- 1Intensive Care Unit, Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, Fujian, China
- 2Blood Transfusion Department, Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, Fujian, China
- 3Department of Laboratory Medicine, The First Affiliated Hospital of Xiamen University, Xiamen Key Laboratory of Genetic Testing, Xiamen, China
- 4Department of Infection Control, Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics & Gynecology and Pediatrics, Fujian Medical University, Fuzhou, Fujian, China
Objective: The constant changes in the control strategies of the Corona Virus Disease 2019 (COVID-19) pandemic have greatly affected the prevention and control of nosocomial infections (NIs). This study assessed the impact of these control strategies on the surveillance of NIs in a regional maternity hospital during the COVID-19 pandemic.
Methods: This retrospective study compared the observation indicators of nosocomial infections and their changing trends in the hospital before and during the COVID-19 pandemic.
Results: In total, 2,56,092 patients were admitted to the hospital during the study. During the COVID-19 pandemic, the main drug-resistant bacteria in hospitals were Escherichia coli, Streptococcus agalactiae, Staphylococcus aureus, Klebsiella pneumoniae, and Enterococcus faecalis. The detection rate of S. agalactiae increased annually, while that of E. faecalis remained the same. The detection rate of multidrug-resistant bacteria decreased during the pandemic (16.86 vs. 11.42%), especially that of CRKP (carbapenem-resistant Klebsiella pneumoniae 13.14 vs. 4.39, P < 0.001). The incidence of nosocomial infections in the pediatric surgery department decreased significantly (OR: 2.031, 95% CI: 1.405–2.934, P < 0.001). Regarding the source of infection, a significant reduction was observed in respiratory infections, followed by gastrointestinal infections. In the routine monitoring of the ICU, the incidence of central line-associated bloodstream infection (CLABSI) decreased significantly (9.4/1,000 catheter days vs. 2.2/1,000 catheter days, P < 0.001).
Conclusion: The incidence of nosocomial infections was lower than that before the COVID-19 pandemic. The prevention and control measures for the COVID-19 pandemic have reduced the number of nosocomial infections, especially respiratory, gastrointestinal, and catheter-related infections.
1. Introduction
Nosocomial infections (NIs), also known as hospital-acquired infections, are infections occurring within 48 h of hospital admission, within 30 days after receiving healthcare, or up to 90 days after undergoing surgery (1). NIs can prolong hospitalization and increase antimicrobial resistance, medical expenses, and mortality rates. NIs affect ~2 million individuals in the United States annually, with 80,000 of them dying as a result, resulting in economic losses of more than $4.6 billion (2). In a tertiary hospital in China, the average hospital stay was longer, and hospitalization costs were higher in patients with multidrug resistance (MDR) (3).
Until now, many countries have established their national or regional nosocomial infection surveillance systems (4–7). A systematic review (8) showed that continuous surveillance had a positive impact on NI, and OR/RR ranged from 0.43 to 0.95. However, in maternity hospitals, patient composition, disease severity, and common pathogens varied from those in general hospitals (9, 10). It is important to understand the current situation of NIs in maternity hospitals to guide further prevention and control.
The global COVID-19 pandemic has further aggravated the severity of NIs. The rate of methicillin-resistant Staphylococcus aureus (MRSA) infections among hospitalized patients during the COVID-19 outbreak was substantially greater in SARS-CoV-2 positive patients than in virus-negative patients (11). There was a significant increase in the incidence of New Delhi metallo-β-lactamase-producing carbapenem-resistant Enterobacterales and carbapenem-resistant Acinetobacter baumannii at some hospital sites when compared with that before the COVID-19 pandemic (12). Hospitals at all levels have strengthened the prevention of COVID-19 transmission and control of NIs.
In the study hospital, strategies were developed to prevent hospital-associated transmission of COVID-19. The outpatient volume was controlled, and screening processes were strictly performed. Emergency surgeries were performed in a negative-pressure room to protect the surgeons. All medical staff, including the logistics staff, received extensive training on awareness and prevention of NIs, which comprised two major parts: theoretical training and skill training. A new computer system was used to record surveillance data and provide timely feedback. However, similar data on the influence of the COVID-19 pandemic on NIs in maternity hospitals have not yet been reported. This study assessed the impact of these control strategies on NI surveillance in a regional maternity hospital during the COVID-19 pandemic.
2. Material and methods
2.1. Study design and sample size
This retrospective study was conducted at a large tertiary maternity hospital in Fuzhou, China between 1 January 2018 and 31 December 2021. The hospital serves as both a tertiary maternity hospital in the Fuzhou region and a tertiary referral center for complicated maternal, pediatric, and neonatal cases. A total of 2,56,092 patients were admitted to the hospital during the study period.
Surveys conducted from 1 January 2018 to 31 December 2019 were defined as the pre-pandemic group, while those conducted from 1 January 2020 to 31 December 2021 were defined as the during-pandemic group.
2.2. Ethics approval
This study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics and Gynecology and Pediatrics, Fujian Medical University (ethics approval number: 2022YJ070). Because this retrospective analysis used routinely collected data, the requirement for informed consent was waived.
2.3. Inclusion and exclusion criteria
The inclusion criteria were as follows:
• Patients admitted to all clinical wards who were present at the time of the survey were included.
The exclusion criteria were as follows:
• All patients who were infected at the time of admission or within 48 h after hospitalization were excluded.
• Patients with duplicate isolates, defined as the same bacteria isolated from the same patient and sample, were excluded.
2.4. Data collection
Identification of NIs was done by (1) nosocomial infection-warning systems triggered in the laboratory, (2) clinicians entering data into a hospital-based data system, and (3) confirmation by staff members of the hospital's infection control department. The diagnostic criteria for NIs were based on the diagnostic criteria for nosocomial infections developed by the Ministry of Health of the People's Republic of China (13), which is a modification of the definition provided by the US Center for Disease Control and Prevention (14).
The device-associated infection (DAI) incidence rate per 1,000 device days was calculated as the total number of DAIs divided by the total number of specific device days (days of indwelling urinary catheterization, central line use, or ventilator treatment) and multiplied by 1,000. DAIs included catheter-associated urinary tract infections (CAUTI), central line-associated bloodstream infections (CLABSI), and ventilator-associated pneumonia (VAP).
Clinical data, including information on pathogenic microorganisms, distribution in clinical wards, source of the infection, and DAIs, were extracted from the laboratory information system and the hospital-based nosocomial infection surveillance system.
2.5. Statistical analysis
All statistical analyses were performed using SPSS Statistics software (version 25.0; IBM Corp., Armonk, NY, USA). Descriptive statistics are reported as numbers (percentages) for categorical data. Chi-square or Fisher's exact tests were used to compare discrete variables. Fisher's exact test was used when one or more expected values were equal to or < 5. Binary logistic regression was used to analyze the relevant data. Statistical significance was set at a p-value of < 0.05. A priori sample size calculations were not performed because of the retrospective nature of the analysis, and all eligible cases were included.
3. Results
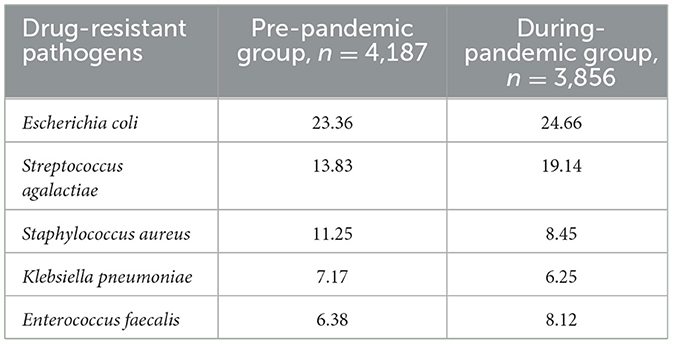
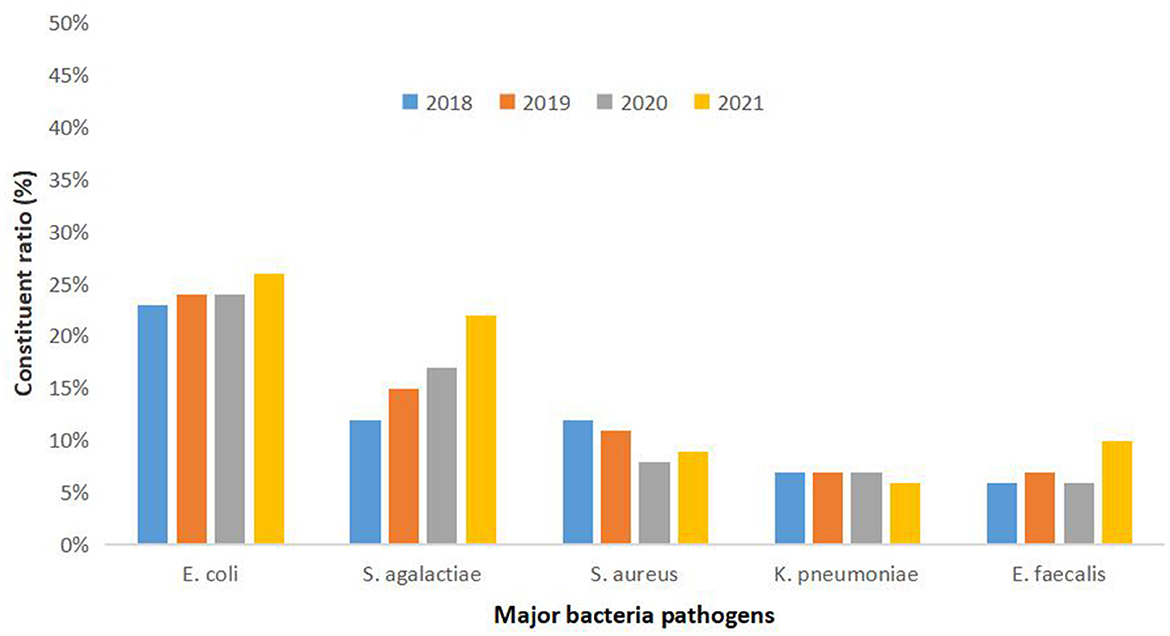
Between January 2018 and December 2021, 2,56,092 patients were admitted to our hospital. In cultures taken from patients who were suspected of infection or before antibiotic administration, 8,043 samples tested positive for drug resistance (n = 4,187 in the pre-pandemic group and n = 3,856 in the during-pandemic group; Table 1). A detailed analysis of the pathogens showed that Escherichia coli, Streptococcus agalactiae, Staphylococcus aureus, Klebsiella pneumoniae, and Enterococcus faecalis were the most prevalent drug-resistant bacteria in NIs in the last 4 years, and the distribution was similar every year. The detection rate of S. agalactiae increased annually (from 12% in 2018 to 22% in 2022) (Figure 1).
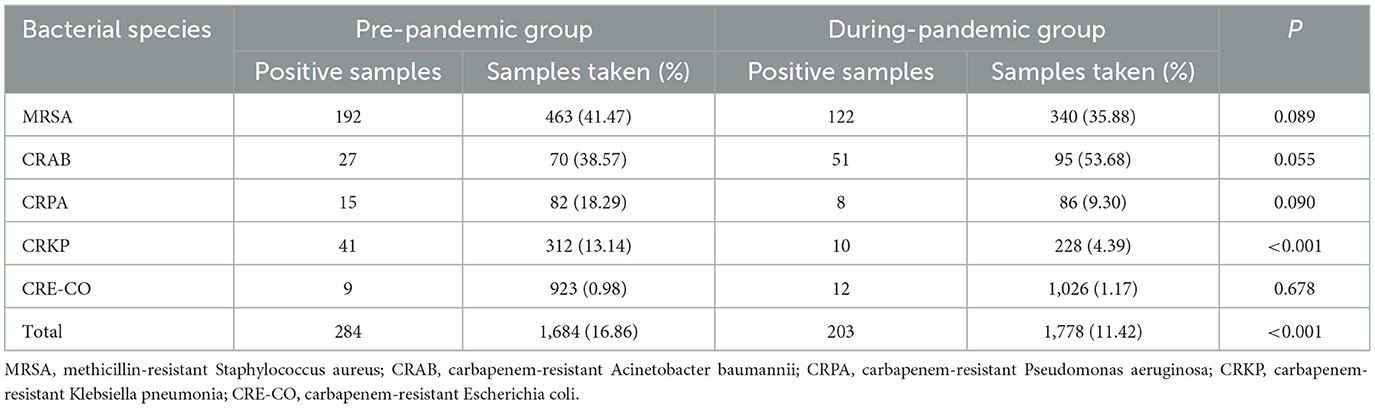
Multidrug-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Pseudomonas aeruginosa (CRPA), carbapenem-resistant Klebsiella pneumoniae (CRKP), and carbapenem-resistant Escherichia coli (CRE-CO) were detected during the study period. The total detection rate of multidrug-resistant bacteria was lower during the COVID-19 pandemic than before (16.86%, pre-pandemic; 11.42%, during-pandemic). Among them, CRKP occurred at a significantly lower rate (P < 0.001) than that before the pandemic (Table 2).
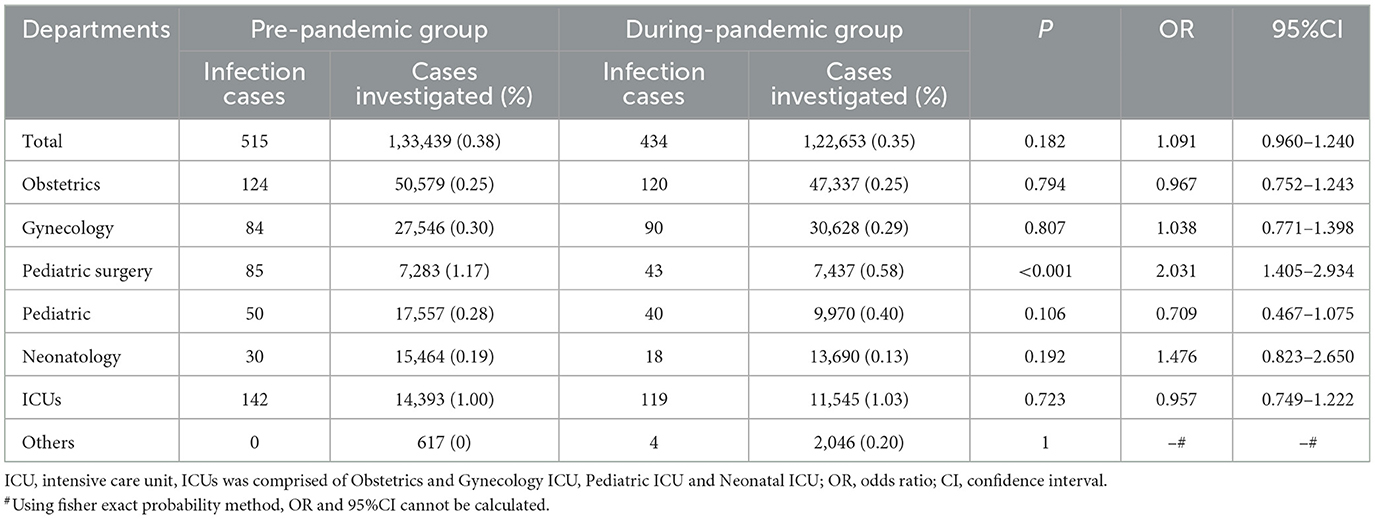
In this study, the incidence of nosocomial infections was lower than the national average. The decline in the prevalence of NIs was most pronounced in the pediatric surgery department. The prevalence was 1.17% (85/7,283) before the pandemic and 0.58% (43/7,437) during the pandemic in the pediatric surgery department. The prevalence of NIs in the other departments during the pandemic did not differ significantly from that before the pandemic (Table 3).
The most obvious decline in the pandemic group was observed in respiratory infections; however, the incidence of NIs at other sites during the pandemic did not change significantly. The tendency declined in gastrointestinal infections, genital tract infections, and bloodstream infections (BSIs) but not in CLABSI; however, there was no statistically significant difference between the two groups (Figure 2).
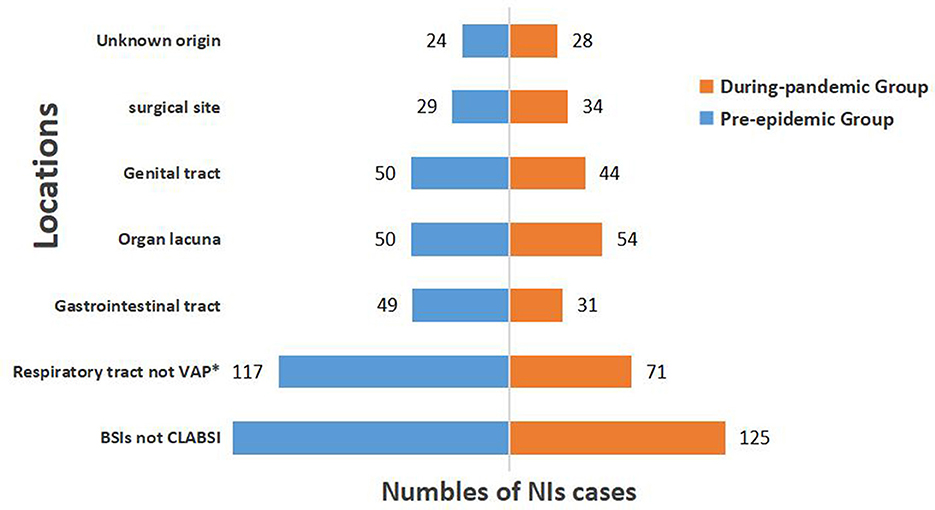
Figure 2. Distribution of locations of NIs before and during COVID-19 pandemic. VAP, ventilator-associated pneumonia; BSIs, bloodstream infections; CLABSI, central line associated-bloodstream infection; *Represents statistically significant difference (P < 0.05).
Device-associated infections (DAIs) are another specific type of NI, and these patients were mostly admitted to the ICU. The incidence rates of ventilator-associated pneumonia (VAP), central line-associated bloodstream infection (CLABSI), and catheter-associated urinary tract infection (CAUTI) were assessed at three ICUs in the study hospital. The incidence of CLABSI during the COVID-19 pandemic decreased significantly compared to that before the pandemic (9.4/1,000 catheterization days vs. 2.2/1,000 catheterization days, P < 0.001) (Table 4).
4. Discussion
Corona Virus Disease 2019 is characterized by rapid transmission, a wide range of infections, and great difficulty in prevention and control, seriously threatening human health and life (15). As the pandemic continues to change, work in hospitals has been greatly affected, especially hospital infection prevention and control work (16). During the post-pandemic period, knowledge of the composition of common pathogens and surveillance of NIs in hospitals will assist clinicians in making better decisions (17). However, previous studies have reported results mostly in tertiary general hospitals or a single department, such as the pediatric and ICU departments, with fewer data available for maternity hospitals (18–20). In this study, which was conducted in a tertiary maternity hospital, we found that between 2020 and 2021, the distribution of the main drug-resistant bacteria was similar to that before, and the detection rate of S. agalactiae increased annually. The detection rate of multidrug-resistant bacteria decreased during the pandemic, especially that of CRKP. In addition, during the pandemic, the incidence of NIs in the entire hospital was much lower than the national average, and the incidence in the pediatric surgery department decreased significantly. The incidences of VAP, CLABSI, and CAUTI were low, and the incidence of CLABSI decreased significantly.
Increased bacterial resistance is often associated with the overuse of antibiotics (21). A systematic analysis showed that there were an estimated 4.95 million deaths associated with bacterial antimicrobial resistance (AMR) in 2019 (22). During the study period, the top five drug-resistant bacteria were Escherichia coli, Streptococcus agalactiae, Staphylococcus aureus, Klebsiella pneumoniae, and Enterococcus faecalis. This is consistent with data from Ireland, where the common maternal pathogens were E. coli and Group B Streptococcus (10). However, unlike the top five bacteria detected in the national reporting data, the ones detected in this study were Escherichia coli, Klebsiella pneumoniae, Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii (7). This may be because no COVID-19 patients had been admitted to the study hospital, and the bacterial composition was mainly related to the environment, season, and composition of the patient population (women, children, and newborns). The prevalence of maternal colonization by Streptococcus agalactiae (Group B Streptococci [GBS]) varies according to the region studied and is approximately between 15 and 30% abroad (23), similar to our results. As a new consensus in China was released, targeted screening was added to the study hospital, and the detection rate of GBS increased annually.
During the COVID-19 pandemic, hospital administrators implemented some measures, such as better management of antibiotic use, better training for physicians, and better environmental hygiene. The detection rate of multidrug-resistant bacteria decreased from 18.86% before the pandemic to 11.42% during the pandemic; among them, methicillin-resistant Staphylococcus aureus (MRSA) was still the most detected bacterium, and the detection rate of CRKP decreased significantly. This declining trend differs from those observed in recent studies. A comprehensive review reported that, in recent years, CR-hvKP (Carbapenem-resistant Hypervirulent Klebsiella pneumoniae) has been increasingly reported in China, the United States, India, Russia, Egypt, Italy, and other countries, represented by the ST11, ST23, and ST258 types (24). Further analysis revealed that CRKP was mainly detected in children with severe disease in the study hospital. However, during the COVID-19 pandemic, pediatric hospitalizations decreased significantly, and antibiotic use was rigorously regulated, which would likely lead to a significant drop in detection rates. NIs caused by CRKP among children are becoming common (25), and it is necessary for pediatric wards to conduct CRKP screening on admission and standardize antibiotic use, which may decrease the incidence of CRKP infections.
With increasing awareness of nosocomial infection prevention, the prevalence rate of NIs in China decreased from 5.36% in 2001 to 1.98% in 2018 (26). During the study period, the incidence of NIs in hospitals was much lower than the national average. Since 2020, a series of recommendations have been made for the management of COVID-19 (27). Based on these guidelines, hospitals have developed hospital-specific strategies to guide clinical departments in their COVID-19 prevention efforts. However, few studies have investigated the relationship between interventions and nosocomial infections. In this study, we observed that NIs in the pediatric surgery department decreased from 1.17 to 0.58% after the response prevention to the COVID-19 pandemic. The incidence of NIs declined in other departments, such as neonatology and gynecology, but the difference was not significant.
When we further studied the locations of NIs, we found the most significant decrease in respiratory infections, followed by gastrointestinal infections, which were related to health habits during the COVID-19 pandemic. In COVID-19 prevention efforts, masks are widely used coupled with adequate social distancing, diligent hand hygiene, and other proven prevention measures (28). These measures may have reduced the spread of respiratory and gastrointestinal infections.
There was a decreasing trend in the monitoring of DAIs in the ICUs during the study period. In particular, the decrease in CLABSI was statistically significant. Similar to a previous study, Costello et al. (29) showed that the education of physicians and nurses and real-time feedback on CLABSI data led to a decrease in CLABSI rates. These results indicate that these measures can be continuously implemented to bEtter prevent and control NIs.
We acknowledge that there were some limitations in the study. First, the data used in this study were retrieved from the hospital infection surveillance system; therefore, there may have been selection bias. Second, without further analysis of the patient's symptoms, recovery, and treatment, the advice provided to different departments will be limited. Third, we only studied the data from 2018 to 2021, and the short year span may lead to limitations in this study.
The incidence of nosocomial infections was lower than that before the COVID-19 pandemic. Active surveillance of MDR, generation of data on etiological agents and their antimicrobial susceptibility patterns, implementation of standard infection control protocols, and re-education of hospital staff are needed to control the occurrence of nosocomial infections.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HH, KW, HC, and JW designed the study. ZL and SL analyzed the clinical data. HH and KW wrote the manuscript. LC revised the manuscript and supervised the study. All authors have seen and approved the manuscript and its submission.
Acknowledgments
The authors would like to thank the Blood Transfusion Department and Department of Infection Control, Fujian Maternity and Child Health Hospital College of Clinical Medicine for Obstetrics and Gynecology and Pediatrics, Fujian Medical University for helping to perform part of this study. We would like to thank Editage for the English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections: an overview. Infect Drug Resist. (2018) 11:2321–33. doi: 10.2147/IDR.S177247
2. Izquierdo-Cubas F, Zambrano A, Frómeta I, Gutiérrez A, Bastanzuri M, Guanche H, et al. National prevalence of nosocomial infections. J Hosp Infect. (2008) 68:234–40. doi: 10.1016/j.jhin.2007.12.006
3. Sun J, Xu H, Zhang J, Gu AM, Jia L, Sun Z, et al. Influence of multidrug-resistant organisms infections on length of hospital stay and hospitalization costs. Chin J Nosocomiol. (2016) 26:4277–9.
4. Schwab F, Gastmeier P, Piening B, Geffers C. The step from a voluntary to a mandatory national nosocomial infection surveillance system: the influence on infection rates and surveillance effect. Antimicrob Resist Infect Control. (2012) 1:24. doi: 10.1186/2047-2994-1-24
5. Schröder C, Schwab F, Behnke M, Breier AC, Maechler F, Piening B, et al. Epidemiology of healthcare associated infections in Germany: nearly 20 years of surveillance. Int J Med Microbiol. (2015) 305:799–806. doi: 10.1016/j.ijmm.2015.08.034
6. Cooke EM, Coello R, Sedgwick J, Ward V, Wilson J, Charlett A, et al. A national surveillance scheme for hospital associated infections in England. Team of the Nosocomial Infection National Surveillance Scheme. J Hosp Infect. (2000) 46:1–3. doi: 10.1053/jhin.2000.0801
7. CHINET. Annual Bacterial Resistance Monitoring Results in 2021. (2021). Available online at: http://www.chinets.com/Document# (accessed October 10, 2022).
8. Li Y, Gong Z, Lu Y, Hu G, Cai R, Chen Z. Impact of nosocomial infections surveillance on nosocomial infection rates: a systematic review. Int J Surg. (2017) 42:164–9. doi: 10.1016/j.ijsu.2017.04.065
9. Xu J, Zhang Y, Ma H, Zhang R, Wu J. Analysis of factors related to neonatal infection and monitoring of bacterial drug resistance. Z Geburtsh Neonatol. (2022) 226:399–404. doi: 10.1055/a-1850-2475
10. Knowles, SJ, O'Sullivan, NP, Meenan, AM, Hanniffy R, Robson M. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG Int J Obstet Gy. (2014) 122:663–71. doi: 10.1111/1471-0528.12892
11. Singh V, Upadhyay P, Reddy J, Granger J. SARS-CoV-2 respiratory co-infections: incidence of viral and bacterial co-pathogens. Int J Infect Dis. (2021) 105:617–20. doi: 10.1016/j.ijid.2021.02.087
12. O'Toole RF. The interface between COVID-19 and bacterial healthcare-associated infections. Clin Microbiol Infect. (2021) 27:1772–6. doi: 10.1016/j.cmi.2021.06.001
13. Ministry of Health of the People's Republic of China. Diagnostic criteria for nosocomial infections. Chin Med J. (2001) 81:314–20
14. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. (1988) 16:128–40. doi: 10.1016/0196-6553(88)90053-3
15. World Health Organization. World Health Assembly. Geneva: WHO (2020). Available online at: https://www.who.int/director-general/speeches/ (accessed May 18, 2020).
16. Lee I, Wang C, Lin M, Kung C, Lan K, Lee C. Effective strategies to prevent coronavirus disease-2019 (COVID-19) outbreak in hospital. J Hosp Infect. (2020) 105:102. doi: 10.1016/j.jhin.2020.02.022
17. Harada S, Uno S, Ando T, Iida M, Takano Y, Ishibashi Y, et al. Control of a nosocomial outbreak of COVID-19 in a university hospital. Open Forum Infect Dis. (2020) 7:ofaa512. doi: 10.1093/ofid/ofaa512
18. Merzougui L, Barhoumi T, Guizani T, Barhoumi H, Hannachi H, Turki E, et al. Nosocomial infections in the Intensive Care Unit: annual incidence rate and clinical aspects. Pan Afr Med J. (2018) 30:143. doi: 10.11604/pamj.2018.30.143.13824
19. Baviskar AS, Khatib KI, Rajpal D, Dongare HC. Nosocomial infections in surgical intensive care unit: a retrospective single-center study. Int J Crit Illn Inj Sci. (2019) 9:16–20. doi: 10.4103/IJCIIS.IJCIIS_57_18
20. Nouetchognou JS, Ateudjieu J, Jemea B, Mesumbe EN, Mbanya D. Surveillance of nosocomial infections in the Yaounde University Teaching Hospital, Cameroon. BMC Res Notes. (2016) 9:505. doi: 10.1186/s13104-016-2310-1
21. Abuhussain SSA, Burak MA, Adams DK, Kohman KN, Tart SB, Hobbs ALV, et al. Variability in emergency medicine provider decisions on hospital admission and antibiotic treatment in a survey study for acute bacterial skin and skin structure infections: opportunities for antimicrobial stewardship education. Open Forum Infect Dis. (2018) 5:206. doi: 10.1093/ofid/ofy206
22. Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. (2022) 399:629–55. doi: 10.1016/S0140-6736(21)02724-0
23. Siqueira F, Ferreira EM, de Matos Calderon I, Dias A. Prevalence of colonisation by group B streptococcus in pregnant patients in Taguatinga, Federal District, Brazil: a cross-sectional study. Arch Gynecol Obstet. (2019) 299:703–11. doi: 10.1007/s00404-019-05040-z
24. Han YL, Wen XH, Zhao W, Cao X-S, Wen J-X, Wang J-R, et al. Epidemiological characteristics and molecular evolution mechanisms of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front Microbiol. (2022) 13 1003783. doi: 10.3389/fmicb.2022.1003783
25. Luo K, Tang J, Qu Y, Yang X, Zhang L, Chan Z, et al. Nosocomial infection by Klebsiella pneumoniae among neonates: a molecular epidemiological study. J Hosp Infect. (2020) 108:174–80. doi: 10.1016/j.jhin.2020.11.028
26. Zong Z, Wu A, Hu B. Infection control in the era of antimicrobial resistance in China: progress, challenges, and opportunities. Clin Infect Dis. (2020) 71(Suppl. 4): S372–8. doi: 10.1093/cid/ciaa1514
27. Li J, Yong, Y. Guidance for Corona Virus Disease 2019: Prevention, Control, Diagnosis Managements. China: People's Medical Publishing House (2020). Available online at: http://www.pmph.com/
28. Tirupathi R, Bharathidasan K, Palabindala V, Salim SA, Al-Tawfiq JA. Comprehensive review of mask utility and challenges during the COVID-19 pandemic. Infez Med. (2020) 28 (suppl 1):57–63.
Keywords: COVID-19 pandemic, nosocomial infection, infection rate, pathogen, epidemiology
Citation: Huang H, Wu K, Chen H, Wang J, Chen L, Lai Z and Lin S (2023) The impact of the COVID-19 pandemic on nosocomial infections: a retrospective analysis in a tertiary maternal and child healthcare hospital. Front. Public Health 11:1132323. doi: 10.3389/fpubh.2023.1132323
Received: 27 December 2022; Accepted: 24 March 2023;
Published: 18 April 2023.
Edited by:
Mahlagha Dehghan, Kerman University of Medical Sciences, IranReviewed by:
Mohammad Ali Zakeri, Rafsanjan University of Medical Sciences, IranPengcheng Zhou, Central South University, China
Copyright © 2023 Huang, Wu, Chen, Wang, Chen, Lai and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiming Chen, 76461792@qq.com; Jing Wang, kingmax34@126.com
†These authors have contributed equally to this work and share first authorship
 Huifang Huang1†
Huifang Huang1† Kunhai Wu
Kunhai Wu