doi: 10.1016/s0002-9343(98)00173-9
40. Boneva RS, Maloney EM, Lin J-M, Jones JF, Wieser F, Nater UM, et al. Gynecological history in chronic fatigue syndrome: a population-based case-control study | journal of Women's health. J Womens Health. (2011) 20:21–8. doi: 10.1089/jwh.2009.1900
41. Boneva RS, Lin J-MS, Unger ER. Early menopause and other gynecologic risk indicators for chronic fatigue syndrome in women. Menopause. (2015) 22:826–34. doi: 10.1097/GME.0000000000000411
42. Boneva RS, Lin J-MS, Wieser F, Nater UM, Ditzen B, Taylor RN, et al. Endometriosis as a comorbid condition in chronic fatigue syndrome (CFS): secondary analysis of data from a CFS case-control study. Front Pediatr. (2019) 7:195. doi: 10.3389/fped.2019.00195
43. Clark K, Del Fante P, Beilby J. Myalgic Encephalopathy/Chronic Fatigue Syndrome Longitudinal Outcomes Pilot Study Report. (2006) http://sacfs.asn.au/download/me_cfs_pilot_study_report.pdf
44. Gimeno Pi I, Guitard Sein-Echaluce ML, Rosselló Aubach L, Torres Puig-Gros J, Fernández Solà J. Stressful events in the onset of chronic fatigue syndrome. Rev Esp Salud Publica. (2016) 90:e1–7.27535808
45. Hira R, Baker JR, Siddiqui T, Ranada SI, Soroush A, Karalasingham K, et al. Objective hemodynamic cardiovascular autonomic abnormalities in post-acute sequelae of COVID-19. Can J Cardiol. (2022) 0. doi: 10.1016/j.cjca.2022.12.002. (In press)
46. Federowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. (2018) 285:352–66. doi: 10.1111/joim.12852
47. Fu Q, VanGundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension. (2010) 56:82–90. doi: 10.1161/HYPERTENSIONAHA.110.151787
48. Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. (2012) 118:242–6. doi: 10.1016/j.ijgo.2012.04.014
49. Knuttinen M-G, Zurcher KS, Khurana N, Patel I, Foxx-Orenstein A, Harris LA, et al. Imaging findings of pelvic venous insufficiency in patients with postural orthostatic tachycardia syndrome. Phlebology. (2021) 36:32–7. doi: 10.1177/0268355520947610
50. Bourne KM, Nerenberg KA, Stiles LE, Shibao CA, Okamoto LE, Garland EM, et al. Symptoms of postural orthostatic tachycardia syndrome in pregnancy: a cross-sectional, community-based survey. BJOG Int J Obstet Gynaecol. (2023). doi: 10.1111/1471-0528.17437. (In press)
51. Blitshteyn S, Poya H, Bett GCL. Pregnancy in postural tachycardia syndrome: clinical course and maternal and fetal outcomes. J Matern Fetal Neonatal Med. (2012) 25:1631–4. doi: 10.3109/14767058.2011.648671
52. Kanjwal K, Karabin B, Kanjwal Y, Grubb BP. Outcomes of pregnancy in patients with preexisting postural tachycardia syndrome. Pacing Clin Electrophysiol. (2009) 32:1000–3. doi: 10.1111/j.1540-8159.2009.02430.x
53. Zhang H, Zang C, Xu Z, Zhang Y, Xu J, Bian J, et al. Data-driven identification of post-acute SARS-CoV-2 infection subphenotypes. Nat Med. (2023) 29:226–35. doi: 10.1038/s41591-022-02116-3
54. CDC. New ICD-10CM code for the 2019 Novel Coronavirus (COVID-19), December 3, 2020. (2021) https://www.cdc.gov/nchs/data/icd/Announcement-New-ICD-code-for-coronavirus-19-508.pdf
55. Gavrilova N, Soprun L, Lukashenko M, Ryabkova V, Fedotkina TV, Churilov LP, et al. New clinical phenotype of the post-COVID syndrome: fibromyalgia and joint hypermobility condition. Pathophysiology. (2022) 29:24–9. doi: 10.3390/pathophysiology29010003
56. Gensemer C, Burks R, Kautz S, Judge DP, Lavallee M, Norris RA. Hypermobile ehlers-danlos syndromes: complex phenotypes, challenging diagnoses, and poorly understood causes. Dev Dyn Off Publ Am Assoc Anat. (2021) 250:318–44. doi: 10.1002/dvdy.220
57. Miller AJ, Stiles LE, Sheehan T, Bascom R, Levy HP, Francomano CA, et al. Prevalence of hypermobile ehlers-danlos syndrome in postural orthostatic tachycardia syndrome. Auton Neurosci. (2020) 224:102637. doi: 10.1016/j.autneu.2020.102637.
58. Bragée B, Michos A, Drum B, Fahlgren M, Szulkin R, Bertilson BC. Signs of intracranial hypertension, hypermobility, and craniocervical obstructions in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front Neurol. (2020) 11:828. Available at: https://www.frontiersin.org/articles/10.3389/fneur.2020.00828 (Accessed November 29, 2022). doi: 10.3389/fneur.2020.00828
59. Eccles JA, Thompson B, Themelis K, Amato ML, Stocks R, Pound A, et al. Beyond bones: the relevance of variants of connective tissue (hypermobility) to fibromyalgia, ME/CFS and controversies surrounding diagnostic classification: an observational study. Clin Med. (2021) 21:53–8. doi: 10.7861/clinmed.2020-0743
60. Monaco A, Choi D, Uzun S, Maitland A, Riley B. Association of mast-cell-related conditions with hypermobile syndromes: a review of the literature. Immunol Res. (2022) 70:419–31. doi: 10.1007/s12026-022-09280-1
61. Fajardo I, Pejler G. Human mast cell β-tryptase is a gelatinase 1. J Immunol. (2003) 171:1493–9. doi: 10.4049/jimmunol.171.3.1493
62. Weinstock LB, Brook JB, Walters AS, Goris A, Afrin LB, Molderings GJ. Mast cell activation symptoms are prevalent in long-COVID. Int J Infect Dis. (2021) 112:217–26. doi: 10.1016/j.ijid.2021.09.043
63. Brock I, Chopra P, Maitland A, Francomano C. Ep305—frequency and co-occurrence of comorbidities in the ehlers-danlos syndromes. Mol Genet Metab. (2021) 132:S194. doi: 10.1016/S1096-7192(21)00387-5
64. Joseph P, Arevalo C, Oliveira RKF, Faria-Urbina M, Felsenstein D, Oaklander AL, et al. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. CHEST. (2021) 160:642–51. doi: 10.1016/j.chest.2021.01.082
65. Kohno R, Cannom DS, Olshansky B, Xi SC, Krishnappa D, Adkisson WO, et al. Mast cell activation disorder and postural orthostatic tachycardia syndrome: a clinical association. J Am Heart Assoc. (2021) 10:e021002. doi: 10.1161/JAHA.121.021002
66. Hernandez AMC, Dietrich JE. Gynecologic management of pediatric and adolescent patients with ehlers-danlos syndrome. J Pediatr Adolesc Gynecol. (2020) 33:291–5. doi: 10.1016/j.jpag.2019.12.011
67. Hugon-Rodin J, Lebègue G, Becourt S, Hamonet C, Gompel A. Gynecologic symptoms and the influence on reproductive life in 386 women with hypermobility type ehlers-danlos syndrome: a cohort study. Orphanet J Rare Dis. (2016) 11:124. doi: 10.1186/s13023-016-0511-2
68. Hurst BS, Lang MB, Kullstam SM, Usadi RS, Matthews ML, Marshburn PB. Reproductive challenges in women with ehlers-danlos syndrome: survey results from over 1350 respondents from the ehlers-danlos national foundation. Fertil Steril. (2012) 98:S112. doi: 10.1016/j.fertnstert.2012.07.411
69. Glayzer JE, McFarlin BL, Castori M, Suarez ML, Meinel MC, Kobak WH, et al. High rate of dyspareunia and probable vulvodynia in ehlers-danlos syndromes and hypermobility spectrum disorders: an online survey. Am J Med Genet C Semin Med Genet. (2021) 187:599–608. doi: 10.1002/ajmg.c.31939
70. Spiegel E, Nicholls-Dempsey L, Czuzoj-Shulman N, Abenhaim HA. Pregnancy outcomes in women with ehlers-danlos syndrome. J Matern Fetal Neonatal Med. (2020) 35:1683–9. doi: 10.1080/14767058.2020.1767574
71. Underhill LA, Barbarita C, Collis S, Tucker R, Lechner BE. Association of maternal versus fetal ehlers-danlos syndrome Status with poor pregnancy outcomes. Reprod Sci. (2022) 29:3459–64. doi: 10.1007/s43032-022-00992-1
72. Subramanian A, Nirantharakumar K, Hughes S, Myles P, Williams T, Gokhale KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. (2022) 28:1706–14. doi: 10.1038/s41591-022-01909-w
74. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. (2021) 397:839–52. doi: 10.1016/S0140-6736(21)00389-5
75. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. (2020) 382:1244–56. doi: 10.1056/NEJMra1810764
76. Laufer MR, Sanfilippo J, Rose G. Adolescent endometriosis: diagnosis and treatment approaches. J Pediatr Adolesc Gynecol. (2003) 16:S3–11. doi: 10.1016/s1083-3188(03)00066-4
77. Secosan C, Balulescu L, Brasoveanu S, Balint O, Pirtea P, Dorin G, et al. Endometriosis in menopause-renewed attention on a controversial disease. Diagn Basel Switz. (2020) 10:134. doi: 10.3390/diagnostics10030134
78. González RS, Vnencak-Jones CL, Shi C, Fadare O. Endomyometriosis (“uterus-like mass”) in an XY male: case report with molecular confirmation and literature review. Int J Surg Pathol. (2014) 22:421–6. doi: 10.1177/1066896913501385
79. Fukunaga M. Paratesticular endometriosis in a man with a prolonged hormonal therapy for prostatic carcinoma. Pathol Res Pract. (2012) 208:59–61. doi: 10.1016/j.prp.2011.10.007.22104297
80. Jabr FI, Mani V. An unusual cause of abdominal pain in a male patient: endometriosis. Avicenna J Med. (2014) 4:99–101. doi: 10.4103/2231-0770.140660
81. Breintoft K, Pinnerup R, Henriksen TB, Rytter D, Uldbjerg N, Forman A, et al. Endometriosis and risk of adverse pregnancy outcome: a systematic review and meta-analysis. J Clin Med. (2021) 10:667. doi: 10.3390/jcm10040667
82. Farland LV, Prescott J, Sasamoto N, Tobias DK, Gaskins AJ, Stuart JJ, et al. Endometriosis and risk of adverse pregnancy outcomes. Obstet Gynecol. (2019) 134:527–36. doi: 10.1097/AOG.0000000000003410
83. Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE Guideline: management of women with endometriosis †. Hum Reprod. (2014) 29:400–12. doi: 10.1093/humrep/det457
84. Hudelist G, Fritzer N, Thomas A, Niehues C, Oppelt P, Haas D, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod Oxf Engl. (2012) 27:3412–6. doi: 10.1093/humrep/des316
85. Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D. Impact of endometriosis on women's lives: a qualitative study. BMC Womens Health. (2014) 14:123. doi: 10.1186/1472-6874-14-123
86. Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. (2011) 96:366–373.e8. doi: 10.1016/j.fertnstert.2011.05.090
87. Pino I, Belloni GM, Barbera V, Solima E, Radice D, Angioni S, et al. “Better late than never but never late is better”, especially in young women. A multicenter Italian study on diagnostic delay for symptomatic endometriosis. Eur J Contracept Reprod. (2022) 28:1–7. doi: 10.1080/13625187.2022.2128644
88. Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod Oxf Engl. (2002) 17:2715–24. doi: 10.1093/humrep/17.10.2715
89. Thiruchelvam U, Wingfield M, O’Farrelly C. Natural killer cells: key players in endometriosis. Am J Reprod Immunol. (2015) 74:291–301. doi: 10.1111/aji.12408
90. Hogg C, Horne AW, Greaves E. Endometriosis-associated macrophages: origin, phenotype, and function. Front Endocrinol. (2020) 11:7. doi: 10.3389/fendo.2020.00007
91. Petrelluzzi KFS, Garcia MC, Petta CA, Grassi-Kassisse DM, Spadari-Bratfisch RC. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress Amst Neth. (2008) 11:390–7. doi: 10.1080/10253890701840610
92. Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, et al. Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell Longev. (2017) 2017:7265238. doi: 10.1155/2017/7265238.29057034
93. Kirchhoff D, Kaulfuss S, Fuhrmann U, Maurer M, Zollner TM. Mast cells in endometriosis: guilty or innocent bystanders? Expert Opin Ther Targets. (2012) 16:237–41. doi: 10.1517/14728222.2012.661415
94. Roerink ME, Roerink SHPP, Skoluda N, van der Schaaf ME, Hermus ARMM, van der Meer JWM, et al. Hair and salivary cortisol in a cohort of women with chronic fatigue syndrome. Horm Behav. (2018) 103:1–6. doi: 10.1016/j.yhbeh.2018.05.016
95. Eaton-Fitch N, du Preez S, Cabanas H, Staines D, Marshall-Gradisnik S. A systematic review of natural killer cells profile and cytotoxic function in myalgic encephalomyelitis/chronic fatigue syndrome. Syst Rev. (2019) 8:279. doi: 10.1186/s13643-019-1202-6
96. Gottschalk G, Peterson D, Knox K, Maynard M, Whelan RJ, Roy A. Elevated ATG13 in serum of patients with ME/CFS stimulates oxidative stress response in microglial cells via activation of receptor for advanced glycation end products (RAGE). Mol Cell Neurosci. (2022) 120:103731. doi: 10.1016/j.mcn.2022.103731
97. Sasso EM, Muraki K, Eaton-Fitch N, Smith P, Lesslar OL, Deed G, et al. Transient receptor potential melastatin 3 dysfunction in post COVID-19 condition and myalgic encephalomyelitis/chronic fatigue syndrome patients. Mol Med Camb Mass. (2022) 28:98. doi: 10.1186/s10020-022-00528-y
98. Klein J, Wood J, Jaycox J, Lu P, Dhodapkar RM, Gehlhausen JR, et al. Distinguishing features of long COVID identified through immune profiling. MedRxiv Prepr Serv Health Sci. (2022) 2022.08.09:22278592. doi: 10.1101/2022.08.09.22278592
99. Villaume WA. Marginal BH4 deficiencies, iNOS, and self-perpetuating oxidative stress in post-acute sequelae of COVID-19. Med Hypotheses. (2022) 163:110842. doi: 10.1016/j.mehy.2022.110842
100. Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. (2014) 2014:179515. doi: 10.1155/2014/179515
101. Cousins FL, McKinnon BD, Mortlock S, Fitzgerald HC, Zhang C, Montgomery GW, et al. New concepts on the etiology of endometriosis. J Obstet Gynaecol Res. (2023) 49:1090–105. doi: 10.1111/jog.15549
102. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, et al. Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol. (2018) 17:125–33. doi: 10.1002/rmb2.12083
103. Khan KN, Kitajima M, Hiraki K, Yamaguchi N, Katamine S, Matsuyama T, et al. Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil Steril. (2010) 94:2860–2863.e1–3. doi: 10.1016/j.fertnstert.2010.04.053
104. Yuan W, Wu Y, Chai X, Wu X. The colonized microbiota composition in the peritoneal fluid in women with endometriosis. Arch Gynecol Obstet. (2022) 305:1573–80. doi: 10.1007/s00404-021-06338-7
105. Noh EJ, Kim DJ, Lee JY, Park JH, Kim J-S, Han JW, et al. Ureaplasma urealyticum infection contributes to the development of pelvic endometriosis through toll-like receptor 2. Front Immunol. (2019) 10:2373. doi: 10.3389/fimmu.2019.02373
106. Proal AD, Lindseth IA, Marshall TG. Microbe-microbe and host-microbe interactions drive microbiome dysbiosis and inflammatory processes. Discov Med. (2017) 23:51–60.28245427
107. Proal A, Marshall T. Myalgic encephalomyelitis/chronic fatigue syndrome in the era of the human microbiome: persistent pathogens drive chronic symptoms by interfering with host metabolism, gene expression, and immunity. Front Pediatr. (2018) 6:373. doi: 10.3389/fped.2018.00373
108. Proal AD, Albert PJ, Marshall TG. The human microbiome and autoimmunity. Curr Opin Rheumatol. (2013) 25:234–40. doi: 10.1097/BOR.0b013e32835cedbf
109. Natarajan A, Zlitni S, Brooks EF, Vance SE, Dahlen A, Hedlin H, et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Med. (2022) 3:371–387.e9. doi: 10.1016/j.medj.2022.04.001
110. Zollner A, Koch R, Jukic A, Pfister A, Meyer M, Rössler A, et al. Postacute COVID-19 is characterized by gut viral antigen persistence in inflammatory bowel diseases. Gastroenterology. (2022) 163:495–506.e8. doi: 10.1053/j.gastro.2022.04.037
111. Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC-Y, Ng SSS, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. (2022) 71:544–52. doi: 10.1136/gutjnl-2021-325989
112. Haran JP, Bradley E, Zeamer AL, Cincotta L, Salive M-C, Dutta P, et al. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight. (2021) 6:e152346. doi: 10.1172/jci.insight.152346
113. Mirin AA, Dimmock ME, Jason LA. Research update: the relation between ME/CFS disease burden and research funding in the USA. Work. (2020) 66:277–82. doi: 10.3233/WOR-203173
114. Pretorius E, Venter C, Laubscher G, Kotze M, Moremi K, Oladejo S, et al. Combined triple treatment of fibrin amyloid microclots and platelet pathology in individuals with long COVID/ post-acute sequelae of COVID-19 (PASC) can resolve their persistent symptoms. (2021). doi: 10.21203/rs.3.rs-1205453/v1
115. Nunes JM, Kruger A, Proal A, Kell DB, Pretorius E. The occurrence of hyperactivated platelets and fibrinaloid microclots in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Pharmaceuticals. (2022) 15:931. doi: 10.3390/ph15080931
116. Swanepoel AC, Visagie A, de Lange Z, Emmerson O, Nielsen VG, Pretorius E. The clinical relevance of altered fibrinogen packaging in the presence of 17β-estradiol and progesterone. Thromb Res. (2016) 146:23–34. doi: 10.1016/j.thromres.2016.08.022
117. Jing Y, Run-Qian L, Hao-Ran W, Hao-Ran C, Ya-Bin L, Yang G, et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod. (2020) 26:367–73. doi: 10.1093/molehr/gaaa030
118. Buonsenso D, Piazza M, Boner AL, Bellanti JA. Long COVID: a proposed hypothesis-driven model of viral persistence for the pathophysiology of the syndrome. Allergy Asthma Proc. (2022) 43:187–93. doi: 10.2500/aap.2022.43.220018
119. Shultz SJ, Sander TC, Kirk SE, Perrin DH. Sex differences in knee joint laxity change across the female menstrual cycle. J Sports Med Phys Fitness. (2005) 45:594–603.16446695
120. Jeziorska M, Salamonsen LA, Woolley DE. Mast cell and eosinophil distribution and activation in human endometrium throughout the menstrual cycle. Biol Reprod. (1995) 53:312–20. doi: 10.1095/biolreprod53.2.312
121. Sugamata M, Ihara T, Uchiide I. Increase of activated mast cells in human endometriosis. Am J Reprod Immunol. (2005) 53:120–5. doi: 10.1111/j.1600-0897.2005.00254.x
122. Binda MM, Donnez J, Dolmans M-M. Targeting mast cells: a new way to treat endometriosis. Expert Opin Ther Targets. (2017) 21:67–75. doi: 10.1080/14728222.2017.1260548
123. Szewczyk G, Pyzlak M, Klimkiewicz J, Smiertka W, Miedzińska-Maciejewska M, Szukiewicz D. Mast cells and histamine: do they influence placental vascular network and development in preeclampsia? Mediators Inflamm. (2012) 2012:307189. doi: 10.1155/2012/307189
124. Leonardi M, Hicks C, El-Assaad F, El-Omar E, Condous G. Endometriosis and the microbiome: a systematic review. BJOG Int J Obstet Gynaecol. (2020) 127:239–49. doi: 10.1111/1471-0528.15916
125. Ata B, Yildiz S, Turkgeldi E, Brocal VP, Dinleyici EC, Moya A, et al. The endobiota study: comparison of vaginal, cervical and gut Microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep. (2019) 9:2204. doi: 10.1038/s41598-019-39700-6
126. Vasundhara D, Raju VN, Hemalatha R, Nagpal R, Kumar M. Vaginal & gut microbiota diversity in pregnant women with bacterial vaginosis & effect of oral probiotics: an exploratory study. Indian J Med Res. (2021) 153:492–502. doi: 10.4103/ijmr.IJMR_350_19
127. Byrne EH, Farcasanu M, Bloom SM, Xulu N, Xu J, Hykes BL, et al. Antigen presenting cells link the female genital tract microbiome to mucosal inflammation, with hormonal contraception as an additional modulator of inflammatory signatures. Front Cell Infect Microbiol. (2021) 11:733619. doi: 10.3389/fcimb.2021.733619
128. Perrotta AR, Borrelli GM, Martins CO, Kallas EG, Sanabani SS, Griffith LG, et al. The vaginal microbiome as a tool to predict rASRM stage of disease in endometriosis: a pilot study. Reprod Sci. (2020) 27:1064–73. doi: 10.1007/s43032-019-00113-5
129. Power ML, Quaglieri C, Schulkin J. Reproductive microbiomes: a new thread in the microbial network. Reprod Sci Thousand Oaks Calif. (2017) 24:1482–92. doi: 10.1177/1933719117698577
130. Hsieh T-C, Edwards NC, Bhattacharyya SK, Nitschelm KD, Burnett AL. The epidemic of COVID-19-related erectile dysfunction: a scoping review and health care perspective. Sex Med Rev. (2022) 10:286–310. doi: 10.1016/j.sxmr.2021.09.002
131. Saini G, Aneja R. Cancer as a prospective sequela of long COVID-19. Bioessays. (2021) 43:2000331. doi: 10.1002/bies.202000331
132. Mecha EO, Njagi JN, Makunja RN, Omwandho COA, Saunders PTK, Horne AW. Endometriosis among African women. Reprod Fertil. (2022) 3:C40–3. doi: 10.1530/RAF-22-0040
133. Bayliss K, Riste L, Fisher L, Wearden A, Peters S, Lovell K, et al. Diagnosis and management of chronic fatigue syndrome/myalgic encephalitis in black and minority ethnic people: a qualitative study. Prim Health Care Res Dev. (2014) 15:143–55. doi: 10.1017/S1463423613000145
134. Varma T, Jones CP, Oladele C, Miller J. Diversity in clinical research: public health and social justice imperatives. J Med Ethics. (2022) 49:200–3. doi: 10.1136/medethics-2021-108068
135. Plesons M, Patkar A, Babb J, Balapitiya A, Carson F, Caruso BA, et al. The state of adolescent menstrual health in low- and middle-income countries and suggestions for future action and research. Reprod Health. (2021) 18:31. doi: 10.1186/s12978-021-01082-2
136. Patterson EJ, Becker A, Baluran DA. Gendered racism on the body: an intersectional approach to maternal mortality in the United States. Popul Res Policy Rev. (2022) 51:1261–94. doi: 10.1007/s11113-021-09691-2
137. National Academies of Sciences E, Division H and M, Practice B on PH and PH, States C on C-BS to PHE in the U, Baciu A, Negussie Y, Geller A, Weinstein JN. The state of health disparities in the United States. Washington (DC): National Academies Press (US) (2017. Available at: https://www.ncbi.nlm.nih.gov/books/NBK425844/ (Accessed December 2, 2022).
138. Zhang L, Losin EAR, Ashar YK, Koban L, Wager TD. Gender biases in estimation of Others’ pain. J Pain. (2021) 22:1048–59. doi: 10.1016/j.jpain.2021.03.001
139. Chen EH, Shofer FS, Dean AJ, Hollander JE, Baxt WG, Robey JL, et al. Gender disparity in analgesic treatment of emergency department patients with acute abdominal pain. Acad Emerg Med. (2008) 15:414–8. doi: 10.1111/j.1553-2712.2008.00100.x
140. As-Sanie S, Black R, Giudice LC, Gray Valbrun T, Gupta J, Jones B, et al. Assessing research gaps and unmet needs in endometriosis. Am J Obstet Gynecol. (2019) 221:86–94. doi: 10.1016/j.ajog.2019.02.033
141. Sims OT, Gupta J, Missmer SA, Aninye IO. Stigma and endometriosis: a brief overview and recommendations to improve psychosocial well-being and diagnostic delay. Int J Environ Res Public Health. (2021) 18:8210. doi: 10.3390/ijerph18158210
142. Avnoon N. Time for women-centred gynaecology. Nat Rev Urol. (2022) 19:689–90. doi: 10.1038/s41585-022-00656-4
143. Au L, Capotescu C, Eyal G, Finestone G. Long COVID and medical gaslighting: dismissal, delayed diagnosis, and deferred treatment. Ssm Qual Res Health. (2022) 2:100167. doi: 10.1016/j.ssmqr.2022.100167
144. Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, et al. Clinical diagnosis of endometriosis: a call to action. Am J Obstet Gynecol. (2019) 220:354.e1–354.e12. doi: 10.1016/j.ajog.2018.12.039
145. Nacul L, Authier FJ, Scheibenbogen C, Lorusso L, Helland IB, Martin JA, et al. European Network on myalgic encephalomyelitis/chronic fatigue syndrome (EUROMENE): expert consensus on the diagnosis, service provision, and care of people with ME/CFS in Europe. Med Kaunas Lith. (2021) 57:510. doi: 10.3390/medicina57050510
Keywords: Long COVID, reproductive health, myalgic encephalomyelitis, endometriosis, postural orthostatic tachycardia syndrome, Ehlers-Danlos sydrome, post-acute sequalae of SARS-CoV-2 infection, female
Citation: Pollack B, von Saltza E, McCorkell L, Santos L, Hultman A, Cohen AK and Soares L (2023) Female reproductive health impacts of Long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: a literature review. Front. Rehabil. Sci. 4:1122673. doi: 10.3389/fresc.2023.1122673
Received: 13 December 2022; Accepted: 3 April 2023;
Published: 28 April 2023.
Edited by:
David Putrino, Icahn School of Medicine at Mount Sinai, United StatesReviewed by:
Mariam Zakhary, Mount Sinai Hospital, United States© 2023 Pollack, von Saltza, McCorkell, Santos, Hultman, Cohen and Soares. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisa McCorkell lisa@patientledresearch.com Letícia Soares leticiassoares@gmail.com
 Beth Pollack
Beth Pollack Emelia von Saltza
Emelia von Saltza Lisa McCorkell
Lisa McCorkell Lucia Santos2
Lucia Santos2  Letícia Soares
Letícia Soares