- 1Laboratory of Biostatistics, Clinical and Epidemiological Research, Faculty of Medicine and Pharmacy, Mohammed V University in Rabat, Rabat, Morocco
- 2Acute Medical Unit, Ibn Sina University Hospital, Rabat, Morocco
- 3Department of Internal Medicine, Military Hospital Bizerte, Bizerte, Tunisia
- 4Faculty of Medicine of Tunis, University El Manar, Tunis, Tunisia
- 5Tunisian Society of Tropical Medicine and Travel, Tunis, Tunisia
- 6Infectious Diseases Department, University Hospital, Monastir, Tunisia
- 7Infectious Diseases Department, Medical School of Casablanca, Casablanca, Morocco
- 8Pfizer Inc., Casablanca, Morocco
- 9Pfizer Inc., Tunis, Tunisia
Vaccine preventable diseases (VPDs) are a prevailing concern among the adult population, despite availability of vaccines. Unlike pediatric vaccination programs, adult vaccination programs lack the required reach, initiative, and awareness. Clinical studies and real-world data have proven that vaccines effectively reduce the disease burden of VPDs and increase life expectancy. In Tunisia and Morocco, the national immunization program (NIP) focuses more on pediatric vaccination and have limited vaccination programs for adults. However, some vaccination campaigns targeting adults are organized. For example, influenza vaccination campaigns prioritizing at risk adults which includes healthcare professionals, elderly, and patients with comorbidities. Women of childbearing age who have never been vaccinated or whose information is uncertain are recommended to receive tetanus vaccination. Tunisia NIP recommends rubella vaccine mainly for women of childbearing age, while in Morocco, national vaccination campaigns were organized for girls and women (up to 24 years of age) to eliminate rubella. Further, travelers from both countries are recommended to follow all requirements and recommendations in the travel destination. The objective of this manuscript is to provide an overview of the global disease burden of common VPDs including (but not limited to) meningococcal diseases, pneumococcal diseases, hepatitis, and influenza. The review also provides an overview of clinical data and guidelines/recommendations on adult vaccination practices, with special focus on Tunisia and Morocco. Some European and North American countries have concrete recommendations and strategies for adult vaccination to keep the VPDs in check. In Morocco and Tunisia, although, there are sporadic adult vaccination initiatives, the efforts still need upscaling and endorsements to boost vaccination awareness and uptake. There is a need to strengthen strategies in both countries to understand the disease burden and spread awareness. Additional studies are needed to generate economic evidence to support cost-effectiveness of vaccines. Integration of private and public healthcare systems may further improve vaccination uptake in adults.
Introduction
Vaccines are an important public health tool used to prevent the spread of contagious diseases and saving lives from preventable diseases (1–3). They have improved the general life expectancy of the global population by combating mortality and morbidity associated with infectious diseases (4).
Most of the vaccination programs have focused on pediatric population (4, 5). Globally, several national and international collaborative initiatives have ensured in achieving a vaccination uptake rate of >90% in the pediatric population across many countries (5). However, the vaccination programs for adults worldwide are comparatively lower, despite the evidence of the increased burden of vaccine-preventable diseases (VPDs) in adults (4, 5).
The life expectancy has improved considerably rising from 64.2 years in 1990 to 72.6 years in 2019, and in many low- and middle-income countries (LMIC), the share of the population aged more than 65 years is expected to be double between 2019 and 2050 (6, 7). In this context, it is imperative to prioritize the health of the adult population and facilitate healthy aging strategies to manage adult mortality and morbidity, improve quality of life, and reduce disability in every way possible to have a positive impact on global wellbeing (7). The susceptibility to infectious diseases is more among the older adults, resulting in higher incidence with increased severity. The possible reason is the onset of immunosenescence (age-related dysregulation of the immune system) in the elderly population (>64 years) (8). Immunosenescence also leads to inadequate response to vaccinations which can be further impacted by other underlying conditions (5, 9). Hence, vaccination at an earlier stage in adulthood may provide appropriate immunity against infectious diseases in older adults (5). Immunization in adults is important to restore their weakening immunity against VPDs and grant immunity against other diseases. Another challenge among the older adults is the high risk for multi-drug resistance infections due to factors such as weak immune system or staying in old age homes. Vaccinations can be an efficient tool in adults that may help decrease disease burden and reduce the use of antibiotics (10). Further, immunization of adults may also prevent the spread of infectious diseases to children and other vulnerable populations.
Several clinical studies on vaccination in adults have proven to be effective (11, 12). Despite the clinical evidence, vaccination coverage in adults is not satisfactory (4) and the impact is further substantial in LMIC (including Tunisia and Morocco), as policies for adult vaccination are not widely established and implemented (13). The obvious lacunae in terms of guidelines/recommendations, and the establishment and/or implementation of vaccination programs need to be addressed. Further, information on the current disease burden of VPDs is important, while constructing preventive measures and prioritization (14). An understanding of the disease burden, existing guidelines/recommendations, and information on ongoing adult vaccination programs will help in leveraging the previous experience and comprehending the gaps to develop robust, effective, and successful vaccination programs in LMIC, where advocacy and implementation are in infancy. Further, emphasis on healthcare education and awareness about the importance of vaccination will also aid in increasing vaccination coverage in adults.
This manuscript aims to provide an overview of the global disease burden of VPDs including meningococcal diseases, pneumococcal diseases, hepatitis, influenza, human papillomavirus, diphtheria, pertussis, tetanus, rabies, measles, and tuberculosis (scope of the review does not cover information on coronavirus disease and associated vaccinations). Additionally, the review focuses on clinical studies related to adult vaccination and established guidelines/recommendations on vaccination practices in adults aged 18 years or above, with special focus on Tunisia and Morocco.
The Burden of Vaccine Preventable Diseases
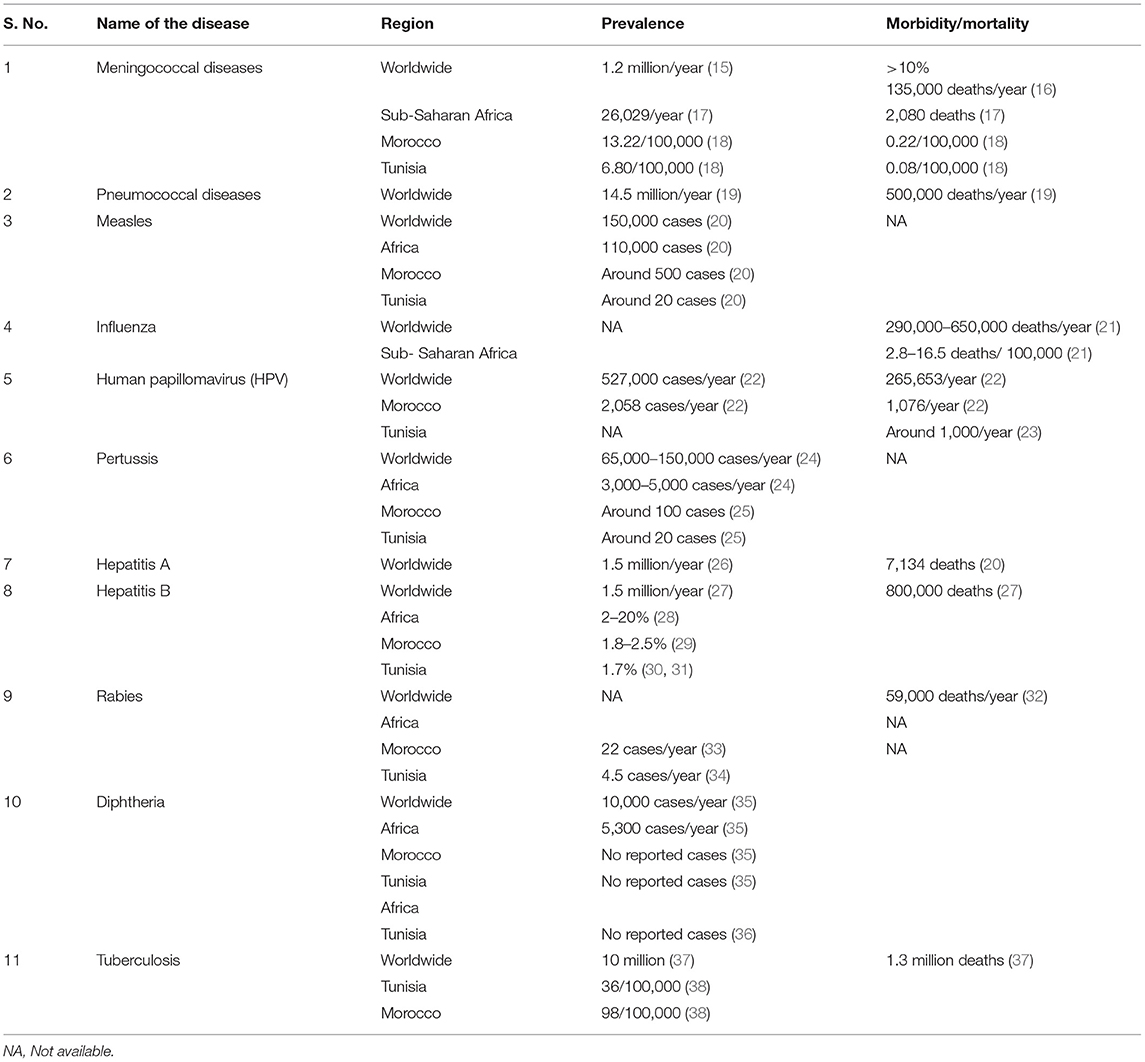
Routine immunization programs conducted worldwide for several decades have drastically reduced or eradicated infectious diseases among children saving millions of lives. In the current scenario, with an increase in aging population, there is an immediate need to focus on adult vaccination to reduce the burden of VPDs in adults. Invasive bacterial diseases and viral diseases are a major public health concern in adult populations worldwide and more specifically in LMIC, such as those in the Middle East and North Africa region, despite the availability of vaccinations for these diseases. The most common VPDs include meningococcal infections caused by Neisseria meningitidis (N. meningitidis), pneumococcal disease, caused by Streptococcus pneumoniae (S. pneumoniae), influenza caused by Haemophilus influenzae (H. influenzae) and Myxovirus influenzae, human papillomavirus (HPV), hepatitis A and B, tuberculosis, diphtheria, tetanus, pertussis, and rabies (15). An overview of the disease burden of the above mentioned VPDs will help strategize vaccination policies and practices to effectively control and prevent these diseases. The burden of VPDs obtained from various studies globally and in Africa, Morocco, and Tunisia has been summarized in Table 1.
International and Regional/Local Epidemiology of Vaccine Preventable Diseases Among Adults
Meningococcal Infection
The distribution of invasive meningococcal disease (IMD) varies by year, region, age group, and causative organism (39, 40). Globally, close to 1.2 million cases of IMD are reported annually with an overall case mortality rate of 5–10%, which includes significantly high contribution from adults aged ≥65 years (15). The highest IMD incidence is observed in infants (due to lack of natural immunity), but a second peak is seen in adolescents and young adults (due to increased risk factors such as social behavior) (41, 42). Based on capsular polysaccharides of N. meningitidis, 12 serogroups are identified and among them six serogroups (A, B, C, W, X, and Y) are responsible for nearly all infections globally (43). In Africa, until recently, most meningitis epidemics in the meningitis belt of sub-Saharan Africa were due to serogroup A. Since the successful rollout of a new serogroup A meningococcal conjugate vaccine in 2010, the incidence of serogroup A is declining, however other serogroups still cause epidemics. Currently, serogroup W is responsible for most cases (79%), followed by serogroup X (11%) (44). In South Africa, the distribution of IMD by serogroup is W (42%), B (21%), “not defined” (15%), and Y (13%) (44). In Morocco, IMD is considered endemic with estimated incidence and death rates for meningococcal meningitis as 13.24/100,000 and 0.22/100,000, respectively (18). While, Tunisia has relatively lower incidence and death rates estimated to be 6.80/100,000 and 0.08/100,000, respectively (18). Meningococcal serogroup B (MenB) is highly prevalent in most of North Africa (45). According to the most recent real-time PCR based surveillance study on invasive bacterial infections in North Africa (Tunisia, Algeria and Morocco), serogroup B meningococci (MenB) was the most prevalent of N. meningitidis ranging between 50 and 90%. Other serogroups detected in these countries were C and Y while serogroup W was not detected (46).Yet, considerable genetic heterogeneity exists across the region. For example, most MenB isolates in Morocco had the B:4:P1.15 phenotype (47), whereas NT:NST was the most frequent in Tunisian children (48). Additionally, MenB isolates in Tunisia were diverse, with six serotypes and eight sero-subtypes identified (18, 49).
Pneumococcal Infection
Pneumococcal infections affect people of all ages, but children younger than 2 years of age and adults aged 65 years and older are at higher risk. A limited number of S. pneumoniae serotypes are responsible for most serious pneumococcal infections in both adults and children around the world (50). Invasive pneumococcal disease (IPD) is often associated with high risk of morbidity and mortality. This risk is further enhanced in immunocompromised patients. A meta-analysis studying the incidence of IPD in adults (≥18 years) reported that the incidence in healthy controls was 10/100,000 person years as compared to a very high incidence range of 65/100,000–812/100,000 in immunocompromised patients with chronic inflammatory diseases, human immunodeficiency virus (HIV) and patients who underwent transplantation (51). Another observational study conducted in the United States reported that the incidence rate of IPD is 10.5 times higher in immunocompromised patients as compared to healthy adults in the age group of 19–64 years (52). The high incidence of IPD in immunocompromised patients highlights the importance of vaccination for this population. In Netherlands, the estimated average annual disease burden expressed using the disability-adjusted life years (DALY) for non-invasive pneumococcal disease was 37,223 DALYs/year, and IPD was 6061 DALYs/year (14). In LMIC, pneumococcal disease, caused by S. pneumoniae is estimated to affect 14.5 million children below 5 years of age, every year with 500,000 deaths (19). Other than young children, adults aged 65 years and older are at higher risk having an incidence of 1 per 1,000 adults per year (53). During Hajj pilgrimage, community-acquired pneumonia (CAP) was the leading cause of hospitalization (15–40% of hospital admissions), accounting for 22–31% of ICU admissions. CAP patients had 10% case-fatality rate, with higher rates in ICU patients (54). S. pneumoniae is the cause of pneumonia in at least 18% of cases during Hajj, with increased risk and higher fatality among individuals with comorbidities and elderly males (54). Other causative pathogens were also reported for CAP, such as Staphylococcus aureus, H. influenzae, and influenza A virus, but S. pneumoniae has a high clinical burden (54).
Influenza
An average of 389,000 (290,000–650,000) deaths due to respiratory illnesses were estimated to be related to influenza, globally each year during 2002–2011. Among these, 67% cases were reported in the adult population ≥65 years. As per the age-mortality patterns, the highest percentage of deaths in older individuals occurred in Europe (84%), and the lowest in sub-Saharan Africa (36%) (21, 55). In the United States, the Centers for Disease Control and Prevention (CDC) estimates ranged from 140,000 to 710,000 for flu-related hospitalizations and about 12,000 to 56,000 deaths since 2010 (56). A study estimating seasonal influenza-associated mortality suggested the highest mortality in sub-Saharan Africa was 2.8–16.5 deaths per 100,000 individuals and among older adults aged ≥75 years (57). Seasonal influenza also causes lower respiratory tract infections (LRTIs), which is the leading cause of infectious disease mortality. According to the global burden of disease study, 2017, influenza was globally responsible for 11.5% cases of LRTI in all age groups, with the highest number in the age group of 30–54 years. In Morocco, the incidence reported was 1,435/100,000 cases and hospitalizations due to LRTI was 237/100,000, while in Tunisia the incidence was 903/100,000 cases and 153/100,000 hospitalizations among all age groups (58).
Human Papillomavirus
HPV is the most common sexually transmissible infection in the world with considerably high prevalence in developing countries (42.2%) compared to more developed countries (22.6%) (59). Asia (45.5%) and Africa (29.6%) have the highest prevalence with serotypes 16 (9.5%) and 18 (6.2%) to be the prevalent type, irrespective of the region of study (60). There is considerable variability in HPV genotypic prevalence based on the geographical region, for example, serotypes 6 and 11 are most common in United States, but has low prevalence in Africa. The most infected age group was adolescent girls and young women <25 years of age, although a rebound in HPV infection in adults >45 years old was seen in Sub-Saharan Africa and American regions (59, 61). The global prevalence rate of HPV infection in men is similar to women (highest in men of the sub-Saharan Africa) (59, 62). The incidence of HPV is higher in men living with HIV and men who have sex with men than in heterosexual men (59).
In 2020, cervical cancer accounted for 604,127 new cases and 341,831 deaths worldwide. Almost all the cases of cervical cancer (99.7%) have been associated with HPV (22, 63). Morocco and Tunisia have high prevalence of HPV with an estimation of ~2,258 new cervical cancer cases annually in Morocco, and around 1,000 cervical cancer deaths occurring each year in Morocco and Tunisia (22, 23).
Hepatitis
Hepatitis A has a high rate of infection, with 1.5 million cases each year (26), but is less likely to cause chronic liver disease. The World Health Organization (WHO) estimated that in 2016, 7,134 patients died from hepatitis A worldwide, with most cases in LMIC (20). In many African countries including Tunisia, prevalence of hepatitis A has decreased with the improvement of sanitary and socioeconomic conditions; they have transitioned from high endemicity to low (64). In Morocco, 222 cases of hepatitis A were reported in 2015 (65).
According to WHO in 2019, 296 million people were reported with chronic hepatitis B infection leading to 820,000 deaths, mostly from cirrhosis and primary liver cancer (27, 29). About 2.7 million (7.4%) of the 36.7 million people living with HIV (PLHIV) are also infected with hepatitis B virus (HBV) of which 71% co-infected patients are from sub-Saharan Africa (66). In Africa, the seroprevalence of HBV infection ranges from 3 to 20% (28). Several studies reported Morocco as low endemic region with seroprevalence around 1.8%, and the males showed higher prevalence than females in all the age groups, especially among aged 30–50 years (29). In Tunisia, seroprevalence was 1.7% with significantly higher prevalence in males and in the ages >20 years (30, 31). Both Morocco and Tunisia are characterized by prevalence of genotype D (29).
Other Vaccine Preventable Diseases
According to the global tuberculosis report of 2021, an estimated 9.9 million people were suffering from tuberculosis with an estimated 1.3 million deaths worldwide (37). Around 8% of infected people are living with HIV, with highest proportion of co-infected cases in African countries. In a 22-year study in Tunisia, annual incidence rate was 13.91/100,000 population/year with mortality rate of 0.39/100,000 population/year. Elderly population (age group >60 years) showed significantly higher incidence and mortality rates as compared to all other age groups (67). In 2020, the World Bank-reported incidence rate in Tunisia was 36/100,000 and in Morocco was 98/100,000 (38). Morocco has made significant progress in the control and management of tuberculosis during the past 30 years, but with COVID-19 pandemic, new challenges resurfaced as health service delivery was disrupted (68).
Pertussis is another VPD, which remains a concern in many regions despite vaccination (69). According to WHO, the prevalence of pertussis has decreased from 150,000 cases to 65,000 cases from 2019 to 2020 (28). Most cases of pertussis are among children <1 year old, however, adults are considered as the main reservoir of transmission of the disease to unvaccinated young children (30). There is a lack of surveillance in Africa and the burden of pertussis among adults is not well-reported, and likely underestimated (70).
Measles can be serious in all age groups. The reported cases of measles globally, as reported by WHO was ~150,000 in 2020 with a majority of cases (115,364) being reported from Africa (36). Specific sub-groups in a population are more likely to suffer from measles complications, which include adults older than 20 years of age, pregnant woman, and people with compromised immune systems, such as leukemia and HIV infection (71). In 2019, according to the World Bank data, Morocco had Africa's highest rates of immunization against measles, at 99% among children. However, with 483 measles cases reported in 2018, efforts are still needed for eliminating the disease (20). For Tunisia, in 2019, the Ministry of Health responded to a large measles outbreak in the country. A total of 909 cases with laboratory confirmation were reported. The two most affected age groups were those older than 15 years (31%) and infants between the ages of 6 and 12 months (28%). Death was reported in 30 cases ranging in age from 15 days to 41 years of age (median, 7.5 months) (72).
Rabies kills around 60,000 people every year worldwide, and the majority of the victims are in developing countries of Africa and Asia (32). Morocco reported 22 cases per year and Tunisia had 4 to 5 cases per year (33, 34). Most of the rabies cases are not even reported, so there is need for more awareness for better rabies control (32). The vaccination coverage for people exposed to risk is also low (33).
The aforementioned clinical disease burden suggests that VPDs contribute to a substantial proportion of infectious diseases globally, especially in African countries. However, the incidence of VPDs in adults is under-reported in most parts of the world, and hence the impact maybe even more than we currently foresee.
Overview on Vaccines for Vaccine Preventable Diseases and Clinical Studies on Adult Vaccination
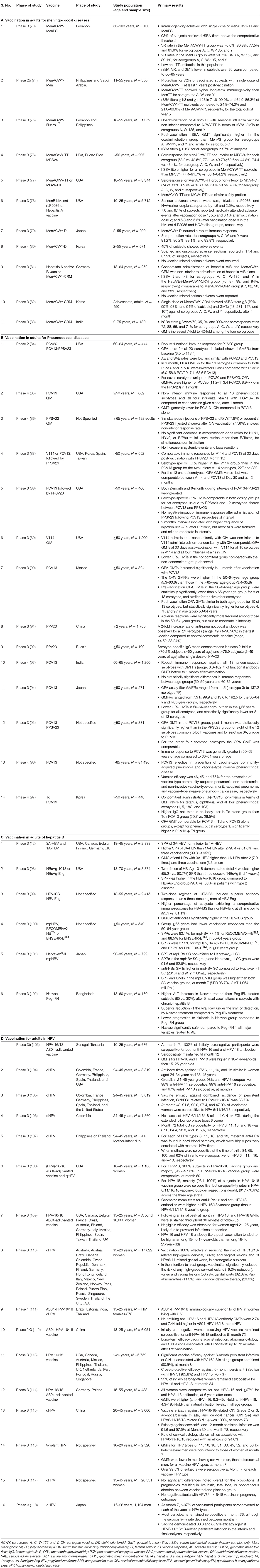
Clinical studies have extensively evaluated the efficacy, safety, and tolerability of vaccines in different age groups. An in-depth understanding of the effectiveness and tolerability of vaccines in adults is essential for both healthcare providers and government bodies to make informed decisions and establish efficient policies and recommendations. This section provides an overview of vaccines available for prevalent diseases in the North African region, which includes vaccines for meningococcal diseases, pneumococcal diseases, hepatitis B, Haemophilus influenzae type b and HPV (Table 2).
Meningococcal Vaccines
N. meningitidis, is associated with many clinical consequences such as meningitis, septicemia, and serious sequelae affecting life physically and psychologically (119). Colonization of the nasopharynx, called meningococcal carriage is common and leads to transmission and development of IMD. Carriage rates are found to be highest in adolescents and young adults (120, 121). Three types of vaccinations are available for IMD; polysaccharide, conjugate, and protein polysaccharide conjugate vaccines (monovalent, bivalent or multivalent). The vaccines are conjugated to carrier proteins such as tetanus toxoid, diphtheria toxoid, or diphtheria toxoid variant CRM197), which enhance immunological memory (18).
Vaccination programs have been highly successful at reducing the burden of serogroup C IMD in many countries, worldwide (122–124). In 1999, in response to the increasing number of cases of serogroup C IMD in the United Kingdom (UK), meningococcal C vaccine was added to the routine infant immunization schedule, increasing in demand for routine vaccination (125). Evidence from published literature suggests that mass vaccination campaigns using conjugated meningococcal vaccines around the world have led to control of serogroup C and a decrease in serogroup B disease (122). The authors noted that data from 10 years of surveillance (in countries with surveillance systems) following the introduction of the quadrivalent (A, C, W, Y) vaccine have led to control of serogroup C IMD in the UK, Canada, Australia, Spain, Belgium, Ireland, and Iceland. Since the introduction of the quadrivalent (A, C, W, Y) vaccine in the UK, there has been a 97% decrease in the incidence of meningococcal serogroup C IMD among both vaccinated and unvaccinated (herd effect) individuals (122). In the UK, there are epidemiologic data that suggest that the use of conjugate meningococcal C vaccines along with mass vaccination and catch-up programs have led to the protection of unvaccinated persons through herd immunity (126). Additionally, serogroup B IMD in New Zealand has also decreased, following the introduction of the serogroup B vaccine (122).
The introduction of mandatory quadrivalent ACWY vaccine in 2002 led to a significant decrease in number of IMD cases in Saudi Arabia. Between 1995 and 1999, the predominant serogroup was A, followed by B. However, between the 2000 and 2001 outbreaks of serogroup W-135 predominated, accounting for 78% of typed isolates. In the post-epidemic period, serogroups A and W were almost equally distributed (36 and 40%, respectively) (127). In England, the introduction of MenACWY vaccination program took place in 2015 and led to a decline of MenW as well as MenY cases' number in the post-immunization period (128). In Netherlands, the incidence of MenW has been reduced after the implementation of MenACWY in the immunization program. In Australia, meningococcal tetanus toxoid conjugate vaccine (MenACWY-TT) has been included into the National Immunization Program (NIP) in 2018, leading to a decline in overall meningococcal disease cases (129).
Quadrivalent polysaccharide vaccine, MenACWY has been conjugated with different carrier proteins, and have shown to be effective for adults in various studies, such as MenACYW-TT and meningococcal Diphtheria Toxoid Conjugate Vaccine (MenACWY-D) in adults aged 11–55 years (77). Other studies confirmed effectiveness of vaccines in adults >56 years (73, 76). In a study in Lebanon, conjugated vaccine (MenACWY-TT) and a quadrivalent polysaccharide vaccine (MensPS), were compared in terms of serum bactericidal activity (rabbit complement source; rSBA) vaccine response (VR) rates after 1 month. High VRs were observed for each serogroup after vaccination (93.3% and 100% in the MenACWY-TT group and MenPS group for serogroup A; 96.3 and 90.9% for serogroup C; 88.7 and 86.4% for W-135, respectively; and for Y, 100% in both groups) (73). The VR tends to decrease with age, as it was lower in subjects >65 years of age as compared to 56–65 years age group. The long-term effectiveness of conjugate vaccines was confirmed with persistence of immune response even after 5 years (74). Another quadrivalent polysaccharide–CRM197 conjugate vaccine (MenACWY-CRM), is currently approved for licensure in 65 countries in all age groups (82, 83, 130). A study in adults (19–65 years) in Latin America, compared MenACWY-CRM with MenACWY-D, and unconjugated meningococcal polysaccharide vaccine (MPSV4). The seroresponse rates were consistently higher in the MenACWY-CRM group than in the MenACWY-D group for all four serogroups, in the 19–55 years age group. Immune response was higher in the MenACWY-CRM group than in the MPSV4 group for serogroups A, C, and Y, in the subjects aged 56–65 years (130). Studies also support co-administration of vaccines, in adolescents and adults, which lowers healthcare visits, provides better adherence by young adults, and helps in accelerating pre-travel multi-vaccination schedules when traveling to endemic areas. MenACWY-CRM has been co-administered with tetanus diphtheria pertussis vaccine (Tdap), hepatitis A and/or B vaccine, and rabies vaccine and shown no safety concerns or interference with immune responses. Immunogenic responses to diphtheria, tetanus, and meningococcal (serogroups A, C, W-135, and Y) antigens were similar regardless of concomitant vaccine administration (81, 131). The conjugate vaccine (Men ACWY-TT) has also been co-administered with seasonal influenza vaccine in adults aged 18–55 years which induced robust immune responses, with at least 76.5% of subjects having a VR to each of the meningococcal serogroups, and a seroconversion rate of at least 61.9% to all three influenza strains (75).
Due to homology of MenB capsular polysaccharides to human neural cells, polysaccharide conjugate vaccines for serogroup B cannot be developed. Alternatively, two meningococcal serogroup B protein vaccines (MenB-4C; MenB-FHbp) can be administered as a 2-dose series (119). Lipoprotein 2086 which is a human complement factor H binding protein (fHBP), is used as a vaccine target for individuals 10–25 years of age. Immunogenicity of the vaccines was evaluated using human serum bactericidal assays (hSBA). Bivalent vaccine showed robust hSBA activity against diverse invasive meningococcus serogroup B disease strains and was well-tolerated (78, 132). Seroconversion rates from 70.0 to 94.7%, following the 3-dose vaccination, confirmed increase with subsequent vaccine doses (133). Coadministration of bivalent rLP2086 with diphtheria, tetanus, and acellular pertussis and inactivated poliovirus vaccine (DTaP/IPV) was immunologically non-inferior to DTaP/IPV administered alone. The vaccine showed robust and broad bactericidal antibody responses to diverse serogroup B strains after 2 and 3 vaccinations in adolescents and young adults (134).
Pneumococcal Vaccines
S. pneumoniae is one of the most common causal pathogens of otitis media, sinusitis, conjunctivitis, and CAP in addition to causing more severe IPDs such as meningitis and sepsis. Other, less frequent infections caused by S. pneumoniae include periorbital cellulitis, osteomyelitis, endocarditis, pericarditis, peritonitis, pyogenic arthritis, soft tissue infections, and neonatal septicemia (53). A large proportion of IPD is vaccine preventable. Currently, two kinds of pneumococcal vaccines are available; namely, pneumococcal polysaccharide vaccines (PPVs) and pneumococcal conjugate vaccines (PCVs). Polyvalent polysaccharide vaccines were first developed and licensed based on the demonstrated efficacy of 6-valent and 13-valent vaccines against pneumococcal pneumonia in adults. Subsequently, both 14-valent and 23-valent vaccines were licensed based on comparable safety and immunogenicity profiles to lower valency vaccines for shared serotypes (88). Both types of vaccines are effective, but PPVs have a short-lived immune response. PCVs which have carrier protein conjugated to polysaccharide, induce B-cell memory response, and provide long-term immunity. 23-valent PPV (PPSV23) showed robust immune response in adults >50 years and individuals 2–49 years having high risk or with chronic conditions (91, 92). Efficacy of 13-valent PCV (PCV13) was observed among adults >65 years of age, in prevention of vaccine-type CAP and vaccine-type IPD which persisted till 4 years (96). Similarly, robust immune response was observed in other studies with single dose of PCV13 in adults >50 years of age (90, 93, 94). Jackson et al. showed PCV13 opsonophagocytic activity (OPA) titers (used to measure antibody response of the vaccine) were non-inferior to PPSV23 for all 12 common serotypes and significantly higher in eight serotypes for PCV13, in 60–65 years age group. PCV13 elicited a substantially greater response to serotype 6A, which is contained only in PCV13. High level of immune response and longer antibody persistence was observed with PCV13 in adults 50–59 years as compared to 60–65 years age group. Enhanced immunogenicity with PCV13 as compared to PPSV23 in adults confirms PCV13 has the potential for improved clinical efficacy against pneumococcal disease (95).
Although PCVs have significantly decreased pneumococcal disease worldwide; expanding serotype coverage may further reduce disease burden. A 15-valent vaccine, PCV15, was found to be non-inferior to PCV13 for 13 common serotypes and superior to PCV13 for two of its unique serotypes (22F and 33F) in adults >50 years of age (135, 136). A 20-valent PCV (PCV20) was developed to expand protection against pneumococcal disease beyond PCV13. PCV20 includes seven additional serotypes than PCV13, therefore providing broader coverage along with all advantages of conjugate vaccine. A study reported robust immune OPA response for PCV20 against all 20 serotypes in adults aged 60–65 years, with no serious adverse events, similar to PCV13 (84). Another Phase 3 study showed comparable OPA titers for all 20 serotypes and non-inferiority with PCV13, in adults aged 14–49 years, thus confirming the efficacy of PCV20 (137).
In adults aged >50 years, sequential dosing with PCV13 and PPSV23 or PCV15 with PPSV23, at 12-month intervals showed comparable immunogenic response, except for two unique serogroups of PCV15 (87, 88). Another study confirmed higher OPA titers with sequential dosing of PCV13 followed by PPSV23 (PCV13/PPSV23) than those following only an initial PPSV23; and with PPSV23 followed by PCV13 (PPSV23/PCV13) (138).
Simultaneous administration of vaccines has also been tested and is a cost-effective and promising strategy. For example, co-administration of PPSV23 with influenza vaccine (86), tetanus diphtheria (Td) with PCV13 (97), influenza vaccine with PCV13 (85) and influenza vaccine with PCV15 (89) have shown to be safe, well-tolerated, and without affecting immunogenicity as compared to separate administrations. This co-administration can have a greater protective effect in the elderly and high-risk groups and prevent hospitalization, morbidity, and mortality from severe respiratory diseases (86). PLHIV are at higher risk of pneumococcal infection and often require hospitalization. A systemic review stated immunization schedule consisting of a sequential combination of PCV13/PPSV23 [PCV13, followed by PPSV23 after minimum 8 weeks recommended by Advisory Committee on Immunization Practices (ACIP)] (139) in PLHIV, or having immunocompromising conditions, aged 19 years or older (140, 141). Also, for optimum vaccination response, vaccination with PCV should be delayed until immunological recovery (CD4 > 200/mm3) (140).
Haemophilus influenzae Vaccines
H. influenzae is both a commensal and invasive pathogen of the upper respiratory tract, oropharynx, and nasopharynx. Major infections/diseases caused by H. influenzae include CAP, acute sinusitis, acute exacerbation of chronic bronchitis, and invasive infections such as bacteremia and meningitis (142, 143). These clinical manifestations are mostly caused by H. influenzae type b (Hib) strain, and/or non-typeable H. influenzae (NTHi) strain (144, 145). Vaccines against the Hib strain was developed as early as 1985 and has evolved over years with better efficacy, better immunogenicity, varied formulation choices and combinations (146). The combination vaccines protect against Hib disease, diphtheria, tetanus, pertussis (whooping cough), and polio, with additional hepatitis B vaccine in one of the combinations. Vaccination of Hib are either made of polysaccharides, the polyribosyl ribitol phosphate (PRP) of the Hib capsule which is less immunogenic in infants or conjugated as PRP linked to carrier proteins such as diphtheria toxoid or tetanus toxoid, making it more efficacious and immunogenic (147).
Hib vaccinations are recommended only for children younger than 5 years, and adults with certain medical conditions such as sickle cell anemia, HIV infection, asplenia, and cancer. Adult population is generally not given Hib vaccine and rely on natural boosting of immune response. The widespread use of Hib conjugate vaccines has drastically reduced invasive Hib disease, however the non-Hib diseases majorly caused by NTHi strain have increased. Invasive H. influenzae disease is now affecting adults, especially elderly and immunocompromised patients, more than children (148, 149). Hence, it is critical to develop and study vaccines against NTHi strain.
Acute exacerbation in patients with chronic obstructive pulmonary disease (COPD) is often associated with NTHi, therefore an investigational NTHi protein vaccine was tested in adults aged 40–80 years with moderate or severe COPD and a history of exacerbation. This study reported higher reactogenicity 7-day post-vaccination as compared to placebo, good immunogenicity, and acceptable safety profile. Further clinical assessments are required to evaluate effects on COPD symptoms and long-term outcomes (150). An early Phase I study was conducted to evaluate the safety and immunogenicity of a combined vaccine, NTHi-Moraxella catarrhalis (MCat) in adults with smoking history ≥10 pack-years, to immunologically represent the COPD population. The investigational NTHi-Mcat vaccine had an acceptable reactogenicity and safety with good immunogenicity in older adults with a smoking history. Further studies are warranted to assess the effectiveness of NTHi-Mcat vaccine (151). These potential vaccines against NTHi and other combination vaccines (S. pneumoniae and H. influenzae triple-protein vaccine) (152) may help to address the increasing burden of NTHi associated diseases.
Hepatitis B Vaccines
The HBV infections lead to complications such as hepatocellular necrosis, cirrhosis, hepatocellular carcinoma, and chronic liver disease. Adults at risk of HBV infection including PLHIV, healthcare workers, and travelers to endemic countries are recommended for vaccination by CDC. Full vaccine coverage is essential to prevent infections, as only 35% of subjects who received one or two doses had protective antibody levels as compared to 65% in subjects who had received full three doses (153).
The first HBV vaccine developed in the early 1980's was plasma-derived which demonstrated effectiveness against HBV infection. However, unproven safety concerns regarding transmission of blood-borne pathogens (including HIV) hindered the acceptance of this vaccine in many populations (154). The recombinant second-generation HBV vaccine was developed to address this concern and to help produce large quantity of vaccines. The recombinant HBV vaccine produced from genetically engineered yeast contains the hepatitis B surface antigen (HBsAg), but lacks the preS domain and glycosylation present in the natural viral particle (155). This vaccine demonstrated excellent immunogenicity in all age groups from neonates to adults with a seroprotection rates (SPR) of 85–100% observed approximately after 1-month of final dose (2-dose schedule for adults) (156). However, SPR was lower in older adults, elderly, smokers, obese individuals, and patients with impaired immune function such as those with malignancies or undergoing hemodialysis (156). A more effective mammalian cell-derived vaccine contains the glycosylated pre-S1 and pre-S2 proteins, in addition to the major HBsAg protein. Currently, new adjuvant formulations have been and are being developed to increase the immunogenicity as compared to the second-generation HBV vaccines.
A few clinical studies comparing effectiveness of different hepatitis B vaccines in adults have been highlighted below. A study comparing tri-antigen to single-antigen vaccine in adults aged 18–45 years revealed robust immune response in tri-antigen vaccine as compared to single-antigen vaccine which took prolonged time to achieve seroprotection (12). Available HBV vaccines show variable immune responses due to presence of different adjuvants. In a study among healthy adults of age 18–55 years, two doses of a vaccine using HBsAg adjuvanted with an immunostimulatory phosphorothioate oligodeoxyribonucleotide (HBV-ISS) compared to three doses of an alum-adjuvanted vaccine (HBV-Eng) demonstrated higher SPR for HBV-ISS (99). One of the clinical studies evaluated the comparable effectiveness of variable dose of different HBV vaccines in high risk population. This study revealed that two doses of HBsAg-1018, which binds to toll-like receptors has higher seroprotection than three doses of HBsAg-Eng in elderly and patients with diabetes, obese, or smokers. HBsAg-1018 was able to induce seroprotection earlier compared to HBsAg-Eng (157). Another study demonstrated that two doses of HBsAg-1018 induced a higher SPR (90.0%) in a month in patients with diabetes than three doses of HBsAg-Eng over 6 months (65.1%) (98). Even in patients with chronic kidney disease and hemodialysis, who have high risk of HBV, three doses of HBsAg-1018 induced significantly higher and more durable seroprotection than four double doses of HBsAg-Eng (158).
Additionally, specific HBV vaccines were also assessed in patients with chronic disease conditions, for example HB-AS02 (an adjuvanted HBV vaccine), was administered in patients with renal insufficiency and other immunocompromised patients with low response to conventional recombinant HBV vaccines (159). In dialysis patients, three doses of HB-AS02 induced rapid and effective protection than a four-dose course of HB-AS04, which is the first adjuvanted HBV vaccine licensed for patients with renal insufficiency, in Europe. Another vaccine formulation consisting of both HBsAg and hepatitis B core antigen (HBcAg) was compared with use of pegylated interferon in chronic hepatitis B and was safer, better tolerated, and showed lower progression to cirrhosis (102). One of the major co-infections observed with HBV is HIV (~30% of PLHIV in sub-Saharan Africa). A study evaluating the response of HBV vaccines in adults with HIV (age group 21–84 years), observed that 80% of patients showed VR and it did not co-relate with age, CD4+ cell count or viral (160). Given the high risk to HBV exposure, the hepatitis B vaccine is strongly advised in PLHIV. The availability and option of different HBV vaccines for healthy adults as well as high risk populations has enabled us to overcome HBV infection, provided necessary vaccination strategies are employed.
Human Papillomavirus (HPV) Vaccine
HPV can cause multiple epithelial lesions and cancers (161). It can clinically manifest as cutaneous and anogenital warts with a possibility to progress into cancer depending on the HPV subtype. Genital warts are most often (90% of cases) caused by infections due to HPV types 6 and 11 (162). Majority of cancers including cervical, anal, penile, oropharyngeal, vulvar, and vaginal cancers are caused by oncogenic HPV 16, 18, 45, and 31. HPV infections are mostly transmitted sexually (162). Currently, there are three vaccines available: bivalent (against HPV types 16 and 18), quadrivalent (against HPV types 6, 11, 16, and 18), and 9-valent (against HPV types 6, 11, 16, 18, 31, 33, 45, 52, and 58). The 9-valent HPV (9vHPV) has a broader coverage and provides immunity against most of the HPV infections. Currently, 9vHPV is recommended in both males and females in the age group of 9–25 years and adults 27–45 years only, if at risk (163).
A high prevalence of HPV has been reported among women in sub-Saharan Africa (164). Hence a clinical study to evaluate the efficacy and safety of bivalent HPV vaccines (2vHPV) was conducted on African girls and young women aged 10–25 years. The subjects were administrated three doses of bivalent, HPV-16/18 AS04-adjuvanted vaccine (AS04HPV-16/18) and all of them developed anti-HPV-16 and anti-HPV-18 antibodies and remained seropositive for 7 months (103). Several other studies have demonstrated high efficacy of 2vHPV against HPV 16/18-associated precancer and a long-term follow-up study (>10 years) have revealed persistence of antibodies ensuring that invasive cervical cancer is preventable with the use of recommended vaccinations (109, 114, 165, 166).
Several studies have showed efficacy of quadrivalent vaccine (4vHPV) in young adults. The HPV6/11/16/18 vaccine was 95–100% effective in reducing HPV16/18-related high-grade vaginal, cervical, and vulvar lesions, and 97% effective in reducing HPV6/11-related genital warts in women 15–26 years (110, 167). The efficacy and immunogenicity of 4vHPV was also seen in women aged 24–45 years (105). Long-term protection was confirmed, even a decade after administration (106, 168). Studies have confirmed that (2vHPV) is well-tolerated in pregnant women and presence of maternal antibodies in infants born to vaccinated women, for each of HPV types 6, 11, 16, and 18 (107, 117), confirming role of vaccination in decreasing prevalence in unvaccinated individuals also. Similar to the 2vHPV and 4vHPV, clinical studies have also confirmed efficacy of 9vHPV vaccines. Studies conducted on women aged 16–26 years have indicated that 9vHPV vaccine aided in preventing infection, cytological abnormalities, and high-grade lesions with sustained vaccine efficacy for 6 years (169, 170). Broader coverage provided by 9vHPV can dramatically decrease genital diseases and cervical cancer cases, worldwide (171, 172).
Women living with HIV are at greater risk of developing HPV infection and cervical cancer. Hence, a comparative study (2vHPV vs. 4vHPV vaccines) was performed on women living with HIV, aged 15–25 years. The outcomes of this study pointed out that though both vaccines were immunogenic, adjuvanted 2vHPV (AS04HPV-16/18) was immunologically superior to 4vHPV (HPV-6/11/16/18 vaccine) with higher HPV-16/18 antibody response and higher seropositivity rates after 24 months (111). Similar results were observed in healthy women of age 18–45 years with higher antibody responses with 2vHPV vs. 4vHPV even in the long-term (108).
The clinical studies in adults and elderly for the above mentioned VPDs have confirmed importance of including vaccination for adults in immunization schedules, with an aim to prevent VPDs and reduce mortality.
Guidelines and Recommendations on Adult Vaccination
The vaccination recommendations for adults are categorized as a) recommendations for the general population and b) recommendations for populations with specific risk factors. The risk factors include pre-existing diseases (e.g., chronic lung or heart disease, diabetes mellitus), immunocompromised populations, travelers, and populations exposed to diseases as occupational risk (5).
The WHO emphasizes on immunization for all age groups. In 2012, WHO made recommendations for annual influenza vaccination defining specific groups at risk of influenza disease. Risk groups include pregnant women, health workers, children aged 6–59 months, persons 65 years of age and older, and individuals with specific chronic medical conditions (173). Effective boosters are recommended throughout older ages, especially in individuals with chronic respiratory comorbidities, can be introduced to lower the disease burden and prevent transmission to more vulnerable populations. For pregnant women, immunization with tetanus toxoid containing vaccine is recommended along with seasonal influenza vaccine. For adolescents aged between 9 and 19 years, immunization with diphtheria booster, HPV, tetanus booster along with typhoid is recommended. For adults and adolescents (9–19 years) immunization against cholera, dengue, rabies, and seasonal influenza are recommended (174). In the United States, the ACIP has made recommendations which are approved by the CDC and Prevention (175) and has been presented in Table 3.
The CDC has also provided recommendations for various immunization practices based on medical conditions. All the vaccines mentioned in Table 3 are recommended for asplenia (complement deficiencies), end-stage renal disease or patients on hemodialysis, patients with heart or lung disease, alcoholic patients, patients with chronic liver disease, diabetic individuals, healthcare personnel, and men engaging in sex with men. Influenza live attenuated vaccine (LAIV4) is not recommended in pregnancy, immunocompromised patients (excluding HIV) and in PLHIV. Measles, mumps, rubella (MMR) and varicella (VAR) are not recommended in pregnant women and immunocompromised patients (excluding HIV), and for PLHIV with a CD4 count of <200 mm3, while HPV is contraindicated in pregnant women. Pregnant women are encouraged to get immunization with Influenza inactivated vaccine (IIV) or Influenza recombinant (RIV4), Tetanus, diphtheria, pertussis (Tdap) or Td, PPSV23, Hepatitis A, HBV, and MenACWY vaccines. Caution is to be exercised for vaccinating pregnant women with MenB. Immunocompromised patients who do not have HIV infection are advised to get vaccinated with IIV or RIV4, Tdap or Td, HPV, PCV13, PPSV23, HepA, HepB, MenACWY, MenB, and Hib. PLHIV can get immunized with IIV or RIV4, HPV, PCV13, PPSV23, HepA, HepB, MenACWy, MenB, and Hib. Additionally, PLHIV with CD4 count of ≥200 mm3 can get vaccinated by MMR and VAR (175).
There are substantial differences country-wise in terms of vaccination schedules for adult population, which depend on many factors such as economic policies and overall incidence of disease including prevalent serotypes in the region. As of 2019, 17 European countries have implemented obligatory vaccination policies for adults. In most of the countries, vaccination policies for influenza, tetanus, diphtheria, pneumococcal disease, hepatitis B, and pertussis are present. Several European countries recommend MenACWY and MenB meningococcal vaccination for adolescents, as MenB is most common cause of IMD in age group 15–25 years (176). The vaccination schedule for the European Union countries can be found at: https://vaccine-schedule.ecdc.europa.eu/. In some countries, such as Australia and New Zealand, meningococcal vaccination is recommended for adolescents and adults only in special conditions, such as residing in accommodations as hostels or in military or having certain medical conditions or traveling to endemic countries. Recommendations and guidelines are also revised every few years based on the clinical requirements and available evidence (119). For instance, recommendations by ACIP, 2014 mandate the use of PCV13 in series with PPSV23 for all adults aged ≥65 years (177). However, this recommendation was revised in 2019, due to the reduced disease burden. According to the updated recommendations, routine administration of PCV13 in adults >65 years are removed and may be administered based on mutual decision considering patient's risk for exposure and underlying medical conditions (178). Additionally, ACIP recommends PCV13 in series with PPSV23 for adults ≥19 years having immunocompromizing conditions, cerebrospinal fluid leak, or cochlear implant (≥1 year between pneumococcal vaccines) (178). Recently, the ACIP updated their recommendations for age-based and risk-based use of pneumococcal vaccines among adults (139) and voted for the following recommendations: (i) Adults aged ≥65 years who have not previously received a PCV or whose previous vaccination history is unknown should receive a PCV (either PCV20 or PCV15). If PCV15 is used, this should be followed by a dose of PPSV23, (ii) Adults aged 19–64 years with certain underlying medical conditions or other risk factors who have not previously received a PCV or whose previous vaccination history is unknown should receive a PCV (either PCV20 or PCV15). If PCV15 is used, this should be followed by a dose of PPSV23 (139).
Overview of Vaccination Practices in Tunisia and Morocco
There is a lack of adult vaccination practices in LMIC of Africa and Asia, but with the emerging trends in adult vaccination globally, there is a paradigm shift. Many countries are adopting vaccination practices from established high-income countries and adapting it based on the regional requirements. Global Alliance for Vaccines and Immunization (GAVI) is bringing together public and private sectors with the shared goal of saving lives and protecting people's health by increasing equitable and sustainable use of vaccines. GAVI supports low-income countries to help introduce vaccines into their NIP. In Tunisia and Morocco, the vaccination drive is usually conducted through the NIP. However, the NIPs are more focused on pediatric vaccination, while the adult vaccination programs are limited.
Meningococcal Vaccines
According to the WHO global summary on VPDs monitoring system in 2019, the Moroccan immunization program does not include meningococcal vaccines (179). However, response plan exists for vaccination in the event of a case of meningitidis or in the event of an epidemic. Also, systematic prevention by the anti-meningococcal vaccine is applied as part of the national control program to protect people living in institutions and pilgrims (180). For people living in institutions, high risk closed communities such as prisons, orphanages, charitable homes, boarding schools, and possibly other closed communities depending on the level of promiscuity, meningococcal vaccines are recommended for all people who have not been vaccinated or who have been vaccinated before 3 years without any age restriction (180). For pilgrims, MenACWY is highly recommended. Moroccan pilgrims are obliged to present a certificate of vaccination against meningitis with a quadrivalent ACW135Y vaccine, received at least 10 days before departure (181). Bivalent polysaccharide vaccine against serogroups A and C, conjugate vaccine against serogroup C, and tetravalent vaccine against serogroups A, C, W, and Y are available. However, no vaccine is available for serogroup B (180).
Similarly in Tunisia, meningococcal vaccines are not included in the NIP (179). Polysaccharide conjugate MenACWY vaccine is the only available meningococcal vaccine in Tunisia and is recommended for Hajj pilgrims and travelers to endemic areas and to children considered at high risk (17, 18).
Pneumococcal Vaccines
Morocco and Tunisia have introduced PCVs in their NIP (182, 183). In Morocco, the 13-valent PCV was introduced in October 2010 and replaced by the 10-valent PCV in July 2012 in the NIP (183). But the NIP does not include adult pneumococcal vaccination.
Hemophilus Influenza Vaccines
Hib vaccine has been introduced in Morocco through its routine immunization program in 2007 for children aged <5 years. Then, in 2012 pentavalent DTC-Hib-HB was introduced including diphtheria, tetanus, pertussis, Hib, and hepatitis B (184, 185).
In Tunisia, Hib vaccine was reintroduced in 2011 as Hib conjugate in combination with hepatitis B, diphtheria, poliomyelitis, and tetanus called the pentavalent vaccine, under the NIP (186).
HPV Vaccines
The HPV vaccine was licensed in Morocco in 2008, but it is not affordable by most and not yet included in NIP. Moreover, its awareness and acceptance among adolescents and adults is very low (22). The HPV vaccine is recommended for young girls aged 11–14 years, with a booster dose after 5 years and ideally completed before the start of sexual activity (187).
The HPV vaccine is not included in NIP of Tunisia in spite of high number of cases in adults (23).
Hepatitis Vaccines
Hepatitis B vaccine is included in Moroccan NIP for children. For adults, it is strongly recommended for healthcare workers, but significant number is still not vaccinated (188).
In Tunisia, as a part of the NIP, hepatitis B vaccination is provided for healthcare workers (189).
Tetanus, Diphtheria, Pertussis, and Poliomyelitis Vaccines
In Morocco, NIP includes national tetanus vaccination schedule for elderly women 15–45 years old. For adolescents and women of childbearing age who have already received a primary vaccination and boosters, according to the NIP (five doses), a 6th dose is recommended to ensure lifelong protection. For adolescents and women of childbearing age who have never been vaccinated or whose information is uncertain, they should be vaccinated according to the national schedule recommended for this group (five doses) (185).
Tunisian NIP covers diphtheria, tetanus, and oral polio vaccine at the age of 18 years and vaccination for rubella and tetanus for women of childbearing age (15–45 years) (190). The Global Pertussis Initiatives have recommended immunization against pertussis for all healthcare workers in African countries (70).
Influenza Vaccines
Morocco joined Partnership for Influenza Vaccine Introduction (PIVI) in 2014 and prioritized influenza vaccines for the elderly, healthcare professionals, healthcare students, and those with chronic illnesses such as diabetes in their 2014 vaccination campaign (191). The National Immunization Technical Advisory Group issued recommendation for the seasonal influenza vaccine and requested continuous burden data generation (21). Also, as overcrowding is considered as a factor favoring the transmission of respiratory pathologies, specifically flu, vaccination against this disease is strongly recommended for all pilgrims, especially the elderly and those with chronic illnesses (diabetics, respiratory failure, etc.) (181). Indeed, an annual dose of influenza vaccine is recommended for high-risk patients (21, 192). Still, it is estimated that only less than half of some risk groups may receive the influenza vaccine annually in Morocco, hence every prospect to promote the vaccination should be undertaken (193).
In Tunisia, influenza vaccine is recommended by Ministry of Health for elderly and healthcare workers, still its uptake is considerably low (192). Influenza vaccine is also recommended for pilgrims (192).
Other Vaccinations
In Morocco, combined measles and rubella vaccine (RR) was introduced in schools in 2003. National campaigns for RR were organized occasionally for children aged 9 months-15 years and girls aged 15–24 years. From 2015, the NIP covers measles and RR for children <5 years (at 9 and 18 months) and includes catch-up vaccination, when needed, for the family of premature children (194).
Pasteur Institute of Morocco manages vaccinations including rabies vaccine and vaccines for travelers, such as yellow fever vaccine. It also provides individual or mass vaccination services through vaccination campaigns (periodically against influenza and throughout the year against viral hepatitis B, tetanus and meningitis) (195).
The Pasteur Institute of Tunisia manages vaccinations for travelers to endemic areas and at high risk. It includes vaccination for meningitis, diphtheria, tetanus, rabies, measles, mumps, tuberculosis, typhoid, hepatitis A, hepatitis B, polio, flu, and Yellow fever (196). There are also special recommendations for vaccination for workers in Tunisia, which includes mandatory immunization for typhoid, hepatitis B, and tetanus.
Despite the aforementioned vaccination guidelines and recommendations, there are still clinical challenges and gaps in the implementation and monitoring of these processes.
Critical Clinical Gaps
Elderly population suffer from more serious consequences of VPDs, being highly sensitive to infections, making the treatment and vaccination programs clinically imperative. Further, antimicrobial resistance (AMR) which represents a global threat, is having increasing incidence. This can be attributed to inappropriate use of antimicrobials, lack of infection and disease control measures, poor access to medicines, vaccines and diagnostics, and lack of awareness (197). Vaccination is an important tool that will help reduce the spread of antimicrobial resistance (10).
The lack or insufficiency in clinical surveillance for adult's VPDs in African countries is another major unmet need. Around 75% of the reported meningococcal infections in the region lack confirmatory laboratory testing (198). To tackle these gaps, there is an immediate need for the development and reinforcement of surveillance systems to enable integration of public/private healthcare with respect to epidemiology, laboratory, and data management (15). This would support in developing enhanced vaccination strategies with improved outcomes.
There are also other factors greatly impacting the clinical applicability of vaccination in adults in the North African countries including gaps in recommendations from health professionals, poor information on the risks of the vaccine compared to the benefits of disease prevention, the gap in coordinated immunization programs for some VPDs in adults and missed opportunities during hospital visits, the absence of clinics or hospital departments for routine adult immunization and the weak basic training of physicians on adult immunization. Availability of certain vaccines at high cost or only in private sectors, also leads to low uptake.
Economic Impact of Vaccine Preventable Diseases
The economic burden of VPDs related to 10 recommended vaccines among the United States adults, aged ≥19 years, was reported to be ~$9 billion in 2015, and about $7.1 billion (80%) was accrued by unvaccinated individuals (199). Another study on the economic impact of VPDs such as influenza, pertussis, herpes zoster, and pneumococcal disease in populations aged >50 years has been projected to increase from ~$35 billion to $49 billion over the next three decades, in the United States (200). Costs associated with VPDs depend on various factors such as geographic region and may be categorized as direct, indirect, and societal. Estimated average costs per IMD case during outbreaks from 1990 to 2010 in high-income and low-income countries were $41,857 to $55,755 and $2,222, respectively. In sub-Saharan Africa, the average cost per household per IMD case was estimated at $90-$244 depending on significant sequelae costs (16). Most of the studies on the economic burden of VPDs and cost-effectiveness of vaccines for pneumococcal infection and influenza have been limited to high-income countries and have demonstrated cost saving and clinical superiority (201, 202). In one of the few studies estimating cost-effectiveness of PCV vaccination in Tunisia, both PCV10 and PCV13 are expected to generate $1.8 million and $2.24 million respectively, in direct medical costs savings, compared to no vaccination (183). Economic evidence supports cost-effectiveness of PCVs in LMIC, including Tunisia (183, 203, 204). Although, the cost-effectiveness of adult vaccination has been established globally, the major challenges are still the lack of awareness and the cost of the vaccines in LMIC. High cost of vaccines, such as Tdap and PCV13 in Tunisia and HPV in Morocco, makes implementation logistically difficult. A major challenge faced by countries not eligible for assistance from GAVI is providing all recommended vaccines for adults through NIP (15). There is an imperative need to support the development of national guidelines and implement effective vaccination policies in LMIC to overcome these challenges.
Additionally, the paucity of data related to health economic assessments of adult vaccination programs triggers the need to develop techniques to access direct and indirect costs of VPDs that significantly affect the adult population. The complex adult vaccination schedule along with limited vaccination infrastructure often act as a barrier. Another challenge was associated with the determination of vaccine coverage and screening coverage causing an economic burden (205).
Conclusion
Infectious diseases are still the leading cause of death in Africa and Asia. Lack of effective vaccination program among adults poses a huge challenge to control and prevent VPDs. Some of the major factors involved in low vaccination coverage among adults include high cost of vaccines, lack of awareness, fear of side effects, lack of management for developing new vaccines, handling, storage, and distribution of vaccines. In Morocco and Tunisia, lack of surveillance techniques, and screening facilities/laboratories are additional clinical gaps that needs to be addressed to boost vaccination. There is also a need of cost-effectiveness studies in Tunisia and Morocco to advocate adult immunization as these can be important analysis tools to understand clinical and economic impact of vaccination which will further emphasize the benefits of vaccination programs, strengthen reliability, and retain public trust.
Implementation of vaccination for adults and elderly would be highly beneficial as it helps decrease mortality, reduce infections, and prevent cancers such as cervical cancer or hepatocellular carcinoma. Initiatives on spreading awareness and clinical benefits of adult vaccination by both government and non-governmental agencies, may help improve adherence to scheduled and routine immunization programs. Overall, effective governance on local, national, and international levels, together with evidence-based development of policies may help to minimize the challenges of adult vaccination and improve coverage.
Author Contributions
JR and HZ contributed to medical writing. RA, MB, MC, and KM have substantially contributed to the development of the manuscript by providing critical insights. All authors helped with conceptualization, data curation, review, editing of the manuscript, and read and approved the final manuscript.
Funding
This work including article processing fee for the journal was sponsored by Pfizer Inc.
Conflict of Interest
JR was employed by Pfizer Inc., Casablanca, Morocco. HZ was employed by Pfizer Inc., Tunis, Tunisia.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to acknowledge Vaidehi Wadhwa (Medical Excellence, Pfizer Ltd.) for providing critical inputs and assisting in drafting the manuscript. The authors would also like to acknowledge IQVIA for their medical writing support.
References
1. Burke M, Rowe T. Vaccinations in older adults. Clin Geriatr Med. (2018) 34:131–43. doi: 10.1016/j.cger.2017.08.006
2. Lahariya C, Bhardwaj P. Adult vaccination in India: status and the way forward. Hum Vaccin Immunother. (2020) 16:1508–10. doi: 10.1080/21645515.2019.1692564
3. Tan L. Adult vaccination: now is the time to realize an unfulfilled potential. Hum Vaccin Immunother. (2015) 11:2158–66. doi: 10.4161/21645515.2014.982998
4. Doherty TM, Del Giudice G, Maggi S. Adult vaccination as part of a healthy lifestyle: moving from medical intervention to health promotion. Ann Med. (2019) 51:128–40. doi: 10.1080/07853890.2019.1588470
5. Swanson KA, Schmitt HJ, Jansen KU, Anderson AS. Adult vaccination. Hum Vaccin Immunother. (2015) 11:150–5. doi: 10.4161/hv.35858
6. United Nations,. World Population Prospects 2019: Highlights. (2019). Available online at: https://www.un.org/development/desa/publications/world-population-prospects-2019-highlights.html (accessed June 17, 2022).
7. Sauer M, Vasudevan P, Meghani A, Luthra K, Garcia C, Knoll MD, et al. Situational assessment of adult vaccine preventable disease and the potential for immunization advocacy and policy in low- and middle-income countries. Vaccine. (2021) 39:1556–64. doi: 10.1016/j.vaccine.2021.01.066
8. Allen JC, Toapanta FR, Chen W, Tennant SM. Understanding immunosenescence and its impact on vaccination of older adults. Vaccine. (2020) 38:8264–72. doi: 10.1016/j.vaccine.2020.11.002
9. Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. (2019) 16:25. doi: 10.1186/s12979-019-0164-9
10. Micoli F, Bagnoli F, Rappuoli R, Serruto D. The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol. (2021) 19:287–302. doi: 10.1038/s41579-020-00506-3
11. Isturiz R, Webber C. Prevention of adult pneumococcal pneumonia with the 13-valent pneumococcal conjugate vaccine: capita, the community-acquired pneumonia immunization trial in adults. Hum Vaccin Immunother. (2015) 11:1825–7. doi: 10.1080/21645515.2015.1043502
12. Vesikari T, Finn A, van Damme P, Leroux-Roels I, Leroux-Roels G, Segall N, et al. Immunogenicity and safety of a 3-antigen hepatitis B vaccine vs a single-antigen hepatitis B vaccine: a phase 3 randomized clinical trial. J Am Med Assoc Netw Open. (2021) 4:e2128652. doi: 10.1001/jamanetworkopen.2021.28652
13. Teresa Aguado M, Barratt J, Beard JR, Blomberg BB, Chen WH, Hickling J, et al. Report on who meeting on immunization in older adults: Geneva, Switzerland, 22-23 March 2017. Vaccine. (2018) 36:921–31. doi: 10.1016/j.vaccine.2017.12.029
14. Kristensen M, van Lier A, Eilers R, McDonald SA, Opstelten W, van der Maas N, et al. Burden of four vaccine preventable diseases in older adults. Vaccine. (2016) 34:942–9. doi: 10.1016/j.vaccine.2015.12.052
15. Bizri AR, Althaqafi A, Kaabi N, Obeidat N, Al Akoury N, Haridy H. The burden of invasive vaccine-preventable diseases in adults in the Middle East and North Africa (Mena) Region. Infect Dis Ther. (2021) 10:663–85. doi: 10.1007/s40121-021-00420-y
16. Martinón-Torres F. Deciphering the burden of meningococcal disease: conventional and under-recognized elements. J Adolesc Health. (2016) 59(2Suppl.):S12–20. doi: 10.1016/j.jadohealth.2016.03.041
17. Borrow R, Caugant DA, Ceyhan M, Christensen H, Dinleyici EC, Findlow J, et al. Meningococcal disease in the Middle East and Africa: findings and updates from the global meningococcal initiative. J Infect. (2017) 75:1–11. doi: 10.1016/j.jinf.2017.04.007
18. Taha M-K, Presa J, Serra L. A review of the epidemiology of invasive meningococcal disease and vaccination strategies in North Africa. Int J Infect Dis. (2021) 104:189–97. doi: 10.1016/j.ijid.2020.11.162
19. Dbaibo G, Tatochenko V, Wutzler P. Issues in pediatric vaccine-preventable diseases in low- to middle-income countries. Hum Vaccin Immunother. (2016) 12:2365–77. doi: 10.1080/21645515.2016.1181243
20. WHO. Global Health Observatory Data Repository Measles. (2021). Available online at: https://apps.who.int/gho/data/view.main.1520_62?lang=en (accessed March 22, 2022).
21. Mantel C, Chu SY, Hyde TB, Lambach P. Seasonal influenza vaccination in middle-income countries: assessment of immunization practices in Belarus, Morocco, and Thailand. Vaccine. (2020) 38:212–9. doi: 10.1016/j.vaccine.2019.10.028
22. Zouheir Y, Daouam S, Hamdi S, Alaoui A, Fechtali T. Knowledge of human papillomavirus and acceptability to vaccinate in adolescents and young adults of the Moroccan population. J Pediatr Adolesc Gynecol. (2016) 29:292–8. doi: 10.1016/j.jpag.2015.11.002
23. Ardhaoui M, Ennaifer E, Letaief H, Salsabil R, Lassili T, Chahed K, et al. Prevalence, genotype distribution and risk factors for cervical human papillomavirus infection in the Grand Tunis Region, Tunisia. PLoS ONE. (2016) 11:e0157432. doi: 10.1371/journal.pone.0157432
24. WHO. Global Health Observatory Data Repository Pertussis. (2021). Available online at: https://apps.who.int/gho/data/view.main.1520_43?lang=en (accessed March 3, 2022).
25. WHO. Vaccine-Preventable Diseases: Monitoring System. 2020 Global Summary. (2020). Available online at: https://apps.who.int/immunization_monitoring/globalsummary (accessed October 14, 2021).
26. Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L. Hepatitis A: epidemiology and prevention in developing countries. World J Hepatol. (2012) 4:68–73. doi: 10.4254/wjh.v4.i3.68
27. WHO. Hepatitis B. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed October 14, 2021).
28. Feindiri M, Kabbaj H, El Mzibri M, Belkadi B, Bouihat N, Filali-Maltouf A, et al. Prevalence of hepatitis B virus infection markers among patients of Ibn Sina University Hospital Center (Rabat, Morocco). Intervirology. (2021) 2021:518618. doi: 10.1159/000518618
29. Madihi S, Syed H, Lazar F, Zyad A, Benani A. A Systematic review of the current hepatitis B viral infection and hepatocellular carcinoma situation in mediterranean countries. Biomed Res Int. (2020) 2020:7027169. doi: 10.1155/2020/7027169
30. Lahchaichi A, Hadj MB, Bouguerra H, Talmoudi K, Bahrini A, Bahri O, et al. Prevalence and risk factors of hepatitis B in Tunisia. Eur J Public Health. (2019) 29(Suppl.4):76. doi: 10.1093/eurpub/ckz187.076
31. Dhouib W, Kacem M, Bennasrallah C, Ben Fredj M, Abroug H, Zemni I, et al. Hepatitis B birth vaccination, cohort study, Tunisia 2000–2017. Libyan J Med. (2020) 15:1809223. doi: 10.1080/19932820.2020.1809223
32. WHO. World Expert Consultation on Rabies. (2018). Available online at: https://apps.who.int/iris/bitstream/handle/10665/272364/9789241210218-eng.pdf (accessed October 14, 2021).
33. Bouaddi K, Bitar A, Bouslikhane M, Ferssiwi A, Fitani A, Mshelbwala PP. Knowledge, attitudes, and practices regarding rabies in El Jadida Region, Morocco. Vet Sci. (2020) 7:10029. doi: 10.3390/vetsci7010029
34. Hassine TB, Ali MB, Ghodhbane I, Said ZB, Hammami S. Rabies in Tunisia: a spatio-temporal analysis in the region of Capbon-Nabeul. Acta Trop. (2021) 216:105822. doi: 10.1016/j.actatropica.2021.105822
35. WHO. Global Health Observatory Data Repository Diphtheria. (2021). Available online at: https://apps.who.int/gho/data/view.main.1520_41?lang=en (accessed March 3, 2022).
36. WHO. Global Health Observatory Data Repository, Tetanus. (2021). Available online at: https://apps.who.int/gho/data/view.main.1520_46 (accessed October 14, 2021).
37. WHO. Global Tuberculosis Report 2021. (2021). Available online at: https://www.who.int/publications/i/item/9789240037021 (accessed March 22, 2022).
38. The World Bank. Incidence of Tuberculosis. (2020). Available online at: https://data.worldbank.org/indicator/SH.TBS.INCD (accessed March 22, 2022).
39. Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunolgy. (2011) 11:865–72. doi: 10.1038/nri3085
40. Purmohamad A, Abasi E, Azimi T, Hosseini S, Safari H, Nasiri MJ, et al. Global estimate of neisseria meningitidis serogroups proportion in invasive meningococcal disease: a systematic review and meta-analysis. Microb Pathog. (2019) 134:103571. doi: 10.1016/j.micpath.2019.103571
41. Pelton SI. The global evolution of meningococcal epidemiology following the introduction of meningococcal vaccines. J Adolesc Health. (2016) 59(2Suppl.):S3–11. doi: 10.1016/j.jadohealth.2016.04.012
42. Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr. (2013) 11:17. doi: 10.1186/1478-7954-11-17
43. Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, et al. The global meningococcal initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines. (2019) 18:15–30. doi: 10.1080/14760584.2019.1557520
44. Oviedo-Orta E, Ahmed S, Rappuoli R, Black S. Prevention and control of meningococcal outbreaks: the emerging role of serogroup B meningococcal vaccines. Vaccine. (2015) 33:3628–35. doi: 10.1016/j.vaccine.2015.06.046
45. Razki A, Hong E, Zerouali K, Belabbes H, Aitmouss K, Terrade A, et al. Molecular characterization of invasive isolates of neisseria meningitidis in Casablanca, Morocco. J Clin Microbiol. (2018) 56:18. doi: 10.1128/JCM.00445-18
46. Smaoui H, Tali-Maamar H, Zouhair S, Bouheraoua S, Mefteh K, Bouskraoui M, et al. Implementation of a prospective study for enhancing surveillance of invasive bacterial infections in North Africa. Int J Infect Dis. (2022) 115:101–5. doi: 10.1016/j.ijid.2021.11.036
47. Zerouali K, Elmdaghri N, Boudouma M, Benbachir M. Serogroups, serotypes, serosubtypes and antimicrobial susceptibility of neisseria meningitidis isolates in Casablanca, Morocco. Eur J Clin Microbiol Infect Dis. (2002) 21:483–5. doi: 10.1007/s10096-002-0736-y
48. Smaoui H, Saguer A, Bouziri A, Fourati S, Chahed M, Ben N, et al. Les Infections Invasives À Neisseria Meningitidis Chez L'enfant À Tunis: À Propos De 79 Cas. J Jebali, Ch Jeanneau, A Bazaa, S Mathieu, M El Ayeb. (2011) p. 35.
49. Saguer A, Smaoui H, Taha MK, Kechrid A. Characterization of invasive neisseria meningitidis strains isolated at the children's hospital of Tunis, Tunisia. Eastern Mediterranean Health J. (2016) 22:343–9. doi: 10.26719/2016.22.5.343
50. Pelton SI, Bornheimer R, Doroff R, Shea KM, Sato R, Weycker D. Decline in pneumococcal disease attenuated in older adults and those with comorbidities following universal childhood Pcv13 immunization. Clin Infect Dis. (2018) 68:1831–8. doi: 10.1093/cid/ciy800
51. Van Aalst M, Lötsch F, Spijker R, van der Meer JTM, Langendam MW, Goorhuis A, et al. Incidence of invasive pneumococcal disease in immunocompromised patients: a systematic review and meta-analysis. Travel Med Infect Dis. (2018) 24:89–100. doi: 10.1016/j.tmaid.2018.05.016
52. Zhang D, Petigara T, Yang X. Clinical and economic burden of pneumococcal disease in us adults aged 19-64 years with chronic or immunocompromising diseases: an observational database study. BMC Infect Dis. (2018) 18:436. doi: 10.1186/s12879-018-3326-z
53. European Centre for Disease Prevention Control. Factsheet About Pneumococcal Disease. (2020). Available online at: https://www.ecdc.europa.eu/en/pneumococcal-disease/facts (accessed October 1, 2021).
54. AlBarrak A, Alotaibi B, Yassin Y, Mushi A, Maashi F, Seedahmed Y, et al. Proportion of adult community-acquired pneumonia cases attributable to streptococcus pneumoniae among Hajj Pilgrims in 2016. Int J Infect Dis. (2018) 69:68–74. doi: 10.1016/j.ijid.2018.02.008
55. Paget J, Spreeuwenberg P, Charu V, Taylor RJ, Iuliano AD, Bresee J, et al. Global mortality associated with seasonal influenza epidemics: new burden estimates and predictors from the glamor project. J Glob Health. (2019) 9:020421. doi: 10.7189/jogh.09.020421
56. Centers for Disease Control Prevention. Vaccine-Preventable Adult Diseases. (2021). Available online at: https://www.cdc.gov/vaccines/adults/vpd.html (accessed July 9, 2021).
57. Iuliano AD, Roguski KM, Chang HH, Muscatello DJ, Palekar R, Tempia S, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. (2018) 391:1285–300. doi: 10.1016/S0140-6736(17)33293-2
58. GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the global burden of disease study 2017. Lancet Respir Med. (2019) 7:69–89. doi: 10.1016/S2213-2600(18)30496-X
59. Kombe Kombe AJ, Li B, Zahid A, Mengist HM, Bounda G-A, Zhou Y, et al. Epidemiology and burden of human papillomavirus and related diseases, molecular pathogenesis, and vaccine evaluation. Front Public Health. (2021) 8:28. doi: 10.3389/fpubh.2020.552028
60. Vinodhini K, Shanmughapriya S, Das BC, Natarajaseenivasan K. Prevalence and risk factors of Hpv infection among women from various provinces of the world. Arch Gynecol Obstet. (2012) 285:771–7. doi: 10.1007/s00404-011-2155-8
61. Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. (2010) 202:1789–99. doi: 10.1086/657321
62. Moreira ED Jr, Giuliano AR, Palefsky J, Flores CA, Goldstone S, Ferris D, et al. Incidence, clearance, and disease progression of genital human papillomavirus infection in heterosexual. Men J Infect Dis. (2014) 210:192–9. doi: 10.1093/infdis/jiu077
63. Benhafid M, Rguig A, Trivedi T, Elqazoui M, Teleb N, Mouane N, et al. Monitoring of rotavirus vaccination in Morocco: establishing the baseline burden of rotavirus disease. Vaccine. (2012) 30:6515–20. doi: 10.1016/j.vaccine.2012.08.058
64. Ayouni K, Naffeti B, Ben Aribi W, Bettaieb J, Hammami W, Ben Salah A, et al. Hepatitis a virus infection in Central-West Tunisia: an age structured model of transmission and vaccination impact. BMC Infect Dis. (2020) 20:627. doi: 10.1186/s12879-020-05318-7
65. Essayagh T, Essayagh M, El Rhaffouli A, Essayagh S. Epidemiologic profile of hepatitis a in Meknès, Morocco, 2013-2016. Med Sante Trop. (2019) 29:92–6.
66. WHO. Global Hepatitis Report, 2017. (2017). Available online at: https://apps.who.int/iris/bitstream/handle/10665/255016/9789241565455-eng.pdf (accessed October 1, 2021).
67. Ben Ayed H, Koubaa M, Gargouri L, Ben Jemaa M, Trigui M, Hammemi F, et al. Epidemiology and disease burden of tuberculosis in South of Tunisia over a 22-year period: current trends and future projections. PLoS ONE. (2019) 14:e0212853. doi: 10.1371/journal.pone.0212853
68. Bouaddi O, Hasan MM, Sahito AM, Shah PA, Mohammed AZA, Essar MY. Tuberculosis in the middle of covid-19 in Morocco: efforts, challenges and recommendations. Trop Med Health. (2021) 49:98. doi: 10.1186/s41182-021-00388-y
69. Ben Fraj I, Smaoui H, Zghal M, Sassi O, Guiso N, Kechrid A. Seroprevalence of pertussis among healthcare workers: a cross-sectional study from Tunisia. Vaccine. (2019) 37:109–12. doi: 10.1016/j.vaccine.2018.11.023
70. Macina D, Evans KE. Bordetella pertussis in school-age children, adolescents, and adults: a systematic review of epidemiology, burden, and mortality in Africa. Infect Dis Ther. (2021) 10:1097–113. doi: 10.1007/s40121-021-00442-6
71. Centers for Disease Control Prevention. Complications of Measles. (2022). Available online at: https://www.cdc.gov/measles/symptoms/complications.html (accessed March 3, 2022).
72. WHO. Measles- Tunisia. (2019). Available online at: https://www.who.int/emergencies/disease-outbreak-news/item/2019-DON150 (accessed March 3, 2022).
73. Dbaibo G, El-Ayoubi N, Ghanem S, Hajar F, Bianco V, Miller JM, et al. Immunogenicity and safety of a quadrivalent meningococcal serogroups a, C, W-135 and Y tetanus toxoid conjugate vaccine (Menacwy-Tt) administered to adults aged 56 years and older: results of an open-label, randomized, controlled trial. Drugs Aging. (2013) 30:309–19. doi: 10.1007/s40266-013-0065-0
74. Borja-Tabora CF, Montalban C, Memish ZA, Boutriau D, Kolhe D, Miller JM, et al. Long-term immunogenicity and safety after a single dose of the quadrivalent meningococcal serogroups a, C, W, and Y tetanus toxoid conjugate vaccine in adolescents and adults: 5-year follow-up of an open, randomized trial. BMC Infect Dis. (2015) 15:409. doi: 10.1186/s12879-015-1138-y
75. Aplasca-De Los Reyes MR, Dimaano E, Macalalad N, Dbaibo G, Bianco V, Baine Y, et al. The investigational meningococcal serogroups a, C, W-135, Y tetanus toxoid conjugate vaccine (Acwy-Tt) and the seasonal influenza virus vaccine are immunogenic and well-tolerated when co-administered in adults. Hum Vaccin Immunother. (2012) 8:881–7. doi: 10.4161/hv.20212
76. Esteves-Jaramillo A, Koehler T, Jeanfreau R, Neveu D, Jordanov E, Singh Dhingra M. Immunogenicity and safety of a quadrivalent meningococcal tetanus toxoid-conjugate vaccine (Menacyw-Tt) in ≥56-year-olds: a phase Iii randomized study. Vaccine. (2020) 38:4405–11. doi: 10.1016/j.vaccine.2020.04.067
77. Dhingra MS, Peterson J, Hedrick J, Pan J, Neveu D, Jordanov E. Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (Menacyw-Tt) in adolescents and adults: a phase Iii randomized study. Vaccine. (2020) 38:5194–201. doi: 10.1016/j.vaccine.2020.06.013
78. Ostergaard L, Lucksinger GH, Absalon J, Beeslaar J, Eiden J, Jansen KU, et al. A phase 3, randomized, active-controlled study to assess the safety and tolerability of meningococcal serogroup B vaccine bivalent Rlp2086 in healthy adolescents and young adults. Vaccine. (2016) 34:1465–71. doi: 10.1016/j.vaccine.2016.01.044
79. Fukushima S, Kikuchi H, Miyazu M, Hamada A, Ouchi K, Takagi H, et al. A safety and immunogenicity study of a single dose of a meningococcal (groups a, C, W, and Y) polysaccharide diphtheria toxoid conjugate vaccine (Men-Acwy-D) in Healthy Japanese Participants. Jpn J Infect Dis. (2018) 71:402–7. doi: 10.7883/yoken.JJID.2017.277
80. Kim HS, Engel S, Neveu D, Thollot Y, Oster P, Yang K. Post-marketing surveillance observational study of quadrivalent meningococcal diphtheria toxoid conjugate vaccine (Menacwy-Dt, Mcv4/Menactra(®)) in the Republic of Korea, 2014-2019. Infect Dis Ther. (2021) 10:399–409. doi: 10.1007/s40121-020-00393-4
81. Alberer M, Burchard G, Jelinek T, Reisinger EC, Meyer S, Forleo-Neto E, et al. Immunogenicity and safety of concomitant administration of a combined hepatitis a/B vaccine and a quadrivalent meningococcal conjugate vaccine in healthy adults. J Travel Med. (2015) 22:105–14. doi: 10.1111/jtm.12180
82. Lee HJ, Chung M-H, Kim WJ, Hong YJ, Choi KM, Lee J, et al. Immunogenicity and safety of a novel quadrivalent meningococcal conjugate vaccine (Menacwy-Crm) in healthy Korean adolescents and adults. Int J Infect Dis. (2014) 28:204–10. doi: 10.1016/j.ijid.2014.06.008
83. Lalwani S, Agarkhedkar S, Gogtay N, Palkar S, Agarkhedkar S, Thatte U, et al. Safety and immunogenicity of an investigational meningococcal Acwy Conjugate Vaccine (Menacwy-Crm) in healthy indian subjects aged 2 to 75 years. Int J Infect Dis. (2015) 38:36–42. doi: 10.1016/j.ijid.2015.07.003
84. Hurley D, Griffin C, Young M, Scott DA, Pride MW, Scully IL, et al. Safety, tolerability, and immunogenicity of a 20-valent pneumococcal conjugate vaccine (Pcv20) in adults 60 to 64 years of age. Clin Infect Dis. (2021) 73:e1489–e97. doi: 10.1093/cid/ciaa1045
85. Thompson AR, Klein NP, Downey HJ, Patterson S, Sundaraiyer V, Watson W, et al. Coadministration of 13-valent pneumococcal conjugate and quadrivalent inactivated influenza vaccines in adults previously immunized with polysaccharide pneumococcal vaccine 23: a randomized clinical trial. Hum Vaccin Immunother. (2019) 15:444–51. doi: 10.1080/21645515.2018.1533777
86. Nakashima K, Aoshima M, Ohfuji S, Yamawaki S, Nemoto M, Hasegawa S, et al. Immunogenicity of simultaneous versus sequential administration of a 23-valent pneumococcal polysaccharide vaccine and a quadrivalent influenza vaccine in older individuals: a randomized, open-label, non-inferiority trial. Hum Vaccin Immunother. (2018) 14:1923–30. doi: 10.1080/21645515.2018.1455476
87. Song J-Y, Chang C-J, Andrews C, Diez-Domingo J, Oh M-d, Dagan R, et al. Safety, tolerability, and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, followed by sequential Ppsv23 vaccination in healthy adults aged ≥50 years: a randomized phase Iii trial (Pneu-Path). Vaccine. (2021) 39:6422–36. doi: 10.1016/j.vaccine.2021.08.038
88. Buchwald UK, Andrews CP, Ervin J, Peterson JT, Tamms GM, Krupa D, et al. Sequential administration of Prevnar 13™ and Pneumovax™ 23 in healthy participants 50 years of age and older. Hum Vaccin Immunother. (2021) 17:2678–90. doi: 10.1080/21645515.2021.1888621
89. Severance R, Schwartz H, Dagan R, Connor L, Li J, Pedley A, et al. Safety, tolerability, and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, administered concomitantly with influenza vaccine in healthy adults aged ≥50 years: a randomized phase 3 trial (Pneu-Flu). Hum Vac Immunotherapeut. (2021) 202:1–14. doi: 10.1080/21645515.2021.1976581
90. Tinoco JC, Juergens C, Ruiz Palacios GM, Vazquez-Narvaez J, Enkerlin-Pauwells HL, Sundaraiyer V, et al. Open-label trial of immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults ≥ 50 years of age in Mexico. Clin Vaccine Immunol. (2015) 22:185–92. doi: 10.1128/CVI.00711-14
91. Huang L, Wang L, Li H, Hu Y, Ru W, Han W, et al. A Phase Iii clinical trial to evaluate the safety and immunogenicity of 23-valent pneumococcal polysaccharide vaccine (Ppv23) in healthy children, adults, and elderly. Hum Vaccin Immunother. (2019) 15:249–55. doi: 10.1080/21645515.2018.1509648
92. Ciprero K, Zykov KA, Briko NI, Shekar T, Sterling TM, Bitieva E, et al. Safety and immunogenicity of a single dose 23-valent pneumococcal polysaccharide vaccine in Russian subjects. Hum Vaccin Immunother. (2016) 12:2142–7. doi: 10.1080/21645515.2016.1165373
93. Solanki BB, Juergens C, Chopada MB, Supe P, Sundaraiyer V, Le Dren-Narayanin N, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine in adults 50 to 65 years of age in India: an open-label trial. Hum Vaccin Immunother. (2017) 13:2065–71. doi: 10.1080/21645515.2017.1331796
94. Shiramoto M, Irie S, Juergens C, Yamaji M, Tamai S, Aizawa M, et al. Immunogenicity and safety of 13-valent pneumococcal conjugate vaccine when administered to healthy japanese adults aged ≥50 years. Hum Vaccin Immunother. (2014) 10:1850–8. doi: 10.4161/hv.28633
95. Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. (2013) 31:3577–84. doi: 10.1016/j.vaccine.2013.04.085
96. Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. (2015) 372:1114–25. doi: 10.1056/NEJMoa1408544
97. Song JY, Cheong HJ, Noh JY, Choi MJ, Yoon JG, Lee SN, et al. Immunogenicity and safety of a tetanus-diphtheria vaccine and a 13-valent pneumococcal conjugate vaccine after concomitant vaccination in ≥ 50-year-old adults. BMC Infect Dis. (2018) 18:628. doi: 10.1186/s12879-018-3479-9
98. Jackson S, Lentino J, Kopp J, Murray L, Ellison W, Rhee M, et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, Hbsag-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults. Vaccine. (2018) 36:668–74. doi: 10.1016/j.vaccine.2017.12.038
99. Halperin SA, Ward B, Cooper C, Predy G, Diaz-Mitoma F, Dionne M, et al. Comparison of safety and immunogenicity of two doses of investigational hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligodeoxyribonucleotide and three doses of a licensed hepatitis B vaccine in healthy adults 18–55 years of age. Vaccine. (2012) 30:2556–63. doi: 10.1016/j.vaccine.2012.01.087
100. Gilbert CL, Klopfer SO, Martin JC, Schödel FP, Bhuyan PK. Safety and immunogenicity of a modified process hepatitis B vaccine in healthy adults ≥50 years. Hum Vaccin. (2011) 7:1336–42. doi: 10.4161/hv.7.12.18333
101. Kishino H, Takahashi K, Sawata M, Tanaka Y. Immunogenicity, safety, and tolerability of a recombinant hepatitis B vaccine manufactured by a modified process in Healthy Young Japanese adults. Hum Vaccin Immunother. (2018) 14:1773–8. doi: 10.1080/21645515.2018.1452578
102. Al Mahtab M, Akbar SMF, Aguilar JC, Guillen G, Penton E, Tuero A, et al. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing Hbs and Hbc antigens (a randomized, open and treatment controlled phase Iii clinical trial). PLoS ONE. (2018) 13:e0201236. doi: 10.1371/journal.pone.0201236
103. Sow PS, Watson-Jones D, Kiviat N, Changalucha J, Mbaye KD, Brown J, et al. Safety and immunogenicity of human papillomavirus-16/18 As04-adjuvanted vaccine: a randomized trial in 10-25-year-old Hiv-seronegative African girls and young women. J Infect Dis. (2013) 207:1753–63. doi: 10.1093/infdis/jis619
104. Muñoz N, Manalastas R Jr, Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet. (2009) 373:1949–57. doi: 10.1016/S0140-6736(09)60691-7
105. Castellsagué X, Muñoz N, Pitisuttithum P, Ferris D, Monsonego J, Ault K, et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent Hpv (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br J Cancer. (2011) 105:28–37. doi: 10.1038/bjc.2011.185
106. Luna J, Plata M, Gonzalez M, Correa A, Maldonado I, Nossa C, et al. Long-term follow-up observation of the safety, immunogenicity, and effectiveness of Gardasil™ in adult women. PLoS ONE. (2013) 8:e83431. doi: 10.1371/journal.pone.0083431
107. Matys K, Mallary S, Bautista O, Vuocolo S, Manalastas R, Pitisuttithum P, et al. Mother-infant transfer of anti-human papillomavirus (Hpv) antibodies following vaccination with the quadrivalent Hpv (type 6/11/16/18) virus-like particle vaccine. Clin Vaccine Immunol. (2012) 19:881–5. doi: 10.1128/CVI.00002-12
108. Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, et al. Comparison of long-term immunogenicity and safety of human papillomavirus (Hpv)-16/18 As04-adjuvanted vaccine and Hpv-6/11/16/18 vaccine in healthy women aged 18-45 years: end-of-study analysis of a phase Iii randomized trial. Hum Vaccin Immunother. (2014) 10:3435–45. doi: 10.4161/hv.36121
109. Apter D, Wheeler CM, Paavonen J, Castellsagué X, Garland SM, Skinner SR, et al. Efficacy of human papillomavirus 16 and 18 (Hpv-16/18) As04-adjuvanted vaccine against cervical infection and precancer in young women: final event-driven analysis of the randomized, double-blind patricia trial. Clin Vaccine Immunol. (2015) 22:361–73. doi: 10.1128/CVI.00591-14
110. Muñoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (Hpv)-6/11/16/18 vaccine on all Hpv-associated genital diseases in young women. J Natl Cancer Inst. (2010) 102:325–39. doi: 10.1093/jnci/djp534
111. Folschweiller N, Teixeira J, Joshi S, Goldani LZ, Supparatpinyo K, Basu P, et al. Immunogenicity and safety of the As04-Hpv-16/18 and Hpv-6/11/16/18 human papillomavirus vaccines in asymptomatic young women living with Hiv aged 15-25 years: a phase Iv randomized comparative study. EClinicalMedicine. (2020) 23:100353. doi: 10.1016/j.eclinm.2020.100353
112. Zhu FC, Hu SY, Hong Y, Hu YM, Zhang X, Zhang YJ, et al. Efficacy, immunogenicity and safety of the As04-Hpv-16/18 vaccine in Chinese women aged 18-25 years: end-of-study results from a phase Ii/Iii, randomised, controlled trial. Cancer Med. (2019) 8:6195–211. doi: 10.1002/cam4.2399
113. Wheeler CM, Skinner SR, Del Rosario-Raymundo MR, Garland SM, Chatterjee A, Lazcano-Ponce E, et al. Efficacy, safety, and immunogenicity of the human papillomavirus 16/18 As04-adjuvanted vaccine in women older than 25 years: 7-year follow-up of the phase 3, double-blind, randomised controlled viviane study. Lancet Infect Dis. (2016) 16:1154–68. doi: 10.1016/S1473-3099(16)30120-7
114. Schwarz T, Spaczynski M, Kaufmann A, Wysocki J, Gałaj A, Schulze K, et al. Persistence of immune responses to the Hpv-16/18 As04-adjuvanted vaccine in women aged 15-55 years and first-time modelling of antibody responses in mature women: results from an open-label 6-year follow-up study. Bjog. (2015) 122:107–18. doi: 10.1111/1471-0528.13070
115. Chen W, Zhao Y, Xie X, Liu J, Li J, Zhao C, et al. Safety of a quadrivalent human papillomavirus vaccine in a phase 3, randomized, double-blind, placebo-controlled clinical trial among Chinese women during 90 months of follow-up. Vaccine. (2019) 37:889–97. doi: 10.1016/j.vaccine.2018.12.030
116. Castellsagué X, Giuliano AR, Goldstone S, Guevara A, Mogensen O, Palefsky JM, et al. Immunogenicity and safety of the 9-valent Hpv vaccine in men. Vaccine. (2015) 33:6892–901. doi: 10.1016/j.vaccine.2015.06.088
117. Garland SM, Ault KA, Gall SA, Paavonen J, Sings HL, Ciprero KL, et al. Pregnancy and infant outcomes in the clinical trials of a human papillomavirus type 6/11/16/18 vaccine: a combined analysis of five randomized controlled trials. Obstet Gynecol. (2009) 114:1179–88. doi: 10.1097/AOG.0b013e3181c2ca21
118. Mikamo H, Yamagishi Y, Murata S, Yokokawa R, Han SR, Wakana A, et al. Efficacy, safety, and immunogenicity of a quadrivalent Hpv vaccine in japanese men: a randomized, phase 3, placebo-controlled study. Vaccine. (2019) 37:1651–8. doi: 10.1016/j.vaccine.2019.01.069
119. Burman C, Serra L, Nuttens C, Presa J, Balmer P, York L. Meningococcal disease in adolescents and young adults: a review of the rationale for prevention through vaccination. Hum Vaccin Immunother. (2019) 15:459–69. doi: 10.1080/21645515.2018.1528831
120. Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis. (2010) 10:853–61. doi: 10.1016/S1473-3099(10)70251-6
121. Read RC. Neisseria meningitidis; clones, carriage, and disease. Clin Microbiol Infect. (2014) 20:391–5. doi: 10.1111/1469-0691.12647
122. Ali A, Jafri RZ, Messonnier N, Tevi-Benissan C, Durrheim D, Eskola J, et al. Global practices of meningococcal vaccine use and impact on invasive disease. Pathog Glob Health. (2014) 108:11–20. doi: 10.1179/2047773214Y.0000000126
123. MacNeil JR, Blain AE, Wang X, Cohn AC. Current epidemiology and trends in meningococcal disease—United States, 1996–2015. Clin Infect Dis. (2017) 66:1276–81. doi: 10.1093/cid/cix993
124. Sridhar S, Greenwood B, Head C, Plotkin SA, Sáfadi MA, Saha S, et al. Global incidence of serogroup B invasive meningococcal disease: a systematic review. Lancet Infect Dis. (2015) 15:1334–46. doi: 10.1016/S1473-3099(15)00217-0
125. Trotter CL, Chandra M, Cano R, Larrauri A, Ramsay ME, Brehony C, et al. A surveillance network for meningococcal disease in Europe. FEMS Microbiol Rev. (2007) 31:27–36. doi: 10.1111/j.1574-6976.2006.00060.x
126. Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. (2010) 17:840–7. doi: 10.1128/CVI.00529-09
127. Memish Z, Al Hakeem R, Al Neel O, Danis K, Jasir A, Eibach D. Laboratory-confirmed invasive meningococcal disease: effect of the Hajj Vaccination Policy, Saudi Arabia, 1995 to 2011. Euro Surveill. (2013) 18:20581. doi: 10.2807/1560-7917.ES2013.18.37.20581
128. Public Health England. Invasive Meningococcal Disease in England: Annual Report for 2018 to 2019 Supplementary Data Tables. (2019). Available online at: https://www.gov.uk/government/publications/meningococcal-disease-laboratory-confirmed-cases-in-england-in-2018-to-2019 (accessed June 17, 2022).
129. National Center for Immunisation Research Surveillance. Significant Events in Meningococcal Vaccination Practice in Australia. (2020). Available online at: https://ncirs.org.au/sites/default/files/2020-03/Meningococcal-history-Mar%202020.pdf (accessed February 14, 2022).
130. Stamboulian D, Lopardo G, Lopez P, Cortes-Barbosa C, Valencia A, Bedell L, et al. Safety and immunogenicity of an investigational quadrivalent meningococcal Crm(197) conjugate vaccine, Menacwy-Crm, compared with licensed vaccines in adults in Latin America. Int J Infect Dis. (2010) 14:e868–75. doi: 10.1016/j.ijid.2010.03.017
131. Gasparini R, Conversano M, Bona G, Gabutti G, Anemona A, Dull PM, et al. Randomized trial on the safety, tolerability, and immunogenicity of Menacwy-Crm, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults. Clin Vaccine Immunol. (2010) 17:537–44. doi: 10.1128/CVI.00436-09
132. Richmond PC, Marshall HS, Nissen MD, Jiang Q, Jansen KU, Garcés-Sánchez M, et al. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. (2012) 12:597–607. doi: 10.1016/S1473-3099(12)70087-7
133. Marshall HS, Richmond PC, Nissen MD, Wouters A, Baber J, Jiang Q, et al. A Phase 2 open-label safety and immunogenicity study of a meningococcal B bivalent Rlp2086 vaccine in healthy adults. Vaccine. (2013) 31:1569–75. doi: 10.1016/j.vaccine.2013.01.021
134. Vesikari T, Wysocki J, Beeslaar J, Eiden J, Jiang Q, Jansen KU, et al. Immunogenicity, safety, and tolerability of bivalent Rlp2086 meningococcal group B vaccine administered concomitantly with diphtheria, tetanus, and acellular pertussis and inactivated poliomyelitis vaccines to healthy adolescents. J Pediatric Infect Dis Soc. (2016) 5:180–7. doi: 10.1093/jpids/piv064
135. Stacey HL, Rosen J, Peterson JT, Williams-Diaz A, Gakhar V, Sterling TM, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (Pcv-15) compared to Pcv-13 in healthy older adults. Hum Vaccin Immunother. (2019) 15:530–9. doi: 10.1080/21645515.2018.1532249
136. Ermlich SJ, Andrews CP, Folkerth S, Rupp R, Greenberg D, McFetridge RD, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naïve adults ≥50 years of age. Vaccine. (2018) 36:6875–82. doi: 10.1016/j.vaccine.2018.03.012
137. Klein NP, Peyrani P, Yacisin K, Caldwell N, Xu X, Scully IL, et al. A phase 3, randomized, double-blind study to evaluate the immunogenicity and safety of 3 lots of 20-valent pneumococcal conjugate vaccine in pneumococcal vaccine-naive adults 18 through 49 years of age. Vaccine. (2021) 39:5428–35. doi: 10.1016/j.vaccine.2021.07.004
138. Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. (2014) 32:2364–74. doi: 10.1016/j.vaccine.2014.02.002
139. Kobayashi M, Farrar JL, Gierke R, Britton A, Childs L, Leidner AJ, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: updated recommendations of the advisory committee on immunization practices - United States, 2022. Morb Mortal Wkly Rep. (2022) 71:109–17. doi: 10.15585/mmwr.mm7104a1
140. Garrido HMG, Schnyder JL, Tanck MWT, Vollaard A, Spijker R, Grobusch MP, et al. Immunogenicity of pneumococcal vaccination in Hiv infected individuals: a systematic review and meta-analysis. EClinicalMedicine. (2020) 29–30:100576. doi: 10.1016/j.eclinm.2020.100576
141. Lee K-Y, Tsai M-S, Kuo K-C, Tsai J-C, Sun H-Y, Cheng AC, et al. Pneumococcal vaccination among Hiv-infected adult patients in the era of combination antiretroviral therapy. Hum Vaccin Immunother. (2014) 10:3700–10. doi: 10.4161/hv.32247
142. Agrawal A, Murphy TF. Haemophilus influenzae infections in the H. influenzae Type B conjugate vaccine. Era J Clin Microbiol. (2011) 49:3728–32. doi: 10.1128/JCM.05476-11
143. Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in haemophilus influenzae. Clin Microbiol Rev. (2007) 20:368–89. doi: 10.1128/CMR.00040-06
145. Slack MPE. A review of the role of haemophilus influenzae in community-acquired pneumonia. Pneumonia. (2015) 6:26–43. doi: 10.15172/pneu.2015.6/520
146. Zarei AE, Almehdar HA, Redwan EM. Hib vaccines: past, present, and future perspectives. J Immunol Res. (2016) 2016:7203587. doi: 10.1155/2016/7203587
147. Kelly DF, Moxon ER, Pollard AJ. Haemophilus influenzae type B conjugate vaccines. Immunology. (2004) 113:163–74. doi: 10.1111/j.1365-2567.2004.01971.x
148. Blain A, MacNeil J, Wang X, Bennett N, Farley MM, Harrison LH, et al. Invasive haemophilus influenzae disease in adults ≥65 years, United States, 2011. Open For Infect Dis. (2014) 1:ofc044. doi: 10.1093/ofid/ofu044
149. Rubach MP, Bender JM, Mottice S, Hanson K, Weng HY, Korgenski K, et al. Increasing incidence of invasive haemophilus influenzae disease in adults, Utah, USA. Emerg Infect Dis. (2011) 17:1645–50. doi: 10.3201/eid1709.101991
150. Wilkinson TMA, Schembri S, Brightling C, Bakerly ND, Lewis K, MacNee W, et al. Non-typeable haemophilus influenzae protein vaccine in adults with copd: a phase 2 clinical trial. Vaccine. (2019) 37:6102–11. doi: 10.1016/j.vaccine.2019.07.100
151. Van Damme P, Leroux-Roels G, Vandermeulen C, De Ryck I, Tasciotti A, Dozot M, et al. Safety and immunogenicity of non-typeable haemophilus influenzae-moraxella catarrhalis vaccine. Vaccine. (2019) 37:3113–22. doi: 10.1016/j.vaccine.2019.04.041
152. Berglund J, Vink P, Tavares Da Silva F, Lestrate P, Boutriau D. Safety, immunogenicity, and antibody persistence following an investigational streptococcus pneumoniae and haemophilus influenzae triple-protein vaccine in a phase 1 randomized controlled study in healthy adults. Clin Vaccine Immunol. (2014) 21:56–65. doi: 10.1128/CVI.00430-13
153. La Fauci V, Riso R, Facciolà A, Ceccio C, Lo Giudice D, Calimeri S, et al. Response to anti-Hbv vaccine and 10-year follow-up of antibody levels in healthcare workers. Public Health. (2016) 139:198–202. doi: 10.1016/j.puhe.2016.08.007
154. Francis DP, Feorino PM, McDougal S, Warfield D, Getchell J, Cabradilla C, et al. The safety of the hepatitis B vaccine. Inactivation of the aids virus during routine vaccine manufacture. J Am Med Assoc. (1986) 256:869–72. doi: 10.1001/jama.1986.03380070075022
155. Pattyn J, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B vaccines. J Infect Dis. (2021) 224(Suppl.4):S343–51. doi: 10.1093/infdis/jiaa668
156. Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs. (2003) 63:1021–51. doi: 10.2165/00003495-200363100-00006
157. Heyward WL, Kyle M, Blumenau J, Davis M, Reisinger K, Kabongo ML, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (Hbsag-1018) compared to a licensed hepatitis B vaccine in healthy adults 40–70 years of age. Vaccine. (2013) 31:5300–5. doi: 10.1016/j.vaccine.2013.05.068
158. Janssen RS, Mangoo-Karim R, Pergola PE, Girndt M, Namini H, Rahman S, et al. Immunogenicity and safety of an investigational hepatitis B vaccine with a toll-like receptor 9 agonist adjuvant (Hbsag-1018) compared with a licensed hepatitis B vaccine in patients with chronic kidney disease. Vaccine. (2013) 31:5306–13. doi: 10.1016/j.vaccine.2013.05.067
159. Beran J, Hobzova L, Wertzova V, Kuriyakose S, Leyssen M, Surquin M, et al. Safety and immunogenicity of an investigational adjuvanted hepatitis B vaccine (Hb-As02v) in healthy adults. Hum Vaccin. (2010) 6:578–84. doi: 10.4161/hv.6.7.11883
160. Rech-Medeiros AF, Marcon PdS, Tovo CdV, de Mattos AA. Evaluation of response to hepatitis B virus vaccine in adults with human immunodeficiency virus. Ann Hepatol. (2019) 18:725–9. doi: 10.1016/j.aohep.2019.03.012
162. Castellsague X. Natural history and epidemiology of Hpv infection and cervical cancer. Gynecol Oncol. (2008) 110(3Suppl.2):S4–7. doi: 10.1016/j.ygyno.2008.07.045
163. Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. Morb Mortal Wkly Rep. (2019) 68:698–702. doi: 10.15585/mmwr.mm6832a3
164. Watson-Jones D, Baisley K, Brown J, Kavishe B, Andreasen A, Changalucha J, et al. High prevalence and incidence of human papillomavirus in a cohort of healthy young african female subjects. Sex Transm Infect. (2013) 89:358–65. doi: 10.1136/sextrans-2012-050685
165. Porras C, Tsang SH, Herrero R, Guillén D, Darragh TM, Stoler MH, et al. Efficacy of the bivalent Hpv vaccine against Hpv 16/18-associated precancer: long-term follow-up results from the costa rica vaccine trial. Lancet Oncol. (2020) 21:1643–52. doi: 10.1016/S1470-2045(20)30524-6
166. Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsagué X, et al. Overall efficacy of Hpv-16/18 As04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind patricia trial. Lancet Oncol. (2012) 13:89–99. doi: 10.1016/S1470-2045(11)70286-8
167. Dillner J, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. (2010) 341:c3493. doi: 10.1136/bmj.c3493
168. Kjaer SK, Nygård M, Dillner J, Brooke Marshall J, Radley D, Li M, et al. A 12-year follow-up on the long-term effectiveness of the quadrivalent human papillomavirus vaccine in 4 nordic countries. Clin Infect Dis. (2018) 66:339–45. doi: 10.1093/cid/cix797
169. Huh WK, Joura EA, Giuliano AR, Iversen OE, de Andrade RP, Ault KA, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. (2017) 390:2143–59. doi: 10.1016/S0140-6736(17)31821-4
170. Kjaer SK, Nygård M, Sundström K, Munk C, Berger S, Dzabic M, et al. Long-term effectiveness of the nine-valent human papillomavirus vaccine in scandinavian women: interim analysis after 8 years of follow-up. Hum Vaccin Immunother. (2021) 17:943–9. doi: 10.1080/21645515.2020.1839292
171. Giuliano AR, Joura EA, Garland SM, Huh WK, Iversen OE, Kjaer SK, et al. Nine-valent Hpv vaccine efficacy against related diseases and definitive therapy: comparison with historic placebo population. Gynecol Oncol. (2019) 154:110–7. doi: 10.1016/j.ygyno.2019.03.253
172. Garland SM, Pitisuttithum P, Ngan HYS, Cho CH, Lee CY, Chen CA, et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine: subgroup analysis of participants from Asian Countries. J Infect Dis. (2018) 218:95–108. doi: 10.1093/infdis/jiy133
173. WHO. Vaccines against influenza who position paper—November 2012. Weekly Epidemiol Rec. (2012) 87:461–76. Available onine at: https://apps.who.int/iris/handle/10665/241993
174. WHO. Working Together: An Integration Resource Guide for Immunization Services Throughout the Life Course. (2018). Available online at: https://www.who.int/publications/i (accessed August 4, 2021).
175. Centre for Disease Control Prevention. Recommended Adult Immunization Schedule for Ages 19 Years or Older, United States, 2021. (2021). Available online at: https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html#table-age (accessed March 22, 2022).
176. Cassimos DC, Effraimidou E, Medic S, Konstantinidis T, Theodoridou M, Maltezou HC. Vaccination programs for adults in Europe, 2019. Vaccines. (2020) 8:10034. doi: 10.3390/vaccines8010034
177. Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the advisory committee on immunization practices (Acip). Morb Mortal Wkly Rep. (2014) 63:822–5.
178. Matanock A, Lee G, Gierke R, Kobayashi M, Leidner A, Pilishvili T. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: updated recommendations of the advisory committee on immunization practices. Morb Mortal Wkly Rep. (2019) 68:1069–75. doi: 10.15585/mmwr.mm6846a5
179. WHO. Vaccine-Preventable Diseases: Monitoring System: 2020 Global Summary. (2020). Available online at: http://apps.who.int/immunization_monitoring/globalsummary/schedules (accessed February 14, 2022).
180. Sante.gov. Guide De La Lutte Contre Les Méningites Bactériennes Communautaires. (2010). Available online at: https://www.sante.gov.ma/Publications/Guides-Manuels/Documents/Guide%20m%C3%A9ningites.pdf (accessed March 3, 2022).
181. Sante.gov. Guide De La Couverture Sanitaire Des Pèlerins Aux Lieux Saints De I' Islam. (2018). Available online at: https://www.sante.gov.ma/Publications/Guides-Manuels/Documents/2018/06/Guide%20de%20la%20couverture%20sanitaire.pdf (accessed March 3, 2022).
182. Diawara I, Zerouali K, Katfy K, Zaki B, Belabbes H, Najib J, et al. Invasive pneumococcal disease among children younger than 5 years of age before and after introduction of pneumococcal conjugate vaccine in Casablanca, Morocco. Int J Infect Dis. (2015) 40:95–101. doi: 10.1016/j.ijid.2015.09.019
183. Pugh SJ, Fletcher MA, Charos A, Imekraz L, Wasserman M, Farkouh R. Cost-effectiveness of the pneumococcal conjugate vaccine (10- or 13-valent) versus no vaccination for a national immunization program in Tunisia or Algeria. Infect Dis Ther. (2019) 8:63–74. doi: 10.1007/s40121-018-0226-x
184. Sante.gov. Programme National D'immunisation. (2013). Available online at: https://www.sante.gov.ma/Documents/Manuel_PNI_29Juin2013_VersionImprime_SIPAMA.pdf (accessed March 3, 2022).
185. Sante.gov. Manuel Pratique Sur La Vaccination À L'intention Des Professionnels De Santé. (2016). Available online at: https://www.capm-sante.ma/uploads/documents/Ebook_manuel%20sur%20la%20vaccination%202016.pdf (accessed March 3, 2022).
186. Kacem M, Dhouib W, Bennasrallah C, Zemni I, Abroug H, Fredj MB, et al. Programme élargie de vaccination aux pays du Maghreb. Etude de cas de la Tunisie. Revue systématique de la literature. La Tunise Medicale. (2018) 96:696–705. Available online at: https://www.researchgate.net/profile/Manel-Ben-Fredj/publication/335774690_Expanded_program_of_immunization_in_the_Maghreb_Case_study_of_TunisiaSystematic_review_of_the_literature/links/5de0fec8299bf10bc32f3b8b/Expanded-program-of-immunization-in-the-Maghreb-Case-study-of-TunisiaSystematic-review-of-the-literature.pdf
187. Mohamed Bouskraoui MB, El Hafidi, N,. Harmonisation Du Calendrier Marocain De Vaccination Propositions De La Société Marocaine D'infectiologie Pédiatrique Et De Vaccinologie. (2014). Available online at: https://www.somipev.ma/congres/2014/resumes/2congres2014_Bouskraoui2.pdf (accessed June 17, 2022).
188. Djeriri K, Laurichesse H, Merle JL, Charof R, Abouyoub A, Fontana L, et al. Hepatitis B in Moroccan health care workers. Occup Med. (2008) 58:419–24. doi: 10.1093/occmed/kqn071
189. Ben Hadj M, Bouguerra H, Saffar F, Chelly S, Hechaichi A, Talmoudi K, et al. Observational study of vaccine effectiveness 20 years after the introduction of universal hepatitis B vaccination in Tunisia. Vaccine. (2018) 36:5858–64. doi: 10.1016/j.vaccine.2018.08.038
190. Tunisia MoPH,. Le Calendrier National De Vaccination-Novembre 2020. (2020). Available online at: http://www.santetunisie.rns.tn/images/vaccin2020.jpg (accessed February 14, 2022).
191. Pivipartners.org. Pivi's Work with Morocco. (2022). Available online at: https://pivipartners.org/morocco/ (accessed March 3, 2022).
192. Al Awaidy S, Althaqafi A, Dbaibo G. A snapshot of influenza surveillance, vaccine recommendations, and vaccine access, drivers, and barriers in selected Middle Eastern and North African Countries. Oman Med J. (2018) 33:283–90. doi: 10.5001/omj.2018.54
193. Zaraket H, Melhem N, Malik M, Khan WM, Dbaibo G, Abubakar A. Review of seasonal influenza vaccination in the eastern mediterranean region: policies, use and barriers. J Infect Public Health. (2019) 12:472–8. doi: 10.1016/j.jiph.2018.10.009
194. Sante.gov. Manuel Pratique Sur La Vaccination 2015. (2015). Available online at: https://www.sante.gov.ma/Publications/Guides-Manuels/Documents/manuel%20pratique%20sur%20la%20vaccination%202015%20.compressed.pdf (accessed March 22, 2022).
195. Institut Pasteur Du Maroc,. Vaccination conseils aux voyageurs. (2022). Available online at: http://www.pasteur.ma/vaccination-internationale-et-conseils-voyageurs.php (accessed March 3, 2022).
196. Institut Pasteur De Tunis,. Vaccins pratiqués à l'Institut Pasteur de Tunis (2012). Available online at: http://www.pasteur.tn/index.php?option=com_content&view=article&id=116&Itemid=180 (accessed March 22, 2022).
197. WHO. Antimicrobial Resistance. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed March 7, 2022).
198. Mustapha MM, Harrison LH. Vaccine prevention of meningococcal disease in Africa: major advances, remaining challenges. Hum Vaccin Immunother. (2018) 14:1107–15. doi: 10.1080/21645515.2017.1412020
199. Ozawa S, Portnoy A, Getaneh H, Clark S, Knoll M, Bishai D, et al. Modeling the economic burden of adult vaccine-preventable diseases in the United States. Health Aff. (2016) 35:2124–32. doi: 10.1377/hlthaff.2016.0462
200. Talbird SE, La EM, Carrico J, Poston S, Poirrier JE, DeMartino JK, et al. Impact of population aging on the burden of vaccine-preventable diseases among older adults in the United States. Hum Vaccin Immunother. (2021) 17:332–43. doi: 10.1080/21645515.2020.1780847
201. Van Hoek AJ, Miller E. Cost-effectiveness of vaccinating immunocompetent ≥65 year olds with the 13-valent pneumococcal conjugate vaccine in England. PLoS ONE. (2016) 11:e0149540. doi: 10.1371/journal.pone.0149540
202. Yamin D, Balicer RD, Galvani AP. Cost-effectiveness of influenza vaccination in prior pneumonia patients in Israel. Vaccine. (2014) 32:4198–205. doi: 10.1016/j.vaccine.2014.05.015
203. Zigmond J, Pecen L, Tichopad A, Roberts CS, Jomaa I. Modeled outcomes and overall costs of the 13-valent pneumococcal conjugate vaccine in the Tunisian National Vaccination Program. Value Health. (2014) 17:A607–8. doi: 10.1016/j.jval.2014.08.2122
204. Nakamura MM, Tasslimi A, Lieu TA, Levine O, Knoll MD, Russell LB, et al. Cost effectiveness of child pneumococcal conjugate vaccination in middle-income countries. Int Health. (2011) 3:270–81. doi: 10.1016/j.inhe.2011.08.004
Keywords: adult vaccination, Tunisia, Morocco, vaccine-preventable diseases, vaccination practices
Citation: Abouqal R, Beji M, Chakroun M, Marhoum El Filali K, Rammaoui J and Zaghden H (2022) Trends in Adult and Elderly Vaccination: Focus on Vaccination Practices in Tunisia and Morocco. Front. Public Health 10:903376. doi: 10.3389/fpubh.2022.903376
Received: 24 March 2022; Accepted: 09 June 2022;
Published: 01 July 2022.
Edited by:
José Tuells, University of Alicante, SpainReviewed by:
Riyadh K. Lafta, Al-Mustansiriya University, IraqDanuta Januszkiewicz-Lewandowska, Poznan University of Medical Sciences, Poland
Copyright © 2022 Abouqal, Beji, Chakroun, Marhoum El Filali, Rammaoui and Zaghden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hela Zaghden, hela.zaghden@pfizer.com
†These authors have contributed equally to this work and share senior authorship
‡These authors have contributed equally to this work and share first authorship
 Redouane Abouqal
Redouane Abouqal Maher Beji
Maher Beji Mohamed Chakroun
Mohamed Chakroun Kamal Marhoum El Filali
Kamal Marhoum El Filali Jihane Rammaoui
Jihane Rammaoui Hela Zaghden
Hela Zaghden