- 1Medical Department, Affiliated Hospital of Nanjing University of Traditional Chinese Medicine, Nanjing, Jiangsu Province, China
- 2Infection Management Department, Affiliated Hospital of Nanjing University of Traditional Chinese Medicine, Nanjing, Jiangsu Province, China
- 3Department of Expanded Program on Immunization, Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, Jiangsu Province, China
- 4Department of Rheumatology and Immunology, The Affiliated Suqian First People’s Hospital of Nanjing Medical University, Suqian, Jiangsu Province, China
Background: The occurrence of surgical site infection (SSI) can prolong the postoperative hospital stay, increase the economic burden of patients, and even endanger their lives. The purpose of this study was to investigate the incidence, risk factors, and microbiology of SSI after colorectal surgery (CRS) and to provide a basis for the prevention and control of SSI.
Methods: A single-center, prospective, cross-sectional study of adult patients undergoing CRS was conducted from 2010–2019. Univariate and multivariate logistic regression models were used to collect and analyze demographic information, hospital characteristics, and potential perioperative risk factors of SSI.
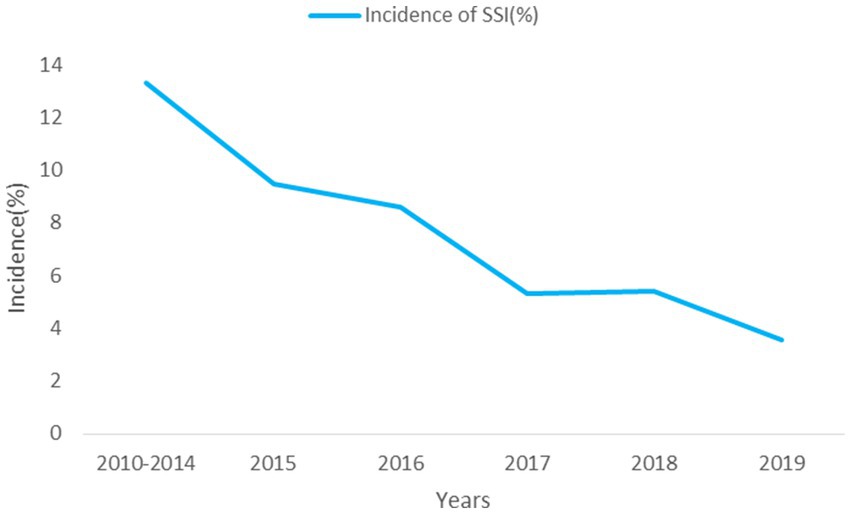
Results: A total of 3,302 eligible patients were included in this study, with 213 cases experiencing SSIs, resulting in an infection rate of 6.45%. Notably, the incidence of SSI decreased from 13.33% in 2010 to 3.56% in 2019 (Ptrend < 0.001). Escherichia coli accounted for the majority of isolated microorganisms (37.09%), with 49 strains exhibiting resistance to one or more antibiotics (35.25%). Multivariate analysis showed that diabetes, anastomosis leakage, wound classification (contaminated/dirty), operation duration, blood loss greater than 200 ml, and NNIS risk index score for 2 or 3 were independent risk factors. Conversely, laparoscopic approach, preoperative bowel preparation and preoperative albumin levels emerged as protective factors against SSI after CRS. Furthermore, compared to non-SSI patients, SSI patients had a significantly higher 30-day mortality rate following surgery (0.23% vs. 2.35%, p < 0.05).
Conclusion: SSI after CRS was susceptible to many factors, and the pathogen of SSI was mainly Escherichia coli. In clinical practice, measures such as correcting preoperative hypoproteinemia, choosing laparoscopic surgery, preoperative bowel preparation and shortening the duration of surgery should be taken to reduce the incidence of SSI.
Introduction
Healthcare-associated infections (HCAIs) refer to infections that occur in patients during the process of care in hospitals or other healthcare facilities (1). These infections mostly occur three or more days after admission, and HCAIs are currently a major public health issue that requires monitoring and control by health departments in various countries. SSIs remain one of the most common types of HCAIs, and according to research by European scholars, SSIs are the main cause of HCAIs (19.6%) (2). The occurrence of SSIs is accompanied by prolonged hospitalization and a higher risk of death. Due to the large number of microorganisms in the rectum and colon, the incidence of postoperative incision SSI is higher in CRS compared with surgery on other parts of the body, but the difference is large (often 10–30%) (3–9). In addition, SSIs also result in a significant increase in economic burden. It is estimated that 157,000 patients in the United States experience SSI each year, resulting in an additional cost of approximately $3.3 billion (10).
SSIs are typically caused by an imbalance between bacteria and the body’s defense, and are related to many endogenous and exogenous factors (11, 12). The most important related factors may include surgical type, degree of contamination, age, length of surgery, comorbidities, and American Society of Anesthesiologists (ASA) classification (13, 14), etc. The presence of a large number of microorganisms in the CRS may cause contamination of the wound, resulting in a particularly high incidence of SSIs. Some studies have found that measures such as maintaining normal blood pressure and blood glucose levels, and appropriate use of antibiotics can reduce the incidence of SSIs (15), while other similar studies have not yielded the same results (16). Therefore, in the population undergoing colorectal surgery, the contribution of patient characteristics needs to be further considered (17–19).
There were international guidelines for the prevention of SSIs in many regions of the world (20–24). A well-performed SSI surveillance system can effectively reduce its incidence and was critical to reducing the burden of infection as well as understanding patient characteristics. Health departments in many countries, including China, are dedicated to developing infection control systems. The US Centers for Disease Control and Prevention’s National Nosocomial Infections Surveillance (NNIS) system can compare the incidence of SSIs among different hospitals, but we still need to understand patients and other risk factors. To this end, we conducted a prospective, single-center, cross-sectional study on the incidence of SSIs and related risk factors, as well as the distribution of pathogens after CRS, at a tertiary hospital in Jiangsu Province, China. our study contributes novel and innovative insights into the prevention, microbiology, risk factors, and outcomes associated with SSIs following colorectal surgery. The comprehensive analysis of a large patient cohort, coupled with the examination of temporal trends and identification of risk and protective factors, enhances our understanding of this complex surgical complication. These findings have important implications for clinical practice, infection control strategies, and patient care, highlighting the significance of our research for the scientific community and the broader healthcare system.
Methods
Study design
A single-center, prospective, cross-sectional study of adult patients undergoing CRS was conducted from January 1, 2010 to December 31, 2019. The follow-up period was defined as 30 days after surgery. The single-center was a tertiary general hospital, the patients were identified using an inpatient SSI Surveillance System database and hospital Information Systems (HIS), and the data on the basic characteristics of patients were collected prospectively. After obtaining approval from the Ethics Review Committee of the Nanjing University of Chinese Medicine Affiliated Hospital, we conducted a retrospective analysis of the data. All patients who underwent colorectal surgery were included in the study, while patients under the age of 18 and deceased patients were excluded.
Data collection
Standardized questionnaires were used to collect information, and all enrolled patients were required to sign a written informed consent before participating in the study. The obtained data includes patient information before, during, and after surgery, with the following data list (1): Basic information (name, age, gender, body mass index (BMI), diabetes, hypertension, etc.) (2); Surgical situation (preoperative: hemoglobin (HB), albumin (ALB), antibiotic use, ASA grade (1–5, or), etc.; intraoperative: surgical method, wound classification (clean or I, clean/ contaminated or II, contaminated or III, and dirty or IV), ways of bowel preparation (oral antibiotics bowel preparation [OABP] with mechanical bowel preparation [MBP], MBP without OABP, none), operation time, etc.; postoperative: diagnosis and classification of SSI (superficial incisional, deep incisional, or organ/space), NNIS risk index(the NNIS risk index represented an internationally recognized stratification method for surgical risk. The NNIS risk index ranges from 0 to 3 and is determined by evaluating factors such as surgical duration, surgical wound classification, and ASA score. Each variable had critical thresholds: a contaminated or dirty surgical incision, a surgical duration of 225 min, and an ASA score of 3. If any of these variables exceed their critical threshold, a score of 1 is assigned.), information on isolated pathogens and antibiotic susceptibility, etc.).
Primary outcome
The primary outcome was the incidence of SSI within 30 days after surgery. SSI was classified according to the Centers for Disease Control and Prevention (CDC) criteria as superficial incisional (Superficial incisional infection occurs within 30 days after surgery and only affects the skin or subcutaneous tissue of the incision), deep incisional (Deep incisional infection occurs within 30 days after surgery for patients without implants and within 1 year after surgery for patients with implants), and organ/space infections (Organ/space infection occurs within 30 days after surgery for patients without implants and within 1 year after surgery for patients with implants) (25, 26). The follow-up period was 30 days after surgery, and if the patient was discharged, follow-up was conducted by phone. However, SSI was diagnosed primarily by physical exam findings documented by the operating surgeon.
Statistical analysis
All data were analyzed by SPSS software (version 22). Non-normally distributed continuous variables were expressed as M (interquartile spacing) and statistical analysis was performed using the Mann–Whitney U test. Fisher’s exact test or Chi-square test were used to compare the qualitative data and the logistic regression models were used to analyze the risk factors for SSI, and factors with p < 0.05 in the single-factor analysis were included in the multifactor analysis. p < 0.05 were considered significant.
Result
The incidence of SSI after CRS, 2010–2019
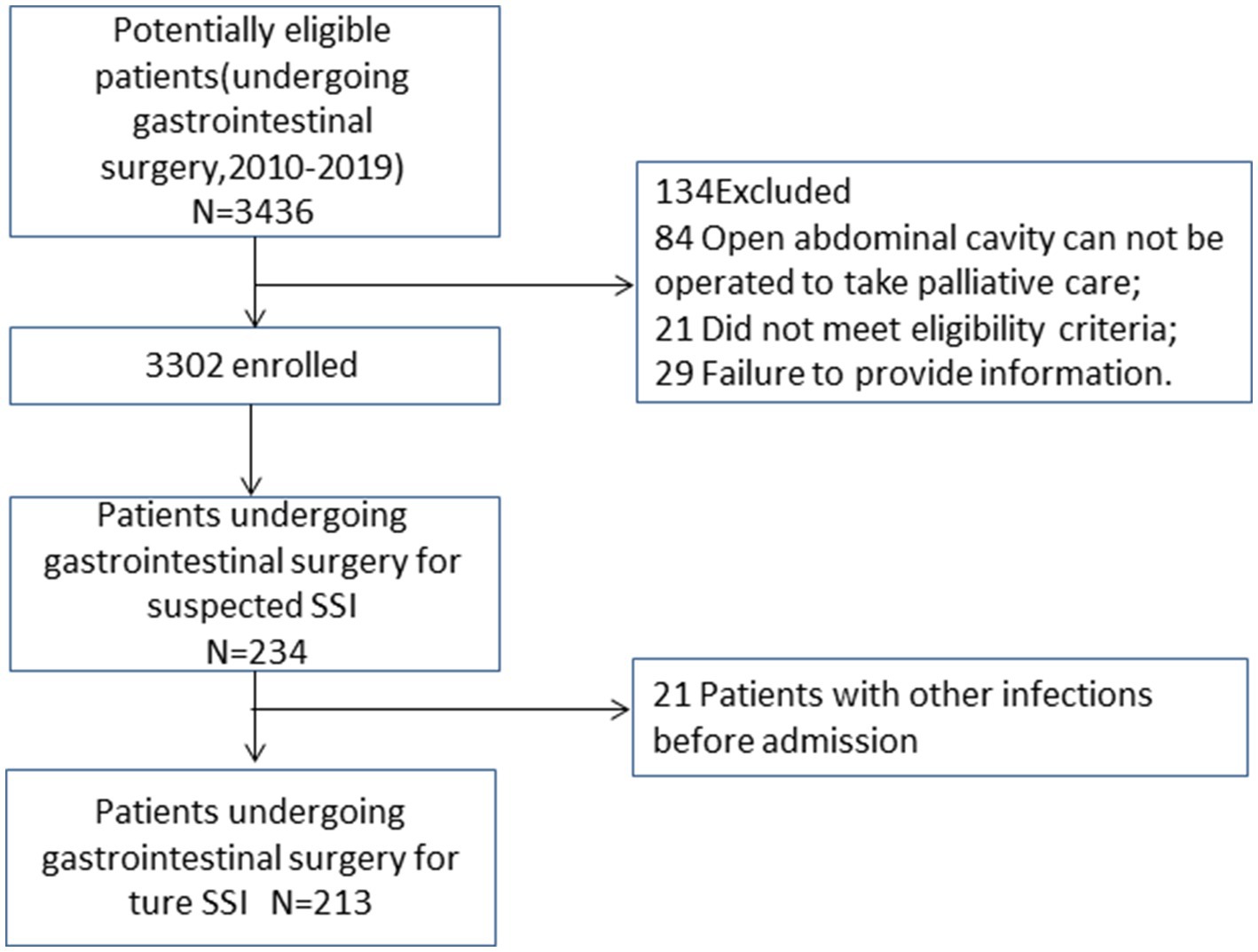
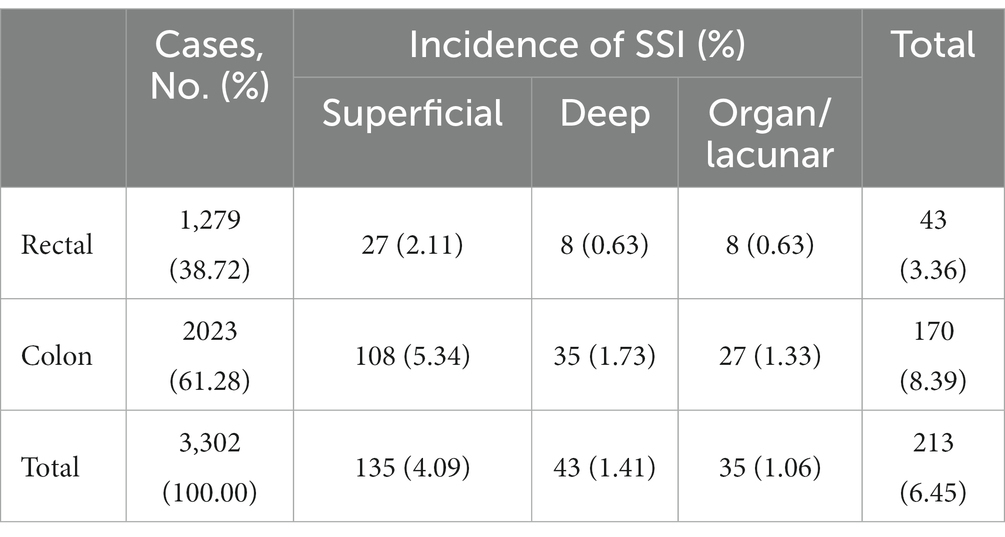
This study included 3,302 eligible patients (Figure 1), of whom 65.2% were male, with a median age of 64 (15) years and a BMI of 23.2 (4.3) kg/m2. A total of 213 patients developed SSI (Figure 1), with an infection rate of 6.45%. The incidence of SSI decreased from 13.33% in 2010 to 3.56% in 2019 (Ptrend < 0.001; Figure 2). Among them, 135 cases (4.09%) were superficial SSIs, 43 cases (1.41%) were deep SSIs, and 35 cases (1.06%) were organ/space SSIs (Table 1).
Analysis of risk factors for SSI after CRS
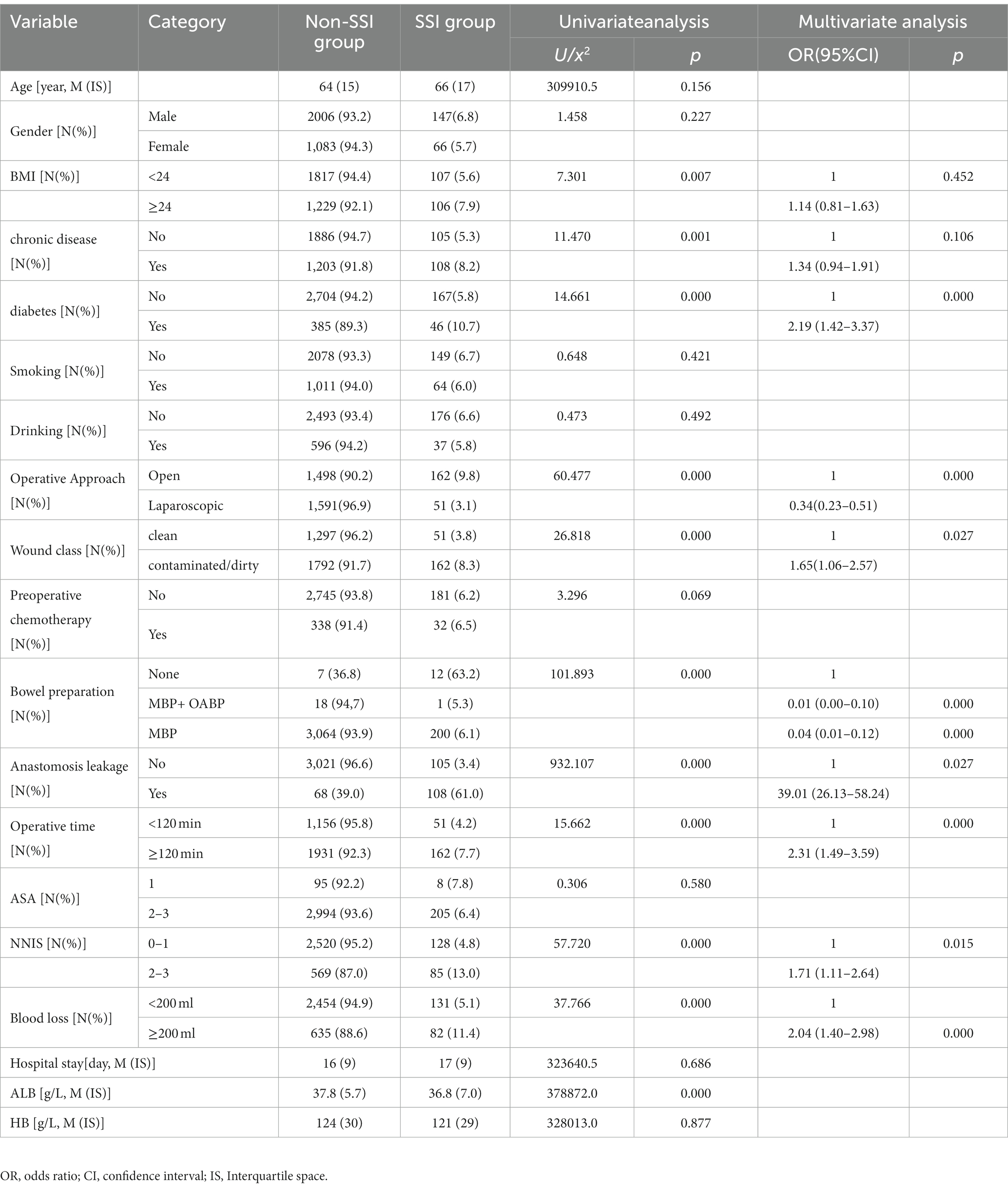
Univariate analysis showed no significant differences between the non-SSI group and the SSI group in terms of gender, age, smoking, drinking, and other factors. However, there were statistically significant differences in 11 variables between the two groups. Furthermore, multivariate analysis showed that diabetes was the most common comorbidities associated with SSI [OR = 2.19 (1.42–3.37)]. Laparoscopic approach, preoperative albumin level and preoperative bowel preparation were protective factors for SSI after colorectal surgery (p < 0.001). Anastomosis leakage, wound classification(contaminated or dirty), operation lasted over 120 min, blood loss greater than 200 ml, and NNIS risk index score for 2 or 3 were independent risk factors for SSIs after CRS (Table 2).
Analysis of pathogen distribution and antibiotic resistance in SSI patients
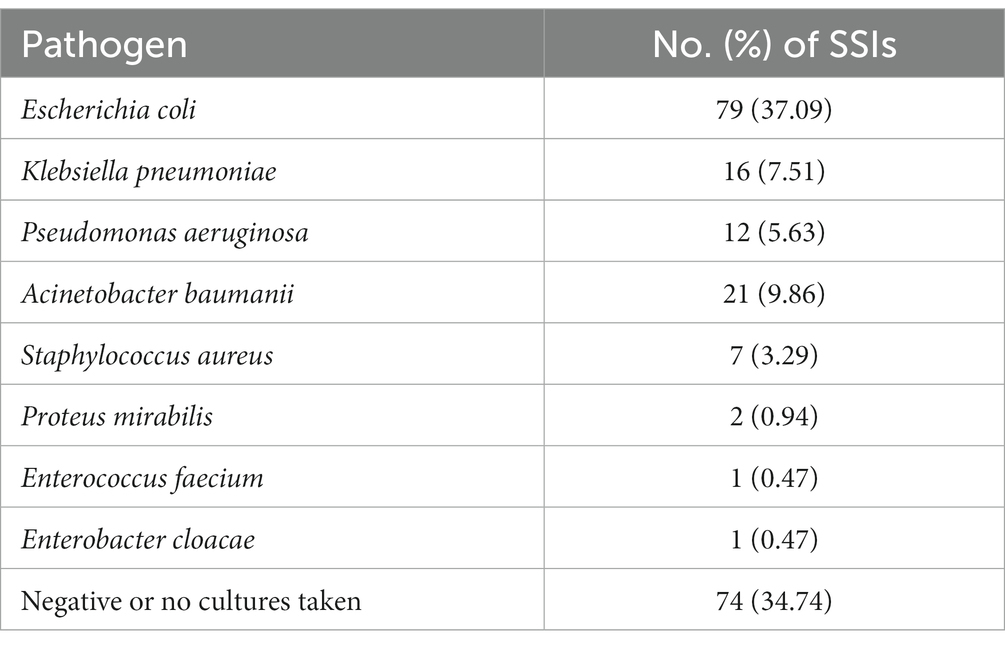
139 pathogens were isolated from the body fluids of 213 patients with SSI, including pus, sputum, and feces. Among them, Escherichia coli was detected in 79 cases (37.09%), followed by Acinetobacter baumannii in 21 cases (9.86%), and the detection rates of Klebsiella pneumonia and Pseudomonas aeruginosa were 7.51 and 5.63%, respectively. The proportion of cultures negative or without any pathogens was 34.74% (Table 3). Although 49 strains of bacteria were resistant to one or more antibiotics (35.25%, 49/139), multidrug-resistant organisms (MRSA) were rare.
Comparison of primary outcomes after CRS
The follow-up data from 2010 to 2019 showed a total of 11 deaths within 30 days after surgery, including 5 cases (2.35%) in the SSI group and 7 cases (0.23%) in the non-SSI group, with a significant statistical difference between the two groups (p < 0.05).
Discussion
Overall, we found 213 cases (6.45%) of SSI among 3,302 colon surgery patients. Compared to other studies (6, 27, 28), there were significant differences in the incidence of SSI, which may be due to differences in the definition of SSI and postoperative follow-up time. In addition, our results showed that the incidence of SSI after colon surgery decreased from 13.33% in 2010 to 3.56% in 2019, and there was a significant decrease in SSI incidence after CRS. According to the “Guideline for the Prevention and Control of Surgical Site Infection (Trial)” issued by the National Health Commission of the People’s Republic of China (NHCPC) (26), prevention of SSI should be carried out preoperatively, intraoperatively, and postoperatively, such as the use of prophylactic antibiotics, preoperative bowel preparation, temperature and blood glucose control. The hospital determines possible risk factors based on the analysis of actively monitored, and regularly provides feedback and summaries of SSI. The active monitoring and preventive control measures of SSI have played a significant role in reducing the incidence.
Previous studies have found that the occurrence of healthcare-associated infections mainly depends on the number of colonizing pathogens and the host’s immune response. Since anaerobic and gram-negative bacteria mainly colonize the rectum and distal ileum, surgery in the colon inevitably causes intestinal bacterial translocation, resulting in a higher incidence of SSI after colorectal surgery (29). In this study, a total of 139 pathogenic bacteria were isolated, with gram-negative bacteria accounting for more than 60% of the isolates, and Escherichia coli, Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa being the most common pathogens. It is noteworthy that 49 strains were found to be resistant to one or more antibiotics, and Emine et al. (30) had reported that the use of antimicrobial agents can reduce the incidence of surgical site infections, but the irrational use of antibiotics can lead to antibiotic resistance, and at the same time increase the risk of SSI.
Multifactorial analysis reveals that diabetes is the most prevalent comorbidity [OR = 2.19(1.42–3.37)]. Furthermore, univariate analysis indicates that chronic conditions (with a primary focus on hypertension in this study) are risk factors for the occurrence of surgical site infections (SSI) after breast cancer and cesarean section procedures (31, 32). Due to increased vascular resistance, hypertension patients are more likely to experience peripheral blood supply changes, thereby increasing the risk of tissue infections. This conclusion was consistent with domestic research, but further studies are needed to confirm our hypothesis as we did not record specific changes in blood pressure values. Diabetes is a metabolic disorder disease, and high blood sugar is conducive to the growth and proliferation of bacteria while also hindering wound healing (33, 34). In the multifactorial analysis, four variables were ultimately confirmed. Our results demonstrated that contaminated or dirty wound classification significantly increases the risk of CRS-associated SSI by 1.65 times when compared to clean wound classification, a finding consistently supported by numerous studies. Anastomotic leakage, a severe complication after rectal cancer surgery, can lead to serious intra-abdominal infections, causing patient distress, prolonged hospitalization, increased treatment costs, and in severe cases, even mortality. Our research also showed that compared to patients without anastomotic leakage, the risk increased by 39.01 times. Prolonged surgical time and increased intraoperative blood loss were independent risk factors for SSI development in CRS, likely due to prolonged exposure to pathogenic microorganisms, potentially leading to an increased risk of infection (29, 35, 36). Therefore, greater attention should be given to the perioperative prevention of SSI in patients with a history of incision contamination in clinical practice. Our results further indicated that NNIS risk index scores of 2 or 3 are independent predictors of SSI, consistent with previous research findings (37).
In recent years, laparoscopic surgery has rapidly gained popularity worldwide due to its small incision and fast recovery features. Laparoscopic surgery can significantly shorten hospitalization and normal intestinal function recovery time in CRS, reduce intraoperative bleeding, and to a certain extent, reduce the incidence of SSI (38–41). This study shows that the SSI incidence rate is only 3.1% in laparoscopic surgery, while it is as high as 9.8% in open surgery, and the difference between the two has statistical significance, which was a protective factor for SSI after CRS. In addition, albumin is a commonly used indicator to evaluate patients’ nutritional status. If a patient’s albumin level is low, it indicates malnutrition, which can be accompanied by tissue edema and decreased resistance, increasing the risk of postoperative complications (42–44). In our study, the preoperative albumin level in the NON-SSI group was higher than that in the SSI group [37.8(5.7) g/L vs. 36.8(7.0) g/L), and it had statistical significance (p < 0.05).
Preoperative bowel preparation remains a topic of great controversy and interest worldwide. The use of MBP and/or OABP has sparked numerous debates. Data collected from this study indicates that since 2014, our hospital has rarely used MBP and OABP. Nevertheless, multifactorial regression analysis still reveals that preoperative intestinal preparation serves as a protective factor against SSI in this research. The World Health Organization (WHO) recommends the adoption of preoperative MBP combined with OABP to reduce the risk of SSI in patients undergoing elective colorectal surgery (24). Due to the limited number of cases with combined preoperative MBP and OABP in this study, a further collection of more cases is needed for analysis.
Our study also had some limitations. Firstly, our study was based on data collected from a single center, which may introduce selection bias. However, it is worth noting that our research center was a prestigious tertiary medical institution located in the eastern region of China known as the Yangtze River Delta, where a considerable number of patients prefer to undergo colorectal surgeries. Secondly, the retrospective analysis of prospectively collected data could introduce bias and does not establish a definitive causal relationship between risk factors and SSI. Thirdly, similar to other comparable studies, our surveillance system database did not collect a sufficient number of risk factors for analysis. It is important to acknowledge that there may be other complications associated with deep or organ/space SSIs that were not accounted for in our study, which could have confounded the primary outcomes. Fourthly, the proportion of cultured samples from SSI patients in our study was relatively low, limiting the availability of comprehensive information on the causative pathogens involved. Lastly, this study lacks a longitudinal analysis, and it is impossible to understand the impact of relevant interventions on individuals by observing their changes at different time points.
Conclusion
In summary, there were many factors that can affect the occurrence of SSIs after CRS. It was hoped that this will help healthcare professionals identify patients at higher risk of developing SSI after surgery, so that further strategies can be implemented to reduce the incidence of SSI. In clinical practice, measures such as correcting preoperative hypoproteinemia, choosing laparoscopic surgery, preoperative bowel preparation and shortening the duration of surgery should be taken to reduce the incidence of SSI.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WW and XS conceived of this study. HS, HJ, Z-WJ, Z-XD and GF collected the data. HS, XS, and ZW analyzed the data and drafted initial manuscript. WW, XS, and ZW critically revised the manuscript and helped interpret data. All authors reviewed the final manuscript.
Funding
This work was supported by the Jiangsu Health Development Research Center Open Project (JSHD2022043) and Jiangsu Provincial Geriatric Health Research Project (LKM2023005).
Acknowledgments
The authors thank all of our colleagues in the studied hospitals for collecting the surveillance data and making this study possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Friedman, ND, Kaye, KS, Stout, JE, McGarry, SA, Trivette, SL, Briggs, JP, et al. Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. (2002) 137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007
2. Badia, JM, Casey, AL, Petrosillo, N, Hudson, PM, Mitchell, SA, and Crosby, C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. (2017) 96:1–15. doi: 10.1016/j.jhin.2017.03.004
3. Pujol, M, Limón, E, López-Contreras, J, Sallés, M, Bella, F, and Gudiol, F. Surveillance of surgical site infections in elective colorectal surgery. Results of the VINCat program (2007-2010). Enferm Infecc Microbiol Clin. (2012) 30:20–5. doi: 10.1016/S0213-005X(12)70092-7
4. Pedroso-Fernandez, Y, Aguirre-Jaime, A, Ramos, MJ, Hernández, M, Cuervo, M, Bravo, A, et al. Prediction of surgical site infection after colorectal surgery. Am J Infect Control. (2016) 44:450–4. doi: 10.1016/j.ajic.2015.10.024
5. Haleem, A, Chiang, HY, Vodela, R, Behan, A, Pottinger, JM, Smucker, J, et al. Risk factors for surgical site infections following adult spine operations. Infect Control Hosp Epidemiol. (2016) 37:1458–67. doi: 10.1017/ice.2016.193
6. Morikane, K, Honda, H, Yamagishi, T, Suzuki, S, and Aminaka, M. Factors associated with surgical site infection in colorectal surgery: the Japan nosocomial infections surveillance. Infect Control Hosp Epidemiol. (2014) 35:660–6. doi: 10.1086/676438
7. Konishi, T, Watanabe, T, Kishimoto, J, and Nagawa, H. Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg. (2006) 244:758–63. doi: 10.1097/01.sla.0000219017.78611.49
8. Tanner, J, Kiernan, M, Hilliam, R, Davey, S, Collins, E, Wood, T, et al. Effectiveness of a care bundle to reduce surgical site infections in patients having open colorectal surgery. Ann R Coll Surg Engl. (2016) 98:270–4. doi: 10.1308/rcsann.2016.0072
9. Zywot, A, Lau, C, Stephen, FH, and Paul, S. Bundles prevent surgical site infections after colorectal surgery: Meta-analysis and systematic review. J Gastrointest Surg. (2017) 21:1915–30. doi: 10.1007/s11605-017-3465-3
10. Anderson, DJ, Pyatt, DG, Weber, DJ, and Rutala, WA. Statewide costs of health care-associated infections: estimates for acute care hospitals in North Carolina. Am J Infect Control. (2013) 41:764–8. doi: 10.1016/j.ajic.2012.11.022
11. Colás-Ruiz, E, Del-Moral-Luque, JA, Gil-Yonte, P, Fernández-Cebrián, JM, Alonso-García, M, Villar-Del-Campo, MC, et al. Incidence of surgical site infection and risk factors in rectal surgery: a prospective cohort study. Cir Esp. (2018) 96:640–7. doi: 10.1016/j.ciresp.2018.06.007
12. Pérez, CD, Rodela, AR, and Monge, JV. The Spanish national health care-associated infection surveillance network (INCLIMECC): data summary January 1997 through December 2006 adapted to the new National Healthcare Safety Network Procedure-associated module codes. Am J Infect Control. (2009) 37:806–12. doi: 10.1016/j.ajic.2009.03.005
13. Pastor, C, Baek, JH, Varma, MG, Kim, E, Indorf, LA, and Garcia-Aguilar, J. Validation of the risk index category as a predictor of surgical site infection in elective colorectal surgery. Dis Colon Rectum. (2010) 53:721–7. doi: 10.1007/DCR.0b013e3181cc573b
14. Wick, EC, Gibbs, L, Indorf, LA, Varma, MG, and Garcia-Aguilar, J. Implementation of quality measures to reduce surgical site infection in colorectal patients. Dis Colon Rectum. (2008) 51:1004–9. doi: 10.1007/s10350-007-9142-y
15. Bull, A, Wilson, J, Worth, LJ, Stuart, RL, Gillespie, E, Waxman, B, et al. A bundle of care to reduce colorectal surgical infections: an Australian experience. J Hosp Infect. (2011) 78:297–301. doi: 10.1016/j.jhin.2011.03.029
16. Serra-Aracil, X, García-Domingo, MI, Parés, D, Espin-Basany, E, Biondo, S, Guirao, X, et al. Surgical site infection in elective operations for colorectal cancer after the application of preventive measures. Arch Surg. (2011) 146:606–12. doi: 10.1001/archsurg.2011.90
17. Wick, EC, Vogel, JD, Church, JM, Remzi, F, and Fazio, VW. Surgical site infections in a "high outlier" institution: are colorectal surgeons to blame? Dis Colon Rectum. (2009) 52:374–9. doi: 10.1007/DCR.0b013e31819a5e45
18. Ho, VP, Stein, SL, Trencheva, K, Barie, PS, Milsom, JW, Lee, SW, et al. Differing risk factors for incisional and organ/space surgical site infections following abdominal colorectal surgery. Dis Colon Rectum. (2011) 54:818–25. doi: 10.1007/DCR.0b013e3182138d47
19. Pendlimari, R, Cima, RR, Wolff, BG, Pemberton, JH, and Huebner, M. Diagnoses influence surgical site infections (SSI) in colorectal surgery: a must consideration for SSI reporting programs? J Am Coll Surg. (2012) 214:580–1. doi: 10.1016/j.jamcollsurg.2011.12.023
20. Ling, ML, Apisarnthanarak, A, Abbas, A, Morikane, K, Lee, KY, Warrier, A, et al. APSIC guidelines for the prevention of surgical site infections. Antimicrob Resist Infect Control. (2019) 8:174. doi: 10.1186/s13756-019-0638-8
21. Seidelman, JL, Mantyh, CR, and Anderson, DJ. Surgical site infection prevention: a review. JAMA. (2023) 329:244–52. doi: 10.1001/jama.2022.24075
22. Allegranzi, B, Bischoff, P, de Jonge, S, Kubilay, NZ, Zayed, B, Gomes, SM, et al. New WHO recommendations on preoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. (2016) 16:e276–87. doi: 10.1016/S1473-3099(16)30398-X
23. Ban, KA, Minei, JP, Laronga, C, Harbrecht, BG, Jensen, EH, Fry, DE, et al. American College of Surgeons and surgical infection society: surgical site infection guidelines, 2016 update. J Am Coll Surg. (2017) 224:59–74. doi: 10.1016/j.jamcollsurg.2016.10.029
24. Leaper, DJ, and Edmiston, CE. World Health Organization: global guidelines for the prevention of surgical site infection. J Hosp Infect. (2017) 95:135–6. doi: 10.1016/j.jhin.2016.12.016
25. Mangram, AJ, Horan, TC, Pearson, ML, Silver, LC, and Jarvis, WR. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control. (1999) 27:97–134. doi: 10.1016/S0196-6553(99)70088-X
26. National Health Commission of the People’s Republic of China (2010). Guidelines for prevention and control of surgical site infection (Trial). Available at: http://www.nhc.gov.cn/ (Accessed December 14, 2010).
27. Gomila, A, Carratalà, J, Camprubí, D, Shaw, E, Badia, JM, Cruz, A, et al. Risk factors and outcomes of organ-space surgical site infections after elective colon and rectal surgery. Antimicrob Resist Infect Control. (2017) 6:40. doi: 10.1186/s13756-017-0198-8
28. Baker, AW, Dicks, KV, Durkin, MJ, Weber, DJ, Lewis, SS, Moehring, RW, et al. Epidemiology of surgical site infection in a community hospital network. Infect Control Hosp Epidemiol. (2016) 37:519–26. doi: 10.1017/ice.2016.13
29. Du, M, Liu, B, Li, M, Cao, J, Liu, D, Wang, Z, et al. Multicenter surveillance study of surgical site infection and its risk factors in radical resection of colon or rectal carcinoma. BMC Infect Dis. (2019) 19:411. doi: 10.1186/s12879-019-4064-6
30. Alp, E, Elmali, F, Ersoy, S, Kucuk, C, and Doganay, M. Incidence and risk factors of surgical site infection in general surgery in a developing country. Surg Today. (2014) 44:685–9. doi: 10.1007/s00595-013-0705-3
31. Krieger, Y, Walfisch, A, and Sheiner, E. Surgical site infection following cesarean deliveries: trends and risk factors. J Matern Fetal Neonatal Med. (2017) 30:8–12. doi: 10.3109/14767058.2016.1163540
32. Yang, S, Liu, G, Tang, D, and Cai, D. Evaluation intravenous drip Cephazolin prophylaxis of breast Cancer surgery site infection. J Craniofac Surg. (2017) 28:e527–31. doi: 10.1097/SCS.0000000000003780
33. Martin, ET, Kaye, KS, Knott, C, Nguyen, H, Santarossa, M, Evans, R, et al. Diabetes and risk of surgical site infection: a systematic review and Meta-analysis. Infect Control Hosp Epidemiol. (2016) 37:88–99. doi: 10.1017/ice.2015.249
34. Park, H, de Virgilio, C, Kim, DY, Shover, AL, and Moazzez, A. Effects of smoking and different BMI cutoff points on surgical site infection after elective open ventral hernia repair. Hernia. (2021) 25:337–43. doi: 10.1007/s10029-020-02190-x
35. Hennessey, DB, Burke, JP, Ni-Dhonochu, T, Shields, C, Winter, DC, and Mealy, K. Risk factors for surgical site infection following colorectal resection: a multi-institutional study. Int J Color Dis. (2016) 31:267–71. doi: 10.1007/s00384-015-2413-5
36. Young, H, Berumen, C, Knepper, B, Miller, A, Silverman, M, Gilmartin, H, et al. Statewide collaboration to evaluate the effects of blood loss and transfusion on surgical site infection after hysterectomy. Infect Control Hosp Epidemiol. (2012) 33:90–3. doi: 10.1086/663341
37. Gaynes, RP, Culver, DH, Horan, TC, Edwards, JR, Richards, C, and Tolson, JS. Surgical site infection (SSI) rates in the United States, 1992-1998: the National Nosocomial Infections Surveillance System basic SSI risk index. Clin Infect Dis. (2001) 33:S69–77. doi: 10.1086/321860
38. Howard, DP, Datta, G, Cunnick, G, Gatzen, C, and Huang, A. Surgical site infection rate is lower in laparoscopic than open colorectal surgery. Color Dis. (2010) 12:423–7. doi: 10.1111/j.1463-1318.2009.01817.x
39. Kulkarni, N, and Arulampalam, T. Laparoscopic surgery reduces the incidence of surgical site infections compared to the open approach for colorectal procedures: a meta-analysis. Tech Coloproctol. (2020) 24:1017–24. doi: 10.1007/s10151-020-02293-8
40. Garbarino, GM, Laracca, GG, Lucarini, A, Piccolino, G, Mercantini, P, Costa, A, et al. Laparoscopic versus open surgery for gastric Cancer in Western countries: a systematic review and Meta-analysis of short-and long-term outcomes. J Clin Med. (2022):11. doi: 10.3390/jcm11133590
41. Benlice, C, Stocchi, L, Sapci, I, Gorgun, E, Kessler, H, Liska, D, et al. Impact of the extraction-site location on wound infections after laparoscopic colorectal resection. Am J Surg. (2019) 217:502–6. doi: 10.1016/j.amjsurg.2018.10.034
42. Hennessey, DB, Burke, JP, Ni-Dhonochu, T, Shields, C, Winter, DC, and Mealy, K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. (2010) 252:325–9. doi: 10.1097/SLA.0b013e3181e9819a
43. Truong, A, Hanna, MH, Moghadamyeghaneh, Z, and Stamos, MJ. Implications of preoperative hypoalbuminemia in colorectal surgery. World J Gastrointest Surg. (2016) 8:353–62. doi: 10.4240/wjgs.v8.i5.353
Keywords: surgical site infection, colorectal surgery, risk factor, prevalence, cross-sectional study
Citation: Sun H, Jiang H, Jiang Z-W, Fang G, Dai Z-X, Wang Z, Sun X and Wang W (2023) Analysis of risk factors for surgical site infection after colorectal surgery: a cross-sectional study in the east of China pre-COVID-19. Front. Public Health. 11:1204337. doi: 10.3389/fpubh.2023.1204337
Edited by:
Francesco Pata, Nicola Giannettasio Hospital, ItalyReviewed by:
Martina Barchitta, University of Catania, ItalyTevfiktolga Sahin, İnönü University, Türkiye
Sergey Achkasov, Ryzhikh National Medical Research Centre of Coloproctology, Russia
Tommaso Sciortino, University at Buffalo, United States
Copyright © 2023 Sun, Jiang, Jiang, Fang, Dai, Wang, Sun and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Wang, 365793124@qq.com; Xiang Sun, sunstone1@163.com; Zhiguo Wang, 120714991@qq.com
†These authors share first authorship
 Hui Sun1†
Hui Sun1† Hua Jiang
Hua Jiang Xiang Sun
Xiang Sun Wen Wang
Wen Wang