- Department of Radiation Biology, Institute for Aerospace Medicine, German Aerospace Center, Cologne, Germany
When we humans travel, our microorganisms come along. These can be harmless but also pathogenic, and are spread by touching surfaces or breathing aerosols in the passenger cabins. As the pandemic with SARS-CoV-2 has shown, those environments display a risk for infection transmission. For a risk reduction, countermeasures such as wearing face masks and distancing were applied in many places, yet had a significant social impact. Nevertheless, the next pandemic will come and additional countermeasures that contribute to the risk reduction are needed to keep commuters safe and reduce the spread of microorganisms and pathogens, but also have as little impact as possible on the daily lives of commuters. This review describes the bacterial microbiome of subways around the world, which is mainly characterized by human-associated genera. We emphasize on healthcare-associated ESKAPE pathogens within public transport, introduce state-of-the art methods to detect common microbes and potential pathogens such as LAMP and next-generation sequencing. Further, we describe and discuss possible countermeasures that could be deployed in public transportation systems, as antimicrobial surfaces or air sterilization using plasma. Commuting in public transport can harbor risks of infection. Improving the safety of travelers can be achieved by effective detection methods, microbial reduction systems, but importantly by hand hygiene and common-sense hygiene guidelines.
1 Introduction and discussion
Viruses play a major role in the spread of infectious diseases, most recently SARS-CoV-2, which was responsible for the COVID-19 pandemic. Even before the occurrence of SARS-CoV-2, the Influenza waves are causing 15,000–70,000 deaths of European citizens every year (1).
However, in addition to viruses, bacteria are also responsible for the spread of infectious diseases. More than half of emerging infectious diseases are caused by bacteria, many of which are drug-resistant (2). Antimicrobial resistance has long been recognized as an acute danger and is also referred to in the literature as a silent pandemic (3). The spread of microorganisms and thus also pathogens does not necessarily begin in hospitals, but rather where people move around.
1.1 Humans represent the main source of bacteria within subways
Subway systems are widely used, especially in big cities and carry millions of passengers every day. The high frequency of passengers using public transportation facilitates an exchange of microorganisms, especially when getting in contact with frequently touched surfaces such as handrails, and sharing the air within a confined space. In this review, the most common taxa within the subway microbiome of different cities are presented, and relevant risk factors are discussed. Further, a range of microbial detection methods are listed and countermeasures that may be applied in public transport are described.
Touching objects, such as handrails, leads to a transfer of the human hand microbiome to the touched object. In recent studies, the transfer of the hand microbiome from test subjects to objects was demonstrated (4, 5), which can also be transferred to the subway environment. The most abundant organisms found in subway microbiome studies in various cities are displayed in Table 1. Among those, most frequently occurring taxa were Acinetobacter, Staphylococcus, Propionibacterium, Corynebacterium, Micrococcus, Streptococcus, and Kocuria, which are common for the human skin microbiome (15–17). These studies were not specifically focused on the detection of pathogens, and only a few were found such as Helicobacter pylori, Acinetobacter species (sp.) (10) as well as opportunistic pathogenic isolates related to the species Propionibacterium acnes and Staphylococcus epidermidis or genera Pseudonocardia and Nesterenkonia (14). An important factor that should be considered is that in most of the studies listed, microbial detection was based on 16S rRNA sequencing, which does not provide adequate detection at the species level (18, 19) and therefore, no pathogenic strains were conclusively detected.
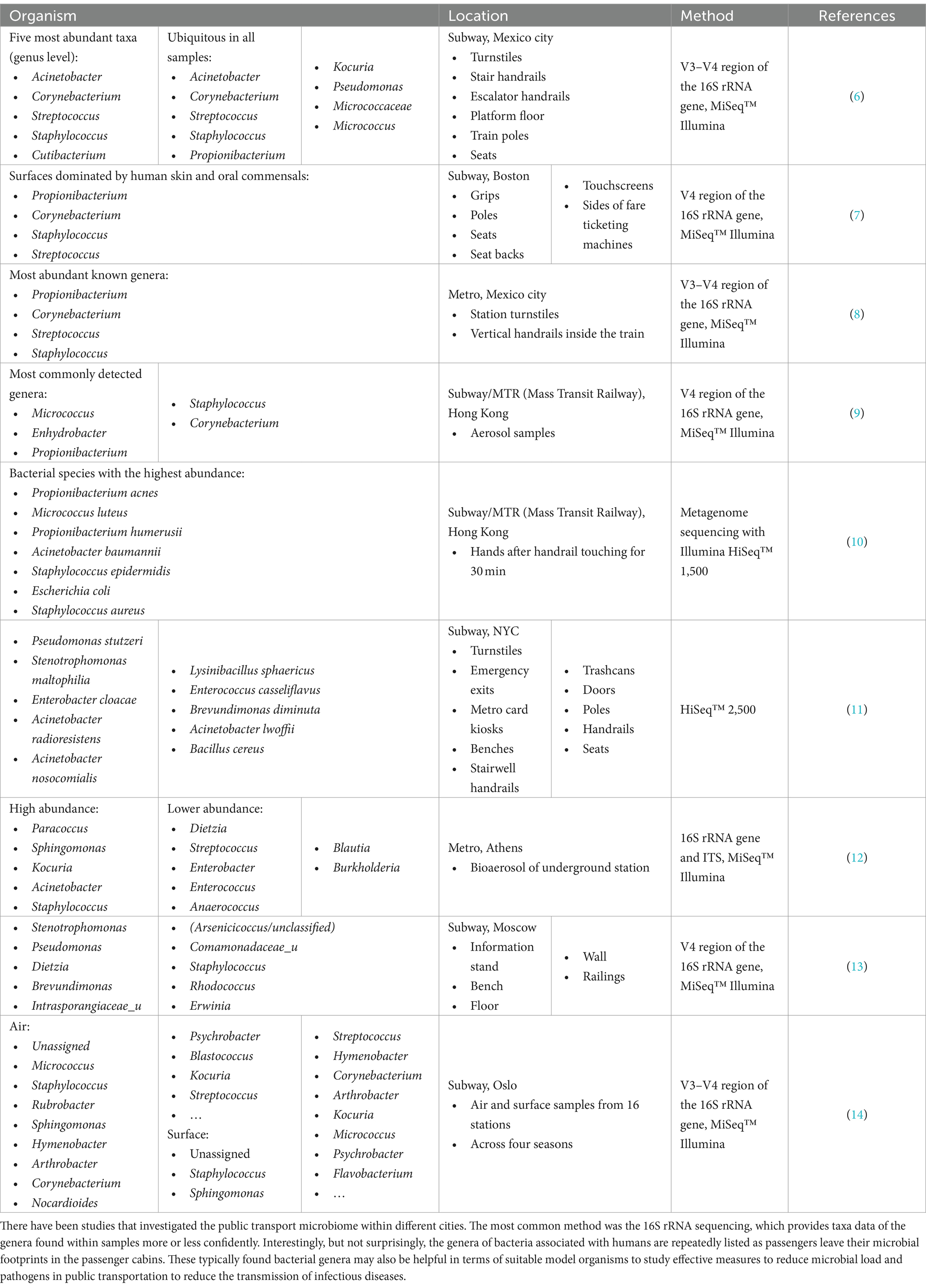
Table 1. Overview of abundant bacteria across subways and subway stations found in multiple studies.
For this section, we reviewed 30 research articles, including nine studies on the subway microbiome, 12 studies on the occurrence of ESKAPE pathogens in public transportation environments, and 7 studies on general information on the human skin microbiome, the association with surfaces and the detection of pathogenic species in general.
1.1.1 ESKAPE pathogens—detected in public transport?
ESKAPE pathogens are the causative agents of most nosocomial infections worldwide. The abbreviation stands for the species Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterococcus faecium/faecalis. Those organisms can be highly virulent and carry or transfer antibiotic resistances (20, 21).
Antibiotic resistance and the spread of multidrug-resistant bacteria (AMR) is a problem that was associated with about 4.9 million deaths worldwide in 2019 (22). However, the spread of these organisms does not occur in hospitals alone, but also in places with a high frequency of people, such as on public transportation. Notably, the following studies were focused on the detection of pathogenic species, mostly based on (selective) cultivation followed by PCR of pathogen-associated marker genes, e.g., the mcr-1 gene for E. coli that mediates colistin resistance.
The most prominent ESKAPE species within the public transport studies is the (opportunistic) pathogen Staphylococcus aureus. In general, due to the natural passenger’s microbiome, the skin-associated species are highly abundant in busses and subways. In a bus, serving both hospital and community routes, methicillin-resistant S. aureus (MRSA) was found (community-associated SCCmec type IV and healthcare-associated SCCmec type II). Of the detected MRSA strains, 65% were multidrug resistant (23). Within this study, seats and seat rails were most contaminated. In subways, S. aureus containing the mecA gene was detected, alongside natural skin-associated species of S. aureus (11). mecA is associated with methicillin-resistant S. aureus (MRSA) and nosocomial infections, but the study concludes no strong evidence for pathogenicity based on the obtained sequences. Other studies showed the prevalence of MRSA in public transport (24–27).
Escherichia coli with a multidrug resistance, including mcr-1 which mediates colistin resistance, was found in public transportation in Guangzhou, China (28). Twenty-three isolates of 737 samples with bacterial growth were positive for mcr-1, most of them were resistant against ampicillin, cefotaxime, fosfomycin, and gentamicin.
For Klebsiella pneumoniae, there were less findings of drug resistant isolates. In the Beijing (China) subway environment, highly touched surfaces were sampled and from a total of 603 samples across 15 metro lines, 11 carbapenem-resistant K. pneumoniae isolates were detected (29).
Enterobacter species were also found in public transport studies. E. faecium was abundant throughout the subway in New York City, United States (11), and multidrug resistant E. faecalis was observed on shared bicycles in Chengdu, China (30).
No studies have been found on the occurrence of multidrug resistant Acinetobacter baumannii and Pseudomonas aeruginosa in public transport.
1.2 Microbial detection methods
There are a number of options for identifying bacteria. Classical biochemical or physiological methods such as microscopy are time-consuming and inefficient when it comes to examining a large number of samples and identifying the organism. Most studies reviewed within this work used the methodology of next-generation sequencing (1.2.1), which displays a modern and high-throughput detection approach, in contrast to cultivation on nutrient media. Latter allows the analysis of organisms that can grow under specific conditions. Several other methods exist, such as matrix-assisted laser desorption ionization coupled to time-of-flight mass spectrometry (MALDI-TOF MS) (31), or tandem mass spectrometry (19).
1.2.1 Next-generation-sequencing
Nowadays, next-generation-sequencing (NGS) is the most used technology for sequencing. With this approach, high throughput analysis is possible and enables the identification of microbes within a high sample size in a cost-effective manner, generating high amounts of data (32, 33). There is a big variation within the NGS DNA sequencing technologies, varying in amplification method, sequencing chemistry, sequencing speed, etc. (32, 33). The most established NGS platform was created by Illumina, followed by Oxford Nanopore, which revolutionized the field by releasing a first portable nanopore sequencing device in 2014. Each technology differs in its output, advantages and limitations. With NGS, not only the microbial identity can be detected, also the diversity within or of all samples can be determined, outshining the limited information obtained by cultivation. The possibilities within NGS and the bioinformatical analysis are rapidly evolving more and more. Nevertheless, there are some shortcomings when it comes to profiling uncharacterized species in environmental microbiomes, as strain-level analyses are usually tested for human metagenomes and the tools are tailored to human metagenomes (34).
1.2.2 Loop-mediated isothermal amplification
Metagenomics is a powerful tool to identify the microbiome of a sample. If specific organisms are to be screened for, such as potential pathogens, there are methods such as loop-mediated isothermal amplification (LAMP) that allow the targeted detection of species. LAMP is a fast, cost effective, and easy tool to detect specific organisms and requires only a few devices, while the evaluation occurs after 30 min.
Using marker genes, which differ for every organism, fast and detailed detection with a high specificity and sensitivity are possible (35). The potential of LAMP has already been established in relation to the detection of pathogens in the food industry (36). It is also useful for hospitals or in human high traffic environments to monitor microbial threats. During the SARS-CoV-2 pandemic, several publications showed the successful application of reverse transcription LAMP for this pathogen (37–39).
To this date and to our knowledge, there is no publication on the use of LAMP for pathogen detection in public transport as microbial monitoring measure. The detection of drug-resistant organisms is an important factor in monitoring the spread of pathogens and has yet to be implemented.
1.3 Countermeasures and feasibility in public transport
A summary of the mentioned countermeasures is displayed in Figure 1. The term antimicrobial includes not only bacteria, but also other groups such as viruses and fungi. But even within the group of bacteria, the effect of countermeasures varies depending on the bacterium, e.g., in the case of spore-forming bacteria, as their spores can be highly resistant to heat, for example (40, 41). Many of the mentioned countermeasures have been tested within hospital settings and in food industry, since the urge of clean and sterile environments is inevitable in those areas. Passenger cabins do not have to be sterile, but to provide an environment that does not promote the transmission of (opportunistic) pathogens, measures are needed.
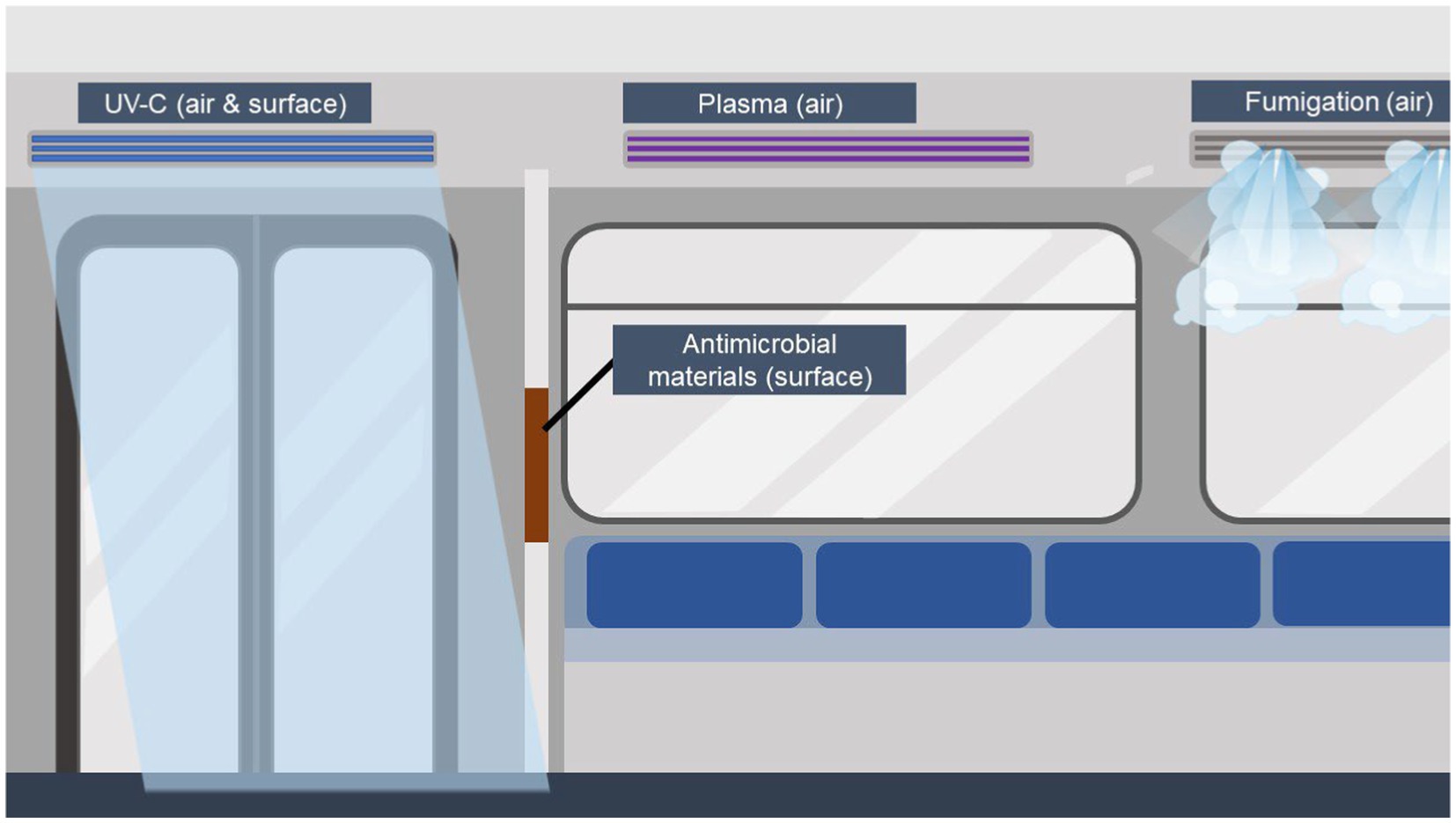
Figure 1. Overview of described microbial countermeasures in public transport. For air cleaning and disinfection, UV-C, fumigation of disinfectants, and plasma air sterilization can be used. Of those, plasma air cleaning is suitable for the use during passenger occurrence. For the reduced microbial burden on (highly) touched surfaces, antimicrobial surfaces can be implemented. UV-C can be installed during cleaning times without any passenger on board, as well as the fumigation of chemicals. Created with BioRender.com.
1.3.1 Antimicrobial surfaces
The transmission of pathogens is especially meaningful when it occurs through surfaces in epidemic and endemic scenarios (42). Although the transmission of pathogens through contact surfaces can be reduced by antimicrobial surfaces, the long-term usage and consequences have to be evaluated. One important factor is the increased risk of the development and transmission of antibiotic resistances between bacteria, when such materials are overused (43).
One of the best investigated antimicrobial material is copper. It causes cell damage by releasing copper ions which causes the cell membrane to rupture, leading to a membrane potential loss and depletion of cytoplasmic subtances (44). Further, copper ions induce reactive oxygen species (ROS), which in turn cause DNA damage (45). While copper as a material is costly, surfaces using the antibacterial effect of copper and integrating it as metal nanoparticles within a polymer matrix makes it cost effective, as reviewed by Tamayo et al. (46), and therefore could be suitable for a broad use.
While the antimicrobial properties of copper have long been known and researched, there are many different antimicrobial surfaces available (47, 48). For example, anti-biofouling surfaces can reduce microbial adhesion to the surfaces, biocidal nanocomposites kill microbes using biocidal species. Physical mechanisms as nanostructured surfaces can rupture bacterial cells, others can even prevent the attachment on the surfaces (49).
Some innovative antimicrobial materials were already tested in public transportation, such as antimicrobial photodynamic coatings, showing an absolute risk reduction of 22.6% for high bacterial counts (50). Other tested materials showed no significant reduction of the microbial burden, using photocatalyst-coated and uncoated hand-contact surfaces (51).
1.3.2 Fumigation of chemicals as an antimicrobial approach
In the process of fumigation, an antimicrobial solution is nebulized in an enclosed environment with the aim to reduce the microbial burden in the air and on surfaces. The nebulization of chemicals, e.g., hydrogen peroxide has been in use (52, 53). There are also different forms of fumigation, that even consider the application in public transport (54). Here, peracetic acid stabilized with acetic acid and hydrogen peroxide showed an effectiveness of disinfection of 81.7% in busses, and even worked against highly resistant spores. Hydrogen peroxide facilitates the penetration of peracetic acid, which contributes to a fortified sporicidal activity of the agents, as tested with Bacillus subtilis spores (55).
The effectiveness of fumigation is highly dependent on the materials to be disinfected (54, 56), e.g., the effect of fogged peracetic acid and hydrogen peroxide was shown to be particularly high on glass windows and doors, and low on fabric materials (56). Further, the efficacy depends on the type of microorganism, the fumigation device and technology and the substance (57–59). One downfall of the fumigation of chemicals is the safety measures, that have to be ensured. Therefore, the usage of fumigation can only occur while the passenger cabins are not in service, but could be performed during night times. Considering the costs of fumigation, it depends on the device and fumigation technologies used. Costs for consumables are low, e.g., ~2 € / L of hydrogen peroxide.
1.3.3 UV-C
Another method for disinfection in public transport, but more commonly employed in hospital settings, is UV-C disinfection. UV radiation causes DNA damage (60), which is mediated by the generation of ROS (61). UV-C operates in a spectrum of 200–280 nm. Because UV-C is also harmful to humans, some efforts have been made to employ mobile robots for disinfection with UV-C radiation (62, 63). In hospitals, there have been several systems using and testing UV-C disinfection, that are combined with disinfectant chemical agents (64, 65). One disadvantage of this approach is the material damage (66) and the incomplete light contact in all areas in a room or cabin. An advantage of UV-C disinfection is the economical aspect. Some low-cost UV-C light devices can be purchased with high efficacy against strains of Candida auris, MRSA, and bacteriophage Phi6 (67). Although UV-C disinfection shows effectiveness against some pathogens, it can cause bacterial mutations (68). A new, LED-based UV-irradiation technology has shown to be effective against some bacteria and viruses, but it is connected to high costs (69), which is uneconomical for use in public transport.
1.3.4 Plasma sterilization
A tool designed to provide both air purification and surface disinfection is plasma. Plasma is also known as the fourth state of matter, which is a particle mix with a high electrical conductivity and is chemically reactive. The use of plasma is well established in the food industry (70) and in the medical field (71, 72), but it may be useful for the application in public transport.
The antimicrobial effect of plasma has been long known and is created by the combination or single effect of charged particles (ions, electrons), reactive species (e.g., ozone, ROS), radiation of UV-C/Vacuum-UV (VUV) as well as heating (73–75).
There are different types of plasma that can be used for disinfection. In a study conducted by Liang and Wu (76), culturable bacterial aerosol diversity loss was observed after using non-thermal plasma. Tested with Aspergillus niger, Bacillus subtilis, and Pseudomonas fluorescens as test organisms, it was described as a highly efficient air decontamination method.
To date, no study has used plasma as a system to reduce the microbial load in public transport. Only plasma related methods, such as a needle-point bipolar ionization system was tested in trams to investigate the reduction of bioaerosols (77). It was shown that environmental bioaerosols were reduced with this method, but it was not sufficient for surfaces. Therefore, more research is needed to test the feasibility of plasma technologies in the public transport context. Regarding cost-efficiency, only publications are available on the use of plasma in water treatment plants or in food industry (78), using plasma activated water (79). But in general, the formation of non-thermal plasma is connected to low energy input, unlike thermal plasma (80).
All approaches that were introduced in this section have their advantages and disadvantages. Different factors have to be considered when finding a best suiting method for a specific environment, such as passenger cabins. These include cost-effectiveness, service life, operation of devices, combined with the effectiveness of microbial reduction. In the end, the aim to apply countermeasures within the passenger cabins is to reduce the microbial load and therefore decrease the spread of potential pathogens, and a combination of some methods may bring all advantages together and ensure passenger safety and comfort.
2 Conclusion
In this review, the most common bacterial organisms from studies of the public transport microbiome were presented. Most studies performed 16S rRNA sequencing to identify the microbiome. The results showed that the public transport microbiome is dominated by human-associated organisms, while no pathogens were detected. However, targeted studies have shown that many of the so-called ESKAPE organisms in particular are found in public transportation and that this can be the place for the transmission of pathogens.
The use of the presented countermeasures in public transport was classified in this review. The purpose of this research is to show what is shaping our microbiome in public transportation and how specific organisms can be detected, but also reduced, to create a safe environment where pathogen transmission is minimized. However, this review also shows that more research is still needed to establish microbial reduction measures in public transportation.
Author contributions
Y-TL: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. SL: Conceptualization, Writing – review & editing. RM: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Y-TL was supported by the DLR grant DLR-VO: GANDALF. RM and SL were supported by the DLR grants: FuE-Projekt “ISS LIFE” (Program RF-FuW, TP 475) and Traffic (RoSto and VMo4Orte). These data will be included in the Ph.D. thesis of Y-TL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. ECDC. (2022). Factsheet about seasonal influenza. Available at: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet (Accessed February 13, 2024).
2. Jones, KE, Patel, NG, Levy, MA, Storeygard, A, Balk, D, Gittleman, JL, et al. Global trends in emerging infectious diseases. Nature. (2008) 451:990–3. doi: 10.1038/nature06536
3. Rehman, S. A parallel and silent emerging pandemic: antimicrobial resistance (AMR) amid COVID-19 pandemic. J Infect Public Health. (2023) 16:611–7. doi: 10.1016/j.jiph.2023.02.021
4. Hoisington, AJ, Stamper, CE, Bates, KL, Stanislawski, MA, Flux, MC, Postolache, TT, et al. Human microbiome transfer in the build environment differs based on occupants, objects, and buildings. Sci Rep. (2023) 13:6446. doi: 10.1038/s41598-023-33719-6
5. Meadow, JF, Altrichter, AE, Kembel, SW, Moriyama, M, O’Connor, TK, Womack, AM, et al. Bacterial communities on classroom surfaces vary with human contact. Microbiome. (2014) 2:2–7. doi: 10.1186/2049-2618-2-7
6. Vargas-Robles, D, Gonzalez-Cedillo, C, Hernandez, AM, Alcaraz, LD, and Peimbert, M. Passenger-surface microbiome interactions in the subway of Mexico City. PLoS One. (2020) 15:e0237272. doi: 10.1371/journal.pone.0237272
7. Hsu, T, Joice, R, Vallarino, J, Abu-Ali, G, Hartmann, EM, Shafquat, A, et al. Urban transit system microbial communities differ by surface type and interaction with humans and the environment. mSystems. (2016) 1:e00018-16. doi: 10.1128/mSystems.00018-16
8. Hernández, AM, Vargas-Robles, D, Alcaraz, LD, and Peimbert, M. Station and train surface microbiomes of Mexico City’s metro (subway/underground). Sci Rep. (2019) 10:8798. doi: 10.1101/735027
9. Leung, MH, Wilkins, D, Li, EK, Kong, FK, and Lee, PK. Indoor-air microbiome in an urban Subway network: diversity and dynamics. Appl Environ Microbiol. (2014) 80:6760–70. doi: 10.1128/AEM.02244-14
10. Kang, K, Ni, Y, Li, J, Imamovic, L, Sarkar, C, Kobler, MD, et al. The environmental exposures and inner- and intercity traffic flows of the metro system may contribute to the skin microbiome and Resistome. Cell Rep. (2018) 24:1190–202 e5. doi: 10.1016/j.celrep.2018.06.109
11. Afshinnekoo, E, Meydan, C, Chowdhury, S, Jaroudi, D, Boyer, C, Bernstein, N, et al. Geospatial resolution of human and bacterial diversity with City-scale metagenomics. Cell Syst. (2015) 1:72–87. doi: 10.1016/j.cels.2015.01.001
12. Grydaki, N, Colbeck, I, Mendes, L, Eleftheriadis, K, and Whitby, C. Bioaerosols in the athens metro: metagenetic insights into the PM(10) microbiome in a naturally ventilated subway station. Environ Int. (2021) 146:106186. doi: 10.1016/j.envint.2020.106186
13. Klimenko, NS, Tyakht, AV, Toshchakov, SV, Shevchenko, MA, Korzhenkov, AA, Afshinnekoo, E, et al. Co-occurrence patterns of Bacteria within microbiome of Moscow Subway. Comput Struct Biotechnol J. (2020) 18:314–22. doi: 10.1016/j.csbj.2020.01.007
14. Gohli, J, Boifot, KO, Moen, LV, Pastuszek, P, Skogan, G, Udekwu, KI, et al. The Subway microbiome: seasonal dynamics and direct comparison of air and surface bacterial communities. Microbiome. (2019) 7:160. doi: 10.1186/s40168-019-0772-9
15. Grice, EA, Kong, HH, Renaud, G, Young, AC, Program, NCS, Bouffard, GG, et al. A diversity profile of the human skin microbiota. Genome Res. (2008) 18:1043–50. doi: 10.1101/gr.075549.107
16. Byrd, AL, Belkaid, Y, and Segre, JA. The human skin microbiome. Nat Rev Microbiol. (2018) 16:143–55. doi: 10.1038/nrmicro.2017.157
17. Wang, Y, Yu, Q, Zhou, R, Feng, T, Hilal, MG, and Li, H. Nationality and body location Alter human skin microbiome. Appl Microbiol Biotechnol. (2021) 105:5241–56. doi: 10.1007/s00253-021-11387-8
18. Winand, R, Bogaerts, B, Hoffman, S, Lefevre, L, Delvoye, M, Braekel, JV, et al. Targeting the 16S rRNA Gene for bacterial identification in complex mixed samples: comparative evaluation of second (Illumina) and third (Oxford Nanopore technologies) generation sequencing technologies. Int J Mol Sci. (2019) 21:298. doi: 10.3390/ijms21010298
19. Runzheimer, K, Lozano, C, Boy, D, Boy, J, Godoy, R, Matus, FJ, et al. Exploring Andean high-altitude Lake extremophiles through advanced Proteotyping. J Proteome Res. (2024). doi: 10.1021/acs.jproteome.3c00538
20. De Oliveira, DM, Forde, BM, Kidd, TJ, Harris, PN, Schembri, MA, Beatson, SA, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. (2020) 33:10–1128. doi: 10.1128/CMR.00181-19
21. Denissen, J, Reyneke, B, Waso-Reyneke, M, Havenga, B, Barnard, T, Khan, S, et al. Prevalence of ESKAPE pathogens in the environment: antibiotic resistance status, community-acquired infection and risk to human health. Int J Hyg Environ Health. (2022) 244:114006. doi: 10.1016/j.ijheh.2022.114006
22. Murray, CJ, Ikuta, KS, Sharara, F, Swetschinski, L, Aguilar, GR, Gray, A, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
23. Lutz, JK, Van Balen, J, Mac Crawford, J, Wilkins, JR III, Lee, J, Nava-Hoet, RC, et al. Methicillin-resistant Staphylococcus aureus in public transportation vehicles (buses): another piece to the epidemiologic puzzle. Am J Infect Control. (2014) 42:1285–90. doi: 10.1016/j.ajic.2014.08.016
24. Mendes, Â, da Costa, PM, Rego, D, Beça, N, Alves, C, Moreira, T, et al. Contamination of public transports by Staphylococcus aureus and its carriage by biomedical students: point-prevalence, related risk factors and molecular characterization of methicillin-resistant strains. Public Health. (2015) 129:1125–31. doi: 10.1016/j.puhe.2015.05.010
25. Lin, JL, Peng, Y, Ou, QT, Lin, DX, Li, Y, Ye, XH, et al. A molecular epidemiological study of methicillin-resistant staphylococci environmental contamination in railway stations and coach stations in Guangzhou of China. Lett Appl Microbiol. (2017) 64:131–7. doi: 10.1111/lam.12700
26. Angbuhang, KB, Neupane, M, Adhikari, A, Binita, K, and Jha, S. Detection of methicillin resistant Staphylococcus aureus in public transportation of Kathmandu Valley, Nepal. Tribhuvan Univ J Microbiol. (2018) 5:51–6. doi: 10.3126/tujm.v5i0.22312
27. Medveďová, A, and Györiová, R. Prevalence of Staphylococcus aureus and antibiotic resistant Staphylococcus aureus in public transport in Bratislava, Slovakia. Acta Chim Slov. (2019) 12:41–5. doi: 10.2478/acs-2019-0007
28. Shen, C, Feng, S, Chen, H, Dai, M, Paterson, DL, Zheng, X, et al. Transmission of mcr-1-producing multidrug-resistant Enterobacteriaceae in public transportation in Guangzhou, China. Clin Infect Dis. (2018) 67:S217–24. doi: 10.1093/cid/ciy661
29. Cao, T, Liu, Y, Li, Y, Wang, Y, Shen, Z, Shao, B, et al. A public health concern: emergence of carbapenem-resistant Klebsiella pneumoniae in a public transportation environment. J Antimicrob Chemother. (2020) 75:2769–72. doi: 10.1093/jac/dkaa260
30. Gu, J, Xie, X-J, Liu, J-X, Shui, J-R, Zhang, H-Y, Feng, G-Y, et al. Prevalence and transmission of antimicrobial-resistant staphylococci and enterococci from shared bicycles in Chengdu, China. Sci Total Environ. (2020) 738:139735. doi: 10.1016/j.scitotenv.2020.139735
31. Torres-Sangiao, E, Leal Rodriguez, C, and García-Riestra, C. Application and perspectives of MADLI-TOF mass spectrometry in clinical microbiology laboratories. Microorganisms. (2021) 9:1539. doi: 10.3390/microorganisms9071539
32. Akintunde, O, Tucker, T, and Carabetta, VJ. The evolution of next-generation sequencing technologies. arXiv [preprint]. (2023). Available at: https://arxiv.org/abs/2305.08724 (Accessed December 2023).
33. Barba, M, Czosnek, H, and Hadidi, A. Historical perspective, development and applications of next-generation sequencing in plant virology. Viruses. (2014) 6:106–36. doi: 10.3390/v6010106
34. Zolfo, M, Asnicar, F, Manghi, P, Pasolli, E, Tett, A, and Segata, N. Profiling microbial strains in urban environments using metagenomic sequencing data. Biol Direct. (2018) 13:9. doi: 10.1186/s13062-018-0211-z
35. Pooja, S, Sudesh, D, Poonam, K, Joginder, SD, and Suresh, KG. Loop-mediated isothermal amplification (LAMP) based detection of bacteria: a review. Afr J Biotechnol. (2014) 13:1920–8. doi: 10.5897/ajb2013.13459
36. Kokkinos, P, Ziros, P, Bellou, M, and Vantarakis, A. Loop-mediated isothermal amplification (LAMP) for the detection of Salmonella in food. Food Anal Methods. (2014) 7:512–26. doi: 10.1007/s12161-013-9748-8
37. El-Kafrawy, SA, El-Daly, MM, Hassan, AM, Harakeh, SM, Alandijany, TA, and Azhar, EI. Rapid and reliable detection of SARS-CoV-2 using direct RT-LAMP. Diagnostics. (2022) 12:828. doi: 10.3390/diagnostics12040828
38. dos Santos, C, de Oliveira, K, Mendes, G, Silva, L, de Souza, JM, Estrela, PF, et al. Detection of SARS-CoV-2 in saliva by RT-LAMP during a screening of Workers in Brazil, including pre-symptomatic carriers. J Braz Chem Soc. (2021). doi: 10.21577/0103-5053.20210098
39. Lamb, LE, Bartolone, SN, Ward, E, and Chancellor, MB. Rapid detection of novel coronavirus/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by reverse transcription-loop-mediated isothermal amplification. PLoS One. (2020) 15:e0234682. doi: 10.1371/journal.pone.0234682
40. Setlow, P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. (2006) 101:514–25. doi: 10.1111/j.1365-2672.2005.02736.x
41. Tu, Z, Setlow, P, Brul, S, and Kramer, G. Molecular physiological characterization of a high heat resistant spore forming Bacillus subtilis food isolate. Microorganisms. (2021) 9:667. doi: 10.3390/microorganisms9030667
42. Otter, JA, and French, GL. Bacterial contamination on touch surfaces in the public transport system and in public areas of a hospital in London. Lett Appl Microbiol. (2009) 49:803–5. doi: 10.1111/j.1472-765X.2009.02728.x
43. Llor, C, and Bjerrum, L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. (2014) 5:229–41. doi: 10.1177/2042098614554919
44. Grass, G, Rensing, C, and Solioz, M. Metallic copper as an antimicrobial surface. Appl Environ Microbiol. (2011) 77:1541–7. doi: 10.1128/AEM.02766-10
45. Hong, Y, Zeng, J, Wang, X, Drlica, K, and Zhao, X. Post-stress bacterial cell death mediated by reactive oxygen speciesProc. Natl Acad Sci USA. (2019) 116:10064–71. doi: 10.1073/pnas.1901730116
46. Tamayo, L, Azócar, M, Kogan, M, Riveros, A, and Páez, M. Copper-polymer nanocomposites: an excellent and cost-effective biocide for use on antibacterial surfaces. Mater Sci Eng. (2016) 69:1391–409. doi: 10.1016/j.msec.2016.08.04141
47. Cassidy, SS, Sanders, DJ, Wade, J, Parkin, IP, Carmalt, CJ, Smith, AM, et al. Antimicrobial surfaces: a need for stewardship? PLoS Pathog. (2020) 16:e1008880. doi: 10.1371/journal.ppat.1008880
48. Mahanta, U, Khandelwal, M, and Deshpande, AS. Antimicrobial surfaces: a review of synthetic approaches, applicability and outlook. J Mater Sci. (2021) 56:17915–41. doi: 10.1007/s10853-021-06404-0
49. Linklater, DP, Baulin, VA, Juodkazis, S, Crawford, RJ, Stoodley, P, and Ivanova, EP. Mechano-bactericidal actions of nanostructured surfaces. Nat Rev Microbiol. (2021) 19:8–22. doi: 10.1038/s41579-020-0414-z
50. Kalb, L, Bassler, P, Schneider-Brachert, W, and Eckl, DB. Antimicrobial photodynamic coatings reduce the microbial burden on environmental surfaces in public transportation-a field study in buses. Int J Environ Res Public Health. (2022) 19:2325. doi: 10.3390/ijerph19042325
51. Eicker, R, and Salomon, W. Investigation of the effectiveness of antimicrobial photocatalyst-coated hand-contact surfaces in passenger transport vehicles under everyday conditions. Int J Infect Control. (2021) 17:20969. doi: 10.3396/ijic.v17.20969
52. Andersen, B, Rasch, M, Hochlin, K, Jensen, F-H, Wismar, P, and Fredriksen, J-E. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J Hosp Infect. (2006) 62:149–55. doi: 10.1016/j.jhin.2005.07.020
53. Møretrø, T, Fanebust, H, Fagerlund, A, and Langsrud, S. Whole room disinfection with hydrogen peroxide mist to control Listeria monocytogenes in food industry related environments. Int J Food Microbiol. (2019) 292:118–25. doi: 10.1016/j.ijfoodmicro.2018.12.015
54. Kruszewska, E, Czupryna, P, Pancewicz, S, Martonik, D, Bukłaha, A, and Moniuszko-Malinowska, A. Is Peracetic acid fumigation effective in public transportation? Int J Environ Res Public Health. (2022) 19:2526. doi: 10.3390/ijerph19052526
55. Leggett, MJ, Schwarz, JS, Burke, PA, McDonnell, G, Denyer, SP, and Maillard, J-Y. Mechanism of sporicidal activity for the synergistic combination of Peracetic acid and hydrogen peroxide. Appl Environ Microbiol. (2016) 82:1035–9. doi: 10.1128/AEM.03010-15
56. Richter, WR, Wood, JP, Wendling, MQ, and Rogers, JV. Inactivation of Bacillus anthracis spores to decontaminate subway railcar and related materials via the fogging of peracetic acid and hydrogen peroxide sporicidal liquids. J Environ Manag. (2018) 206:800–6. doi: 10.1016/j.jenvman.2017.11.027
57. Matys, J, Gedrange, T, Dominiak, M, and Grzech-Leśniak, K. The impact of hydrogen peroxide (H2O2) fumigation on bacterial levels in dental office environments: a randomized clinical trial investigation. J Clin Med. (2023) 12:7551. doi: 10.3390/jcm12247551
58. Beswick, AJ, Farrant, J, Makison, C, Gawn, J, Frost, G, Crook, B, et al. Comparison of multiple systems for laboratory whole room fumigation. Appl Biosaf. (2011) 16:139–57. doi: 10.1177/153567601101600303
59. Rutala, WA, and Weber, DJ. Disinfectants used for environmental disinfection and new room decontamination technology. Am J Infect Control. (2013) 41:S36–41. doi: 10.1016/j.ajic.2012.11.006
60. Murphy, T. Nucleic acids: interaction with solar UV radiation. Curr Top Radiat Res Q. (1975) 10:199–228.
61. Peak, MJ, Peak, JG, and Jones, CA. Different (direct and indirect) mechanisms for the induction of DNA-protein crosslinks in human cells by far-and near-ultraviolet radiations (290 and 405 nm). Photochem Photobiol. (1985) 42:141–6. doi: 10.1111/j.1751-1097.1985.tb01552.x
62. Hurtado, M, Marquez, J, Sotelo, P, Cornejo, J, and Palomares, R. Mechanic design and kinematic simulation of tri-star wheeled Mobile robot for COVID-19 using UV-C disinfection for public transport. 2022 First International Conference on Electrical, Electronics, Information and Communication Technologies (ICEEICT) (2022). p. 1–8.
63. Rakib, SH, Masum, S, Farhana, A, Islam, MA, Islam, MF, and Reza, MT. Design of a low cost ultraviolet disinfection unit to minimize the cross-contamination of COVID-19 in transport. 2022 International Conference on Advancement in Electrical and Electronic Engineering (ICAEEE) (2022).
64. Guettari, M, Gharbi, I, and Hamza, S. UVC disinfection robot. Environ Sci Pollut Res Int. (2021) 28:40394–9. doi: 10.1007/s11356-020-11184-2
65. Dos Santos, T, and de Castro, LF. Evaluation of a portable ultraviolet C (UV-C) device for hospital surface decontamination. Photodiagn Photodyn Ther. (2021) 33:102161. doi: 10.1016/j.pdpdt.2020.102161
66. Teska, P, Dayton, R, Li, X, Lamb, J, and Strader, P. Damage to common healthcare polymer Surfaces from UV exposure. Nano Life. (2020) 10:2050001. doi: 10.1142/S1793984420500014
67. Pearlmutter, BS, Haq, MF, Cadnum, JL, Jencson, AL, Carlisle, M, and Donskey, CJ. Efficacy of relatively low-cost ultraviolet-C light devices against Candida auris. Infect Control Hosp Epidemiol. (2022) 43:747–51. doi: 10.1017/ice.2021.206
68. Mora, M, Mahnert, A, Koskinen, K, Pausan, MR, Oberauner-Wappis, L, Krause, R, et al. Microorganisms in confined habitats: microbial monitoring and control of intensive care units, operating rooms, cleanrooms and the international space station. Front Microbiol. (2016) 7:1573. doi: 10.3389/fmicb.2016.01573
69. Schöbel, H, Diem, G, Kiechl, J, Chistè, D, Bertacchi, G, Mayr, A, et al. Antimicrobial efficacy and inactivation kinetics of a novel LED-based UV-irradiation technology. J Hosp Infect. (2023) 135:11–7. doi: 10.1016/j.jhin.2022.12.023
70. Deng, L-Z, Tao, Y, Mujumdar, AS, Pan, Z, Chen, C, Yang, X-H, et al. Recent advances in non-thermal decontamination technologies for microorganisms and mycotoxins in low-moisture foods. Trends Food Sci Technol. (2020) 106:104–12. doi: 10.1016/j.tifs.2020.10.012
71. Bernhardt, T, Semmler, ML, Schäfer, M, Bekeschus, S, Emmert, S, and Boeckmann, L. Plasma medicine: applications of cold atmospheric pressure plasma in dermatology. Oxidative Med Cell Longev. (2019) 2019:1–10. doi: 10.1155/2019/3873928
72. Borges, AC, Kostov, KG, Pessoa, RS, de Abreu, GM, Lima, GDM, Figueira, LW, et al. Applications of cold atmospheric pressure plasma in dentistry. Appl Sci. (2021) 11:1975. doi: 10.3390/app11051975
73. Gallagher, MJ, Vaze, N, Gangoli, S, Vasilets, VN, Gutsol, AF, Milovanova, TN, et al. Rapid inactivation of airborne Bacteria using atmospheric pressure dielectric barrier grating discharge. IEEE Trans Plasma Sci. (2007) 35:1501–10. doi: 10.1109/TPS.2007.905209
74. Scholtz, V, Pazlarova, J, Souskova, H, Khun, J, and Julak, J. Non-thermal plasma—a tool for decontamination and disinfection. Biotechnol Adv. (2015) 33:1108–19. doi: 10.1016/j.biotechadv.2015.01.002
75. Laroussi, M. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym. (2005) 2:391–400. doi: 10.1002/ppap.200400078
76. Liang, Y, Wu, Y, Sun, K, Chen, Q, Shen, F, Zhang, J, et al. Rapid inactivation of biological species in the air using atmospheric pressure non-thermal plasma. Environ Sci Technol. (2012) 46:3360–8. doi: 10.1021/es203770q
77. Baselga, M, Alba, JJ, and Schuhmacher, AJ. Impact of needle-point bipolar ionization system in the reduction of bioaerosols in collective transport. Sci Total Environ. (2023) 855:158965. doi: 10.1016/j.scitotenv.2022.158965
78. Soni, A, Choi, J, and Brightwell, G. Plasma-activated water (PAW) as a disinfection technology for bacterial inactivation with a focus on fruit and vegetables. Food Secur. (2021) 10:166. doi: 10.3390/foods10010166
79. Naicker, KI, Kaweesa, P, Daramola, MO, and Iwarere, SA. Non-thermal plasma review: assessment and improvement of feasibility as a retrofitted Technology in Tertiary Wastewater Purification. Appl Sci. (2023) 13:6243. doi: 10.3390/app13106243
Keywords: microbial community, public transport, microbial detection, countermeasures, microbial spread
Citation: Ly Y-T, Leuko S and Moeller R (2024) An overview of the bacterial microbiome of public transportation systems—risks, detection, and countermeasures. Front. Public Health. 12:1367324. doi: 10.3389/fpubh.2024.1367324
Edited by:
Faris Lami, University of Baghdad, IraqCopyright © 2024 Ly, Leuko and Moeller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ralf Moeller, ralf.moeller@dlr.de
 Yen-Tran Ly
Yen-Tran Ly Stefan Leuko
Stefan Leuko Ralf Moeller
Ralf Moeller