- 1Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China
- 2Department of Psychiatry, The Third People’s Hospital of Huai’an, Huaian, China
- 3Department of Neurology, Affiliated ZhongDa Hospital, School of Medicine Southeast University, Nanjing, China
- 4Institute of Neuropsychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, China
- 5The Affiliated Xuzhou Oriental Hospital of Xuzhou Medical University, Xuzhou, China
Background: Persistent negative symptoms (PNS) include both primary and secondary negative symptoms that persist after adequate treatment, and represent an unmet therapeutic need. Published magnetic resonance imaging (MRI) evidence of structural and resting-state functional brain abnormalities in schizophrenia with PNS has been inconsistent. Thus, the purpose of this meta-analysis is to identify abnormalities in structural and functional brain regions in patients with PNS compared to healthy controls.
Methods: We systematically searched PubMed, Web of Science, and Embase for structural and functional imaging studies based on five research methods, including voxel-based morphometry (VBM), diffusion tensor imaging (DTI), functional connectivity (FC), the amplitude of low-frequency fluctuation or fractional amplitude of low-frequency fluctuation (ALFF/fALFF), and regional homogeneity (ReHo). Afterward, we conducted a coordinate-based meta-analysis by using the activation likelihood estimation algorithm.
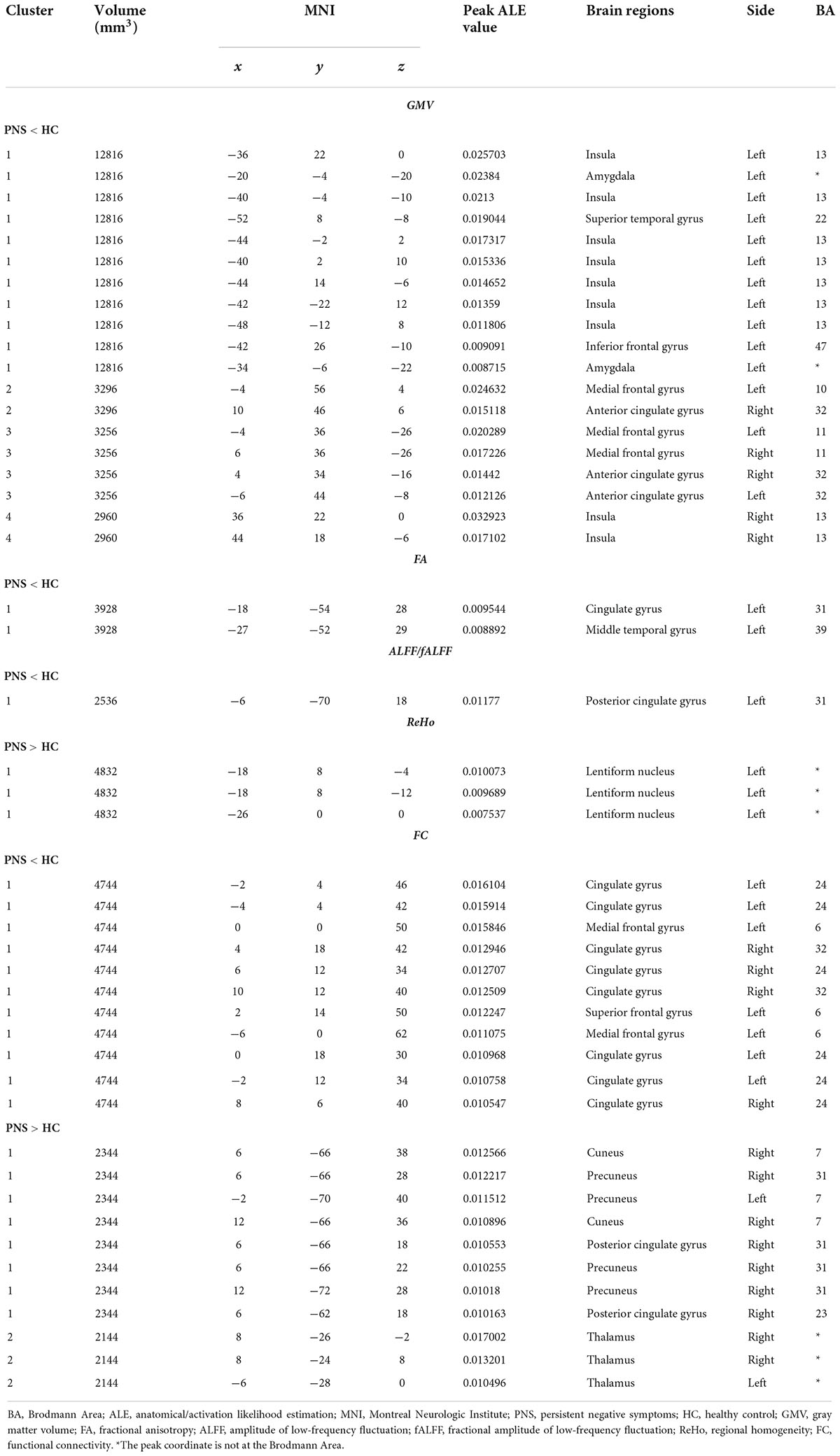
Results: Twenty-five structural MRI studies and thirty-two functional MRI studies were included in the meta-analyses. Our analysis revealed the presence of structural alterations in patients with PNS in some brain regions including the bilateral insula, medial frontal gyrus, anterior cingulate gyrus, left amygdala, superior temporal gyrus, inferior frontal gyrus, cingulate gyrus and middle temporal gyrus, as well as functional differences in some brain regions including the bilateral precuneus, thalamus, left lentiform nucleus, posterior cingulate gyrus, medial frontal gyrus, and superior frontal gyrus.
Conclusion: Our study suggests that structural brain abnormalities are consistently located in the prefrontal, temporal, limbic and subcortical regions, and functional alterations are concentrated in the thalamo-cortical circuits and the default mode network (DMN). This study provides new insights for targeted treatment and intervention to delay further progression of negative symptoms.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/], identifier [CRD42022338669].
Introduction
Schizophrenia is a severe mental illness characterized by positive and negative symptoms. Negative symptoms, including blunted affect, alogia, asociality, anhedonia, and avolition (1), have often been found to contribute to poor community and social functioning and negatively influence recovery and general health outcomes (2). Due to the significance of negative symptoms in schizophrenia, Buchanan (3) coined the term persistent negative symptoms (PNS) to describe negative symptoms that are enduring, trait-like and resistant to currently available treatments. Previous studies have indicated that the estimated prevalence of PNS is above 20% amongst patients with schizophrenia, and 23–40% in first episode psychosis (4, 5). Therefore, it is crucial to develop effective diagnosis and appropriate interventions for schizophrenia patients with PNS, which could remediate the substantial functional disability exhibited by these patients.
PNS represents a broader concept that requires at least moderate negative symptoms, a defined threshold of positive symptoms, none or low depressive and extrapyramidal symptoms (all defined on validated scales), with demonstrated clinical stability (3). The National Institute of Mental Health consensus statement recommends the use of PNS criteria in clinical research designs, especially those targeting therapeutic interventions (1). However, there is currently no assessment instrument specifically designed for PNS. Commonly accepted and validated rating scales, such as the Scale for the Assessment of Negative Symptoms (SANS) (6), Positive and Negative Syndrome Scale (PANSS) (7), Negative Symptoms Assessment (8) or newer scales like the Brief Negative Symptom Scale (9) and Clinical Assessment Interview for Negative Symptoms (10) are often used instead. Different researchers have employed distinct scales with diverse criteria to identify PNS, leading to heterogeneous results.
Magnetic resonance imaging (MRI) research has offered a significantly advanced understanding of brain structural and functional changes associated with schizophrenia (11). In recent years, advances in clinical brain imaging research have been made possible by improvements in the measurement of the distinct aspects of brain anatomy and function. However, the generalization of Task-based findings is limited since different groups utilized various tasks to capture a wide range of emotional states and behaviors. Therefore, for this present review, we chose to focus on task-free studies—namely structure (volume and morphometry), structural connectivity, and resting-state functional MRI findings, which are stable across mental states and hence allow better comparability across independent study settings and populations.
Structural MRI analytic approaches which are used to quantify brain abnormalities include voxel-based morphometry (VBM) for gray matter volume (GMV) and diffusion tensor imaging (DTI) for white matter. The VBM technique involves spatial normalization of the MRI structural images, extraction of gray matter from the normalized images, smoothing, and finally, statistical analyses comparing healthy controls (HCs) and patients (12). Numerous studies have indicated structural alterations in the prefrontal lobes, temporal lobes and limbic regions in schizophrenia (13, 14) which were associated with the severity of negative symptoms (15–17). DTI is also a non-invasive brain imaging method that allows indirect measurements of white matter microstructure by recording the diffusion of water molecules (18). Fractional anisotropy (FA) is the most commonly used index that quantifies the directionality of water diffusion in fiber bundles (19). A recent study reported that the FA value between the right caudate nucleus and putamen was inversely correlated with negative symptoms in schizophrenia (20), while a prior experiment found that the FA value of the anterior part of the corpus callosum was negatively correlated with the avolition score in schizophrenia (21). The inconsistency of these results demonstrates the need to evaluate structural changes in schizophrenia patients with PNS.
Resting-state functional MRI analytical methods that define the local features of the spontaneous blood oxygen level-dependent signal include the amplitude of low-frequency fluctuation (ALFF)/fractional amplitude of low-frequency fluctuation (fALFF) and regional homogeneity (ReHo). ALFF quantifies the intensity of low-frequency oscillations in spontaneous neural activity, which pinpoints the spontaneous neural activity of specific regions and physiological states of the brain (22). fALFF is defined as the total power in the low-frequency range (0.01–0.1 Hz) relative to the total power across all measurable frequencies. As such, fALFF is a normalized version of ALFF and is less susceptible to artifactual signals in regions located within the vicinity of vessels and/or significant pulsatile motion (23). Although many previous studies have found ALFF alternations in schizophrenia, including increased or decreased ALFF in the cingulate gyrus, temporal gyrus, lentiform nuclei, inferior parietal lobes and frontal gyrus (24–27), few studies have been carried out in schizophrenia patients with PNS. ReHo assumes that a given voxel is temporally similar to those of its neighbors, and can be used to detect the localized functional connectivity or synchronization of information processing with little interference from external stimuli (28). Moreover, increasing evidence shows that local functional homogeneity has neurobiological relevance to anatomical, developmental and neurocognitive factors, which could serve as a neuroimaging marker to investigate the human brain function, behaviors and neuropsychiatric disorder (29, 30). In fact, ReHo analysis has been successfully used to detect the abnormalities of regional functional synchronization in subjects with different psychiatric disorders (31–33). A recent ReHo study demonstrated that hyperactivation in the right inferior frontal gyrus/insula was positively associated with negative symptom scores (34). Resting-state functional connectivity (FC) is a powerful and reliable analysis method in which synchronous activity of brain regions can be examined in task-free conditions (35). FC is particularly useful in elucidating patterns of functional integration throughout the brain (i.e., how different brain regions function together) (36). Resting-state studies in schizophrenia have reported increased FC in the left orbital medial frontal cortex and right putamen regions, and reduced FC between the striatum and the right medial orbitofrontal cortex, which were significantly associated with negative symptom severity (37, 38). These findings from functional MRI studies using ALFF, ReHo, or FC support the statement that negative symptoms are associated with aberrant activation or dysconnectivity in extensive brain regions.
Published meta-analyses of VBM studies have focused more on alternations of GMV in schizophrenia patients (39, 40) or the relationship between GMV changes and positive symptoms, such as hallucinations (41, 42). Similarly, numerous meta-analyses have shown an activation or inactivation of functional connectivity in different brain regions in schizophrenia (43–47). However, the meta-analysis of structural and functional MRI studies in patients with PNS is limited. Only one meta-analysis of VMB studies focused on schizophrenia with PNS, and it reveals reduced GMV in the brain regions of the reward network, especially the left caudate nucleus (48). While a large number of existing negative symptom imaging studies have analyzed the relationship between structural or functional brain abnormalities and negative symptoms in schizophrenia patients from a symptomatological perspective, there is a paucity of studies pertaining to the differences in structural and functional brain alterations between the PNS subgroup and HCs. Therefore, this review aims to examine brain regions that show alterations in either structure or function in schizophrenia with PNS via a meta-analysis of structural MRI and functional MRI studies.
Materials and methods
Data sources and searches
The current meta-analysis was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines (PRISMA) (49). A systematic selection of appropriate peer-reviewed studies was undertaken by searching the databases of PubMed, Web of Science, and Embase databases for structural and functional imaging studies. Search keywords were as follows: (1) [(gray matter) OR (cerebellar gray matter)] AND [(schizophrenia) AND (voxel-based morphometry)]; (2) [(white matter) AND (schizophrenia)] AND (diffusion tensor imaging); (3) [(functional magnetic resonance imaging) OR (RESTING STATE)] AND [(schizophrenia) AND (functional connectivity)]; (4) [(functional magnetic resonance imaging) OR (RESTING STATE)] AND [(schizophrenia) AND (regional homogeneity)]; (5) [(functional magnetic resonance imaging) OR (RESTING STATE)] AND [(schizophrenia) AND ((fractional amplitude of low frequency fluctuation) OR (amplitude of low frequency fluctuation))]. We included studies published in these databases up to September 2021. Figure 1 shows the flowchart of the literature search and eligibility assessment.
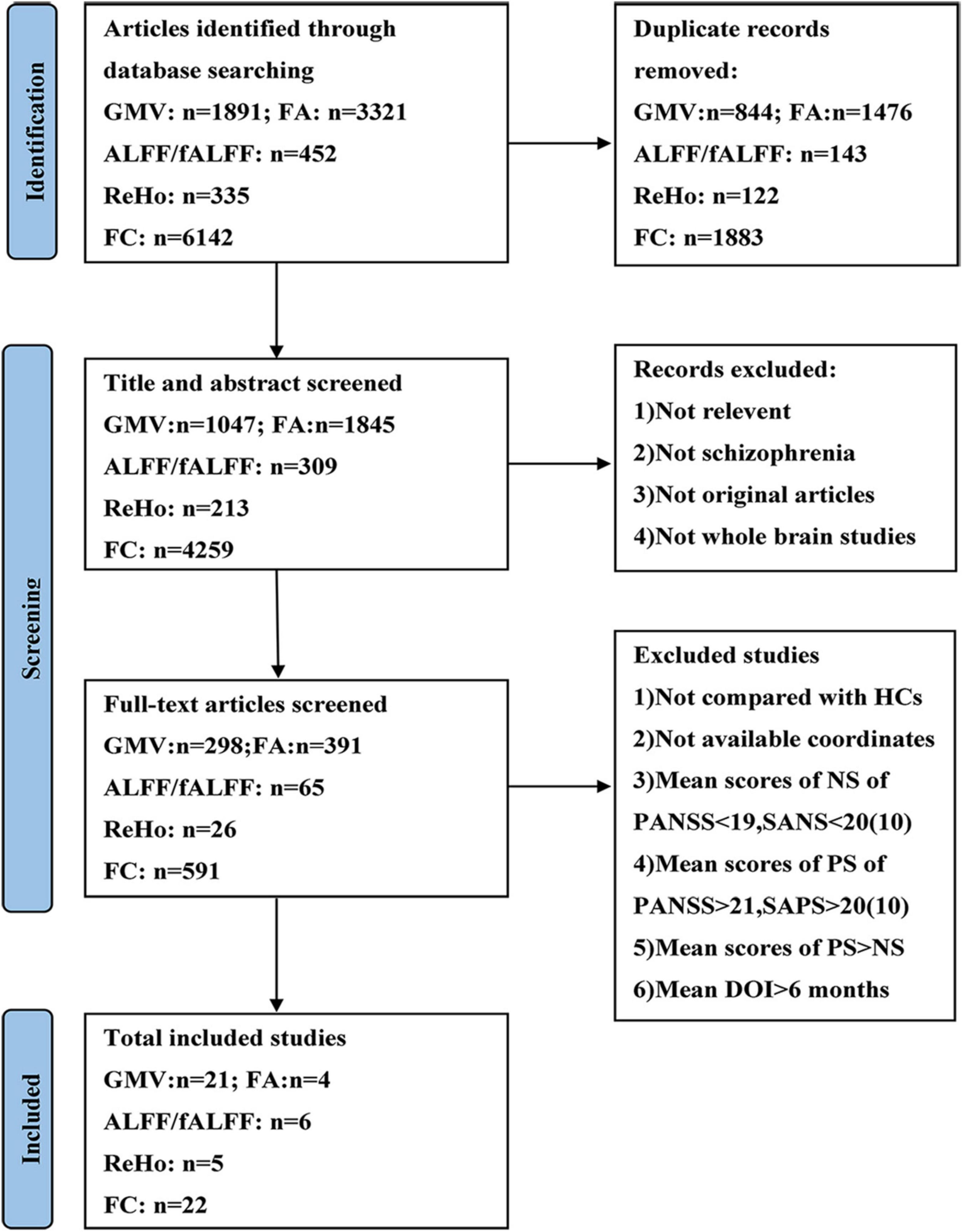
Figure 1. Flow diagram showing the process of identifying relevant studies. GMV, gray matter volume; FA, fractional anisotropy; ALFF, amplitude of low-frequency fluctuation; fALFF, fractional amplitude of low-frequency fluctuation; ReHo, regional homogeneity; FC, functional connectivity; HCs, healthy controls; NS, negative symptoms; PS, positive symptoms; PANSS, Positive and Negative Syndrome Scale; SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; DOI, duration of illness.
Eligible criteria and quality assessment
Inclusion and exclusion criteria were adopted to screen literature. The following inclusion criteria were used to select eligible studies: (1) original articles written in English; (2) the schizophrenia diagnosis for patients was based on DSM criteria (negative symptoms must be measured by validated rating scales such as the PANSS and SANS, and must reach at least mild or moderate severity); (3) the duration of illness must be longer than or equal to 6 months; (4) used whole-brain structural imaging (VBM and DTI) or functional imaging (ALFF/fALFF, ReHo, FC) in schizophrenia patients; (5) reported whole-brain results in stereotactic (x, y, z) coordinates; (6) compared schizophrenia subjects with HCs; and (7) aged 19 years and above.
Before Buchanan developed the criteria of PNS, many researchers used different terms and criteria to identify patients with PNS, which complicated the search. To address this problem, we adopted the following exclusion criteria formulated by Li et al. (48) to identify the relevant studies: (1) mean PANSS negative score of <19, or mean SANS total score of <20; (2) mean PANSS positive subscale score of >21, or the mean Assessment of Positive Symptoms (SAPS) total score of >20; (3) studies in which the mean positive symptom scores exceeded the mean negative symptom scores.
Two authors (Tingting Zhu and Zixu Wang) independently selected eligible studies according to the abovementioned criteria and assessed the quality of the included studies. A 12-point checklist was used to estimate the quality of each included study, based on the reported demographic and clinical characteristics of the participants and the imaging methodology (50). Each point was scored as 0, 0.5, or 1 if the criteria were unfulfilled, partially met or fully met, respectively, and any study scoring >6.0 was included in the meta-analysis (see Supplementary material).
Data extraction
The research results were screened independently by two authors (Tingting Zhu and Zixu Wang) according to the inclusion and exclusion criteria. In case of disagreement, the reviewers (Xiangrong Zhang and Jiu Chen) evaluated and made the final decision. Preliminary screening of the titles and abstracts was conducted so as to evaluate whether they conform to the research content being explored. For articles that conformed to the research content or with content that could not be determined according to the title and abstract, the full text was reviewed for a more extensive assessment. Articles obtained after the preliminary screening were re-examined to assess whether they meet the inclusion criteria. Finally, we crosschecked the references of all the retrieved results to find any missing studies.
Data analysis
Ginger ALE version 2.3.61 was used for the coordinate-based meta-analyses of the neuroimaging data. The algorithm estimated the convergence of activation based on significant foci extracted from selected studies. Localization probability distributions for all foci were modeled as the center of 3D Gaussian functions. The width of the Gaussian probability distribution was determined individually for each experiment based on empirical estimates of between-subject variability taking into account the number of subjects in each experiment (51). Gaussian distributions were pooled voxel-wise within experimental contrasts and across contrasts within a group to create a whole-brain ALE map. Within this whole-brain ALE map, each voxel was assigned a unique ALE value that represents the likelihood of experimental effects in that voxel (52). For ALE Map creation, coordinates and cluster sizes associated with significant activation or deactivation were first converted to Talairach space using the MNI to Talairach conversion tool provided by the Ginger ALE toolbox. The false discovery rate method was employed to correct for multiple comparisons at a significance threshold (p < 0.01, 1000 permutations). ALE results were overlaid into the MNI 152 template and viewed using the Mango2 and DPABI software3.
To test the replicability of the results, we performed a systematic whole-brain jackknife sensitivity analysis in the meta-analysis by repeating the main analysis n times (n = the number of datasets included), dropping one study at a time to determine whether the results remained detectable. However, due to the limitations in the number of included studies involving different metrics, sensitivity analyses were performed only for the group with GMV and FC in the PNS patients.
Results
Search results
The search results and inclusion procedures are shown in Figure 1. The study characteristics and results are summarized in Tables 1, 2. A total of 57 studies were eventually eligible for inclusion and quality assessment. Among these studies, 21 used VBM to analyze gray matter abnormalities and four studies employed DTI to examine white matter abnormalities and the remaining 32 articles comprised resting-state functional MRI studies (6 used the ALFF method, 5 employed the ReHo method, and 22 utilized whole-brain FC method). The results of the quality assessment and jackknife sensitivity analysis are available in the Supplementary material.
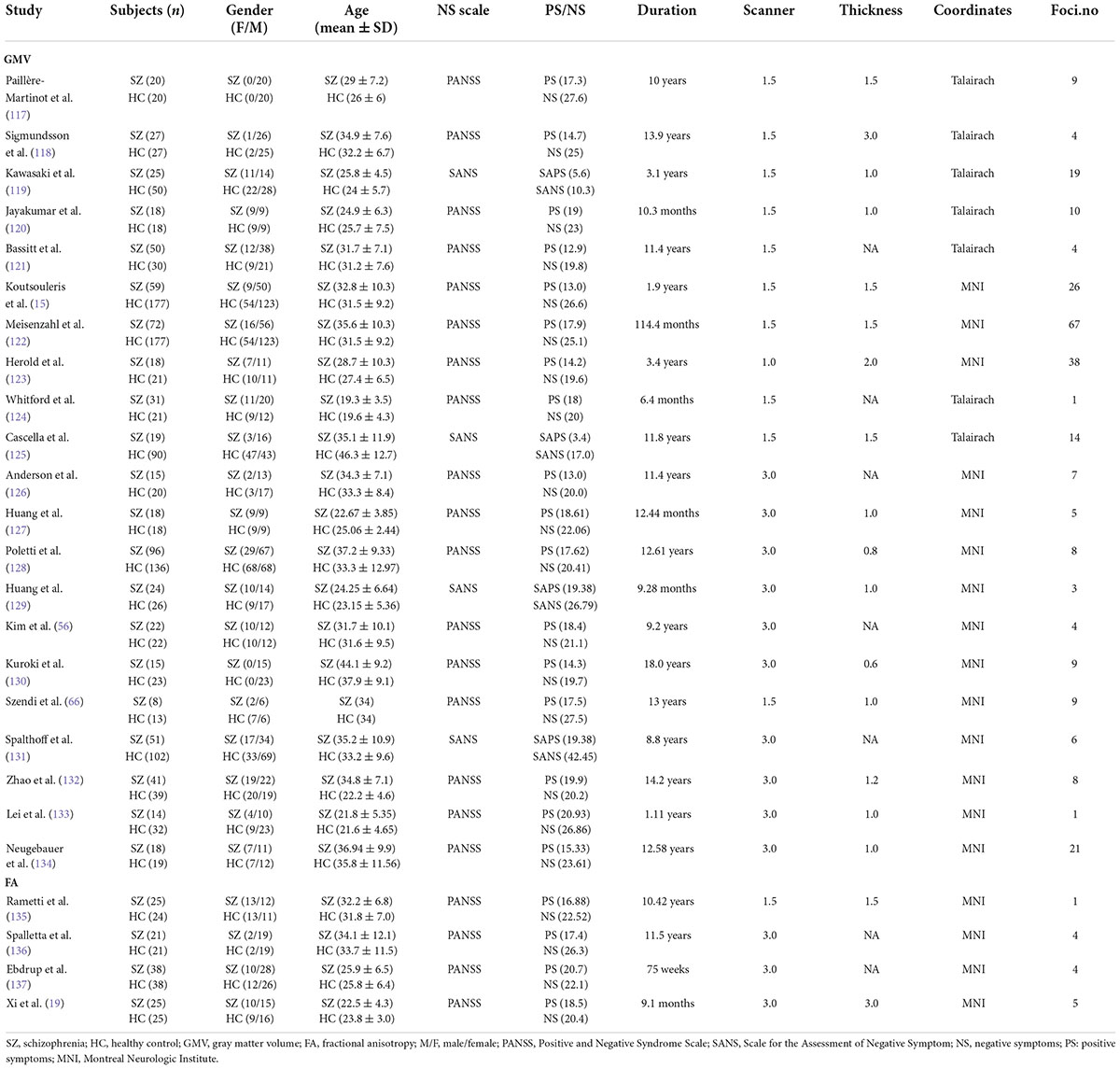
Table 1. Demographic and clinical information for the structural MRI studies included in the meta-analysis.
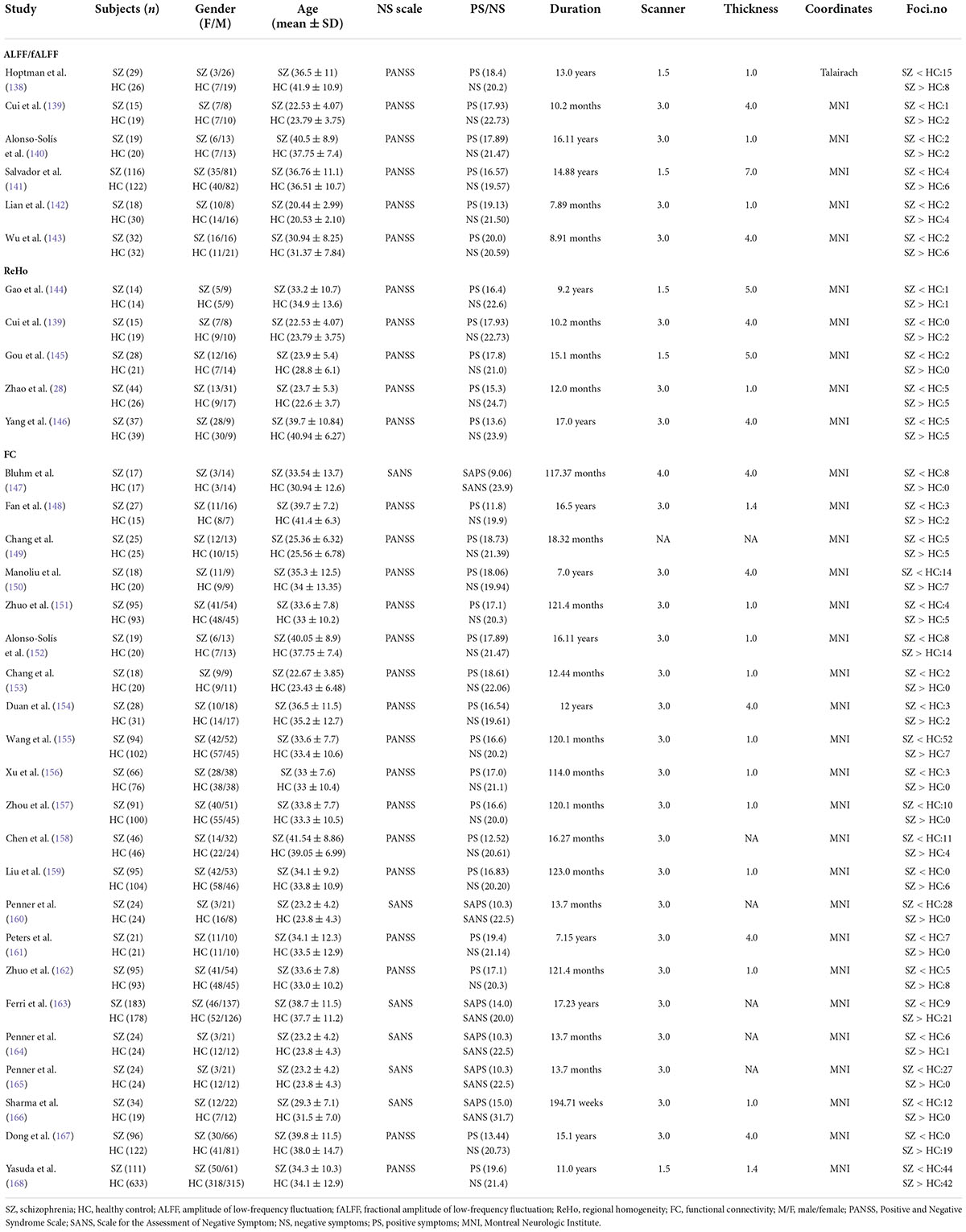
Table 2. Demographic and clinical information for the functional MRI studies included in the meta-analysis.
Meta-analysis results
Activation likelihood estimation (ALE) analysis indicated that schizophrenia with PNS, compared with HCs, showed significant GMV reductions in the bilateral insula, bilateral medial frontal gyrus (MFG), bilateral anterior cingulate gyrus (ACG), left amygdala, left superior temporal gyrus (STG), and left inferior frontal gyrus compared with HCs (Table 3 and Figure 2A). Analysis of the DTI studies revealed that schizophrenia patients with PNS showed reduced fractional anisotropy values in the left cingulate gyrus and middle temporal gyrus (Table 3 and Figure 2B).
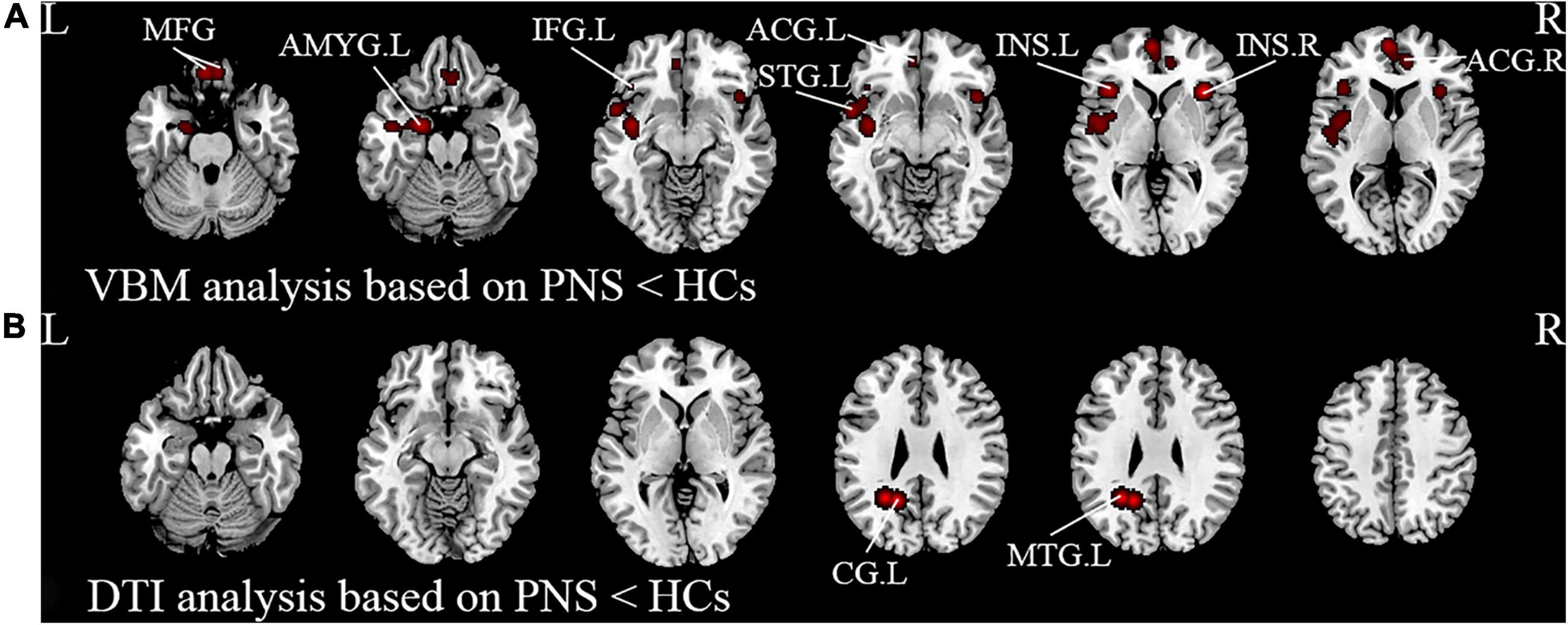
Figure 2. Results from the activation likelihood estimation (ALE) meta-analysis of structural abnormalities in schizophrenia with PNS. Brain regions showing (A) decreased GMV and (B) decreased FA in PNS patients compared with HCs. Significance threshold with a false discovery rate at p < 0.01. PNS, persistent negative symptoms; HCs, healthy controls; GMV, gray matter volume; FA, fractional anisotropy; VBM, voxel-based morphometry; DTI, diffusion tensor imaging; MFG, medial frontal gyrus; AMYG, amygdala; IFG, inferior frontal gyrus; STG, superior temporal gyrus; INS, insula; ACG, anterior cingulate gyrus; CG, cingulate gyrus; MTG, middle temporal gyrus; R, right; L, left.
Schizophrenia patients with PNS exhibited decreased ALFF/fALFF in the left posterior cingulate gyrus (PCG). Additionally, these patients also showed increased ReHo in the left lentiform nucleus and decreased FC in the bilateral cingulate gyrus, left MFG and left superior frontal gyrus. Compared to HCs, PNS patients presented increased FC in the bilateral precuneus, bilateral thalamus, right cuneus and right PCG (Table 3 and Figure 3).
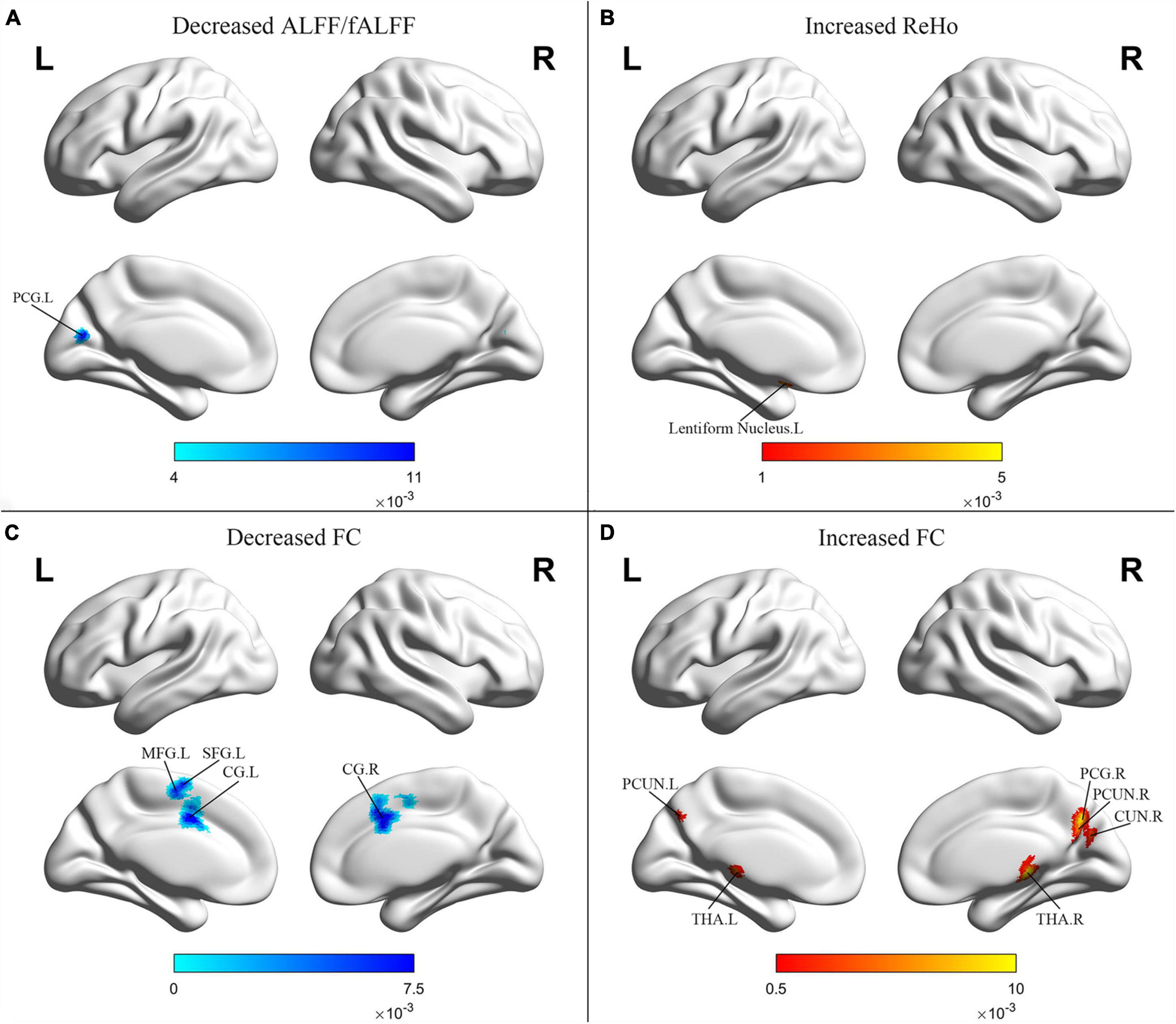
Figure 3. Results from the activation likelihood estimation (ALE) meta-analysis of functional abnormalities in schizophrenia with PNS. Brain regions showing (A) decreased ALFF/fALFF; (B) increased ReHo; (C) decreased FC; (D) increased FC, in schizophrenia with PNS compared with HCs. Significance threshold with a false discovery rate at p < 0.01. PNS, persistent negative symptoms; HCs, healthy controls; ALFF, amplitude of low-frequency fluctuation; fALFF, fractional amplitude of low-frequency fluctuation; ReHo, regional homogeneity; FC, functional connectivity; PCG, posterior cingulate gyrus; MFG, medial frontal gyrus; SFG, superior frontal gyrus; CG, cingulate gyrus; PCUN, precuneus; THA, thalamus; CUN, cuneus; R, right; L, left.
Discussion
To the best of our knowledge, this is the first meta-analysis of whole-brain structural and functional MRI findings for schizophrenia with PNS. Specifically, the main findings of the present article comprise: (1) decreased GMV in bilateral insula, bilateral MFG, bilateral ACG, left amygdala, left STG, and left inferior frontal gyrus in the PNS group; (2) reduced FA values in the left cingulate gyrus and middle temporal gyrus; (3) increased ReHo in the left lentiform nucleus and enhanced FC in the bilateral precuneus, bilateral thalamus, right cuneus, right PCG; and (4) decreased ALFF/fALFF in the left PCG and reduced FC in the bilateral cingulate gyrus, left MFG and left superior frontal gyrus.
Structural magnetic resonance imaging results
In the present study, the VBM meta-analysis results revealed reduced GMV in the prefrontal gyrus and ACG in schizophrenia with PNS compared to HCs. Several neuroimaging studies have reported that decreased GMV in the prefrontal gyrus was negatively correlated with negative symptom severity (53, 54), which might be related to impaired self-reference processing and social cognition (55). Our findings are in line with a previous study that reported a negative correlation between reduced GMV in the ACG and negative symptoms in schizophrenia patients (56) as well as a study that showed right hemispheric ACG volume reduction in schizophrenia with PNS compared to HCs (57). It was previously demonstrated that the medial frontal lobe wall, composed of the ACG and medial prefrontal gyrus (58), plays a key role in social cognitive processing, particularly in mentalizing others’ intentions (59), thereby suggesting that abnormalities in this region could lead to difficulties in interacting with others. Consequently, we speculate that the reduced GMV in the medial frontal lobe wall might underlie the increasing social withdrawal that is characteristic of negative symptoms. However, these findings were not replicated in other studies (60, 61). These inconsistent results might be attributed to variable criteria in defining PNS as well as the heterogeneity of schizophrenia, including disease courses, and the use of antipsychotics.
In our meta-analysis, apart from the frontal gyrus, subcortical regions such as the insula and amygdala have also shown reduced GMV in schizophrenia with PNS. The insula plays a key role in monitoring internal emotional states (62) and regulating the influences of emotion on cognitive processes (63, 64). Many studies have reported abnormalities in the insula which were related to negative symptoms in schizophrenia (17, 65). These are in accordance with findings that a volumetric decrease of the insula relative to controls could be detected in schizophrenia with PNS (66, 67). Similarly, structural abnormalities in the left amygdala were found to be significantly associated with PANSS negative symptoms (68), which is consistent with studies that found a negative correlation between the volume of the hippocampus-amygdala complex and clinical ratings of negative symptoms and thought disturbances (69, 70). Nevertheless, the increased GMV in the amygdala and its negative correlation with PANSS scores have also been reported (71). Considering the function of the amygdala in regulating emotional and motivational behavior (72), it is reasonable that there is an association between the amygdala and negative symptoms of reduced expression in schizophrenia. Together, these findings might explain the various symptom profiles of patients and psychopathology, such as the loss of boundaries, lack of emotional reactivity, and poor empathy.
Alterations of the temporal lobe in schizophrenia have been investigated by a considerable number of neuroimaging studies. We also observed a significant decrease in the GMV of the STG in patients with PNS. The STG is involved in emotion processing, particularly negative emotions as shown in studies of facial emotion perception (73, 74). Previous articles reported that schizophrenia patients had significantly smaller bilateral STG volumes than HCs (75), which was negatively correlated with the severity of auditory hallucinations and thought disorder (76, 77). Consistent with our results, one study of schizophrenia with PNS found a reduction of gray matter in the left STG (78). Several VBM analyses found that the PNS patients showed more prominent and extended alterations affecting the prefrontal, temporal, limbic and subcortical regions compared to the non-PNS patients (15, 79). Altogether, these findings suggest that smaller GMV in these regions appear to be a substrate for schizophrenia with PNS. It remains to be seen whether these regions contribute directly to the pathophysiological process of patients with PNS.
White matter abnormalities have long been reported in schizophrenia patients with inconsistent results (80, 81), and the correlation between the negative symptoms and white matter defects has also been confirmed (82–84). The present meta-analysis additionally observed decreased FA in the left cingulate gyrus and middle temporal gyrus in schizophrenia with PNS. The cingulate cortex is a critical region in the saliency and cognitive motor circuit, with the ACG involved in the decision-making circuit and emotional processing (85). It has been previously reported that there is a significant association between reduced FA in the ACG and avolition-apathy and anhedonia in schizophrenia (86, 87). The middle temporal gyrus is a critical component of the neural network involved in pleasure and reward (88). Our results align with previous reports of FA deficits in the deep temporal lobe in patients with PNS (89, 90). Hence, the decreased white matter FA in the middle temporal lobe might reflect impairments in reward-related processing in schizophrenia with PNS.
Functional magnetic resonance imaging results
The finding of decreased ALFF/fALFF in the left PCG in schizophrenia with PNS is consistent with previous data demonstrating a negative correlation of ALFF in the left PCG with negative symptoms and withdrawal on the PANSS (91). These observations are in accordance with the notion that a dysregulation between the striatum and PCG is associated with cognitive-affective control (92), which might provide a neurophysiological basis for negative symptoms. ReHo abnormalities were also detected in the lentiform nucleus that is involved in the basal ganglia-thalamocortical circuitry (93). Nevertheless, an increased ReHo in the lentiform nucleus in patients with PNS is seldom reported and might represent a protective or compensatory phenomenon. One study indicated that increased ReHo in the lentiform nucleus was not related to negative symptoms (28), while other studies found that increased ReHo values in the right inferior frontal gyrus/insula may reflect the severity of negative symptoms and verbal learning abilities (34). However, in our study, we did not observe consistent results from the ALE analysis. This could be explained by the fact that there exist limited functional MRI studies investigating ReHo changes in PNS patients.
In this study, a decreased FC was detected in the MFG and superior frontal gyrus whilst an increased FC was found in the right PCG and bilateral precuneus. Interestingly, these areas overlap with the DMN, which is involved in the processing of task-independent thoughts, attention to internal emotional states, self-inspection, and future planning (36, 94). Decreased connectivity in the DMN was observed in previous studies (95, 96), and related to clinical symptoms and cognitive performance (97, 98). Although several studies have reported that the DMN connectivity in the prefrontal cortices correlated negatively with the severity of positive and mood symptoms in patients with schizophrenia (99), the connectivity between the prefrontal cortices and PCG was differentially related to social attainment and social competence (100). The results of the present meta-analysis showed hyperconnectivity (right PCG and bilateral precuneus) as well as hypoconnectivity (MFG and superior frontal gyrus) in the DMN. These findings are in line with other recent studies which indicated associations between high DMN resting-state connectivity and negative symptoms in schizophrenia patients (101, 102). Previous evidence has also suggested that the transition probability from a state with weak precuneus/PCG connectivity to stronger connectivity increased with symptom severity (103), thereby demonstrating the functional significance of the relationship between negative symptoms and increased DMN connectivity in schizophrenia. These results suggest that the DMN is often hyperconnected in schizophrenia with PNS, which and might be related to the overly intense self-reference and impairments in attention and working memory observed in these patients (104, 105).
In our study, increased FC was mainly observed in the thalamo-cortical network, including the bilateral thalamus, bilateral precuneus, and right PCG. The thalamus, which is involved in a great variety of cognitive functions and mental activities including memory, language, perception and emotion, represents a key node in distributed neuronal circuits involving various regions of the cerebral cortex, striatum and cerebellum (106–109). Individuals at high clinical risk for psychosis have enhanced connectivity in cerebellar-thalamo-cortical circuits which was significantly associated with positive symptoms (110). Similar findings were also found in patients with schizophrenia through a study of an independent clinical sample (111). Consistent with our findings, Anticevic et al. reported a positive correlation between schizophrenia total symptom severity and all regions displaying hyperconnectivity with the thalamus (112). In a recent study, higher cerebello-thalamo-cortical connectivity at baseline significantly predicted poorer long-term reduction in negative symptoms (113). Numerous functional MRI studies have also reported reduced thalamic-prefrontal connectivity and increased coupling with somatomotor and temporal regions in schizophrenia (114–116). These findings support the theory that thalamo-cortical interactions are critical for optimal brain functioning and provide further evidence for the role of thalamo-cortical interactions in the pathophysiology of schizophrenia.
Clinical implications
The present findings have a few implications for our understanding of both the neural mechanisms of PNS patients and the development of the intervention. Firstly, altered GMV in the prefrontal, temporal, limbic, and subcortical regions might be the key anatomical basis for PNS since these regions were consistently identified in different meta-analyses. Moreover, patients with PNS can benefit from more thorough assessment with multiple imaging techniques, as these data can help researchers to design individualized interventions to achieve better treatment outcomes. Taken together, our findings reveal provide evidence of the specificity of the affected brain regions and provide new insights for targeted treatment and follow-up care.
Limitations
The present study has several limitations. Firstly, due to different terms and definitions of PNS, the included studies did not fully conform to the PNS criteria proposed by Buchanan (3). The assessment of negative symptoms of patients with PNS by different scales may lead to heterogeneity of results. Secondly, the ALE methodology we used had certain limitations. For example, the ALE software could not analyze the correlation between the severity of negative symptoms and these brain regions, and it failed to provide any solving approach to analyze the confidence interval to increase the robustness of our findings. Thirdly, the literature on whole-brain ALFF, ReHo, and DTI data in schizophrenia patients with PNS is very limited, and the small sample size of available articles weakens the validity of our meta-analysis. Next, there was substantial heterogeneity among patients with PNS, including time to the first episode, antipsychotic medication, and duration of negative symptoms. Another inherent limitation of this meta-analysis approach is the heterogeneity of the results, which might arise from differences in methodology across studies, including imaging acquisition and analysis pipelines, clinical assessments, and small sample sizes.
Conclusion
By performing ALE meta-analysis in PNS patients to identify structural and functional alterations, we found that structural brain abnormalities were consistently located in the insula, medial and inferior frontal gyrus, anterior cingulate gyrus, amygdala, superior temporal gyrus and middle temporal gyrus, and functional alterations were concentrated in the thalamo-cortical circuits and the DMN. In addition, we observed that enhanced functional alterations were detected in thalamo-cortical circuits in patients with PNS, thereby demonstrating that it plays an important role in the diagnosis and prediction of negative symptoms in schizophrenia. These findings help to elucidate the brain abnormalities specific to schizophrenia patients with PNS, which are important for understanding their underlying the pathophysiology and may ultimately contribute to the development of future behavioral, pharmacological, or neurotherapeutic treatments.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
TZ guided by XZ and JC designed the study. TZ and ZW performed the meta-analysis and drafted the manuscript. CZ, XF, CH, CX, HG, and ZY helped in literature extraction and data analyses. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFC1314300); the National Natural Science Foundation of China (Grant Nos. 81971255 and 82101572); the Social Development Foundation of Jiangsu Province, China (Grant No. BE2019610); the Jiangsu Provincial Medical Talent Project of China (ZDRCA2016075); the Special Project of Basic Research on Frontier Leading Technology of Jiangsu Province, China (BK20192004D); the Key Project supported by Medical Science and Technology Development Foundation, Nanjing Department of Health (YKK20090); the National Natural Science Foundation of China (Grant No. 81701675); the Key Project supported by Medical Science and technology development Foundation, Nanjing Department of Health (Grant No. JQX18005); the Key Research and Development Plan (Social Development) Project of Jiangsu Province (Grant No. BE2018608); the Top-Notch Talent Program of the Jiangsu Province High-Level Healthcare Talent “Six-Ones” Project (Grant No. LGY2020058); and the Science and Technology Development Program of Nanjing Medical University (NMUB2019107).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.957685/full#supplementary-material
Footnotes
References
1. Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. (2006) 32:214–9. doi: 10.1093/schbul/sbj053
2. Ventura J, Subotnik KL, Gitlin MJ, Gretchen-Doorly D, Ered A, Villa KF, et al. Negative symptoms and functioning during the first year after a recent onset of schizophrenia and 8 years later. Schizophr Res. (2015) 161:407–13. doi: 10.1016/j.schres.2014.10.043
3. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. (2007) 33:1013–22. doi: 10.1093/schbul/sbl057
4. Mäkinen J, Miettunen J, Isohanni M, Koponen H. Negative symptoms in schizophrenia: a review. Nord J Psychiatry. (2008) 62:334–41. doi: 10.1080/08039480801959307
5. Malla AK, Norman RM, Takhar J, Manchanda R, Townsend L, Scholten D, et al. Can patients at risk for persistent negative symptoms be identified during their first episode of psychosis? J Nerv Ment Dis. (2004) 192:455–63. doi: 10.1097/01.nmd.0000131804.34977.c1
6. Andreasen NC. The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl. (1989) 7:49–58.
7. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
8. Axelrod BN, Goldman RS, Alphs LD. Validation of the 16-item negative symptom assessment. J Psychiatr Res. (1993) 27:253–8. doi: 10.1016/0022-3956(93)90036-2
9. Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. (2011) 37:300–5. doi: 10.1093/schbul/sbq059
10. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psychiatry. (2013) 170:165–72. doi: 10.1176/appi.ajp.2012.12010109
11. Schultz SK, Andreasen NC. Schizophrenia. Lancet. (1999) 353:1425–30. doi: 10.1016/s0140-6736(98)07549-7
12. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. (2000) 11(6 Pt. 1):805–21. doi: 10.1006/nimg.2000.0582
13. Li C, Liu W, Guo F, Wang X, Kang X, Xu Y, et al. Voxel-based morphometry results in first-episode schizophrenia: a comparison of publicly available software packages. Brain Imaging Behav. (2020) 14:2224–31. doi: 10.1007/s11682-019-00172-x
14. Shah C, Zhang W, Xiao Y, Yao L, Zhao Y, Gao X, et al. Common pattern of gray-matter abnormalities in drug-naive and medicated first-episode schizophrenia: a multimodal meta-analysis. Psychol Med. (2017) 47:401–13. doi: 10.1017/s0033291716002683
15. Koutsouleris N, Gaser C, Jäger M, Bottlender R, Frodl T, Holzinger S, et al. Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage. (2008) 39:1600–12. doi: 10.1016/j.neuroimage.2007.10.029
16. Lei W, Deng W, Li M, He Z, Han Y, Huang C, et al. Gray matter volume alterations in first-episode drug-naïve patients with deficit and nondeficit schizophrenia. Psychiatry Res. (2015) 234:219–26. doi: 10.1016/j.pscychresns.2015.09.015
17. Takahashi T, Kido M, Sasabayashi D, Nakamura M, Furuichi A, Takayanagi Y, et al. Gray matter changes in the insular cortex during the course of the schizophrenia spectrum. Front Psychiatry. (2020) 11:659. doi: 10.3389/fpsyt.2020.00659
18. Viswanath H, Velasquez KM, Thompson-Lake DG, Savjani R, Carter AQ, Eagleman D, et al. Alterations in interhemispheric functional and anatomical connectivity are associated with tobacco smoking in humans. Front Hum Neurosci. (2015) 9:116. doi: 10.3389/fnhum.2015.00116
19. Xi YB, Guo F, Li H, Chang X, Sun JB, Zhu YQ, et al. The structural connectivity pathology of first-episode schizophrenia based on the cardinal symptom of auditory verbal hallucinations. Psychiatry Res Neuroimaging. (2016) 257:25–30. doi: 10.1016/j.pscychresns.2016.09.011
20. Liu J, Yao L, Zhang W, Deng W, Xiao Y, Li F, et al. Dissociation of fractional anisotropy and resting-state functional connectivity alterations in antipsychotic-naive first-episode schizophrenia. Schizophr Res. (2019) 204:230–7. doi: 10.1016/j.schres.2018.08.005
21. Nakamura K, Kawasaki Y, Takahashi T, Furuichi A, Noguchi K, Seto H, et al. Reduced white matter fractional anisotropy and clinical symptoms in schizophrenia: a voxel-based diffusion tensor imaging study. Psychiatry Res. (2012) 202:233–8. doi: 10.1016/j.pscychresns.2011.09.006
22. Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. (2007) 29:83–91. doi: 10.1016/j.braindev.2006.07.002
23. Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. The oscillating brain: complex and reliable. Neuroimage. (2010) 49:1432–45. doi: 10.1016/j.neuroimage.2009.09.037
24. Athanassiou M, Dumais A, Tikasz A, Lipp O, Dubreucq JL, Potvin S. Increased cingulo-orbital connectivity is associated with violent behaviours in schizophrenia. J Psychiatr Res. (2022) 147:183–9. doi: 10.1016/j.jpsychires.2022.01.001
25. Guo P, Hu S, Jiang X, Zheng H, Mo D, Cao X, et al. Associations of neurocognition and social cognition with brain structure and function in early-onset schizophrenia. Front Psychiatry. (2022) 13:798105. doi: 10.3389/fpsyt.2022.798105
26. Yu L, Guo L, Fang X, Yang F, Chen Y, Wang Y, et al. Altered brain activity in the bilateral frontal cortices and neural correlation with cognitive impairment in schizophrenia. Brain Imaging Behav. (2022) 16:415–23. doi: 10.1007/s11682-021-00516-6
27. Yu XM, Qiu LL, Huang HX, Zuo X, Zhou ZH, Wang S, et al. Comparison of resting-state spontaneous brain activity between treatment-naive schizophrenia and obsessive-compulsive disorder. BMC Psychiatry. (2021) 21:544. doi: 10.1186/s12888-021-03554-y
28. Zhao X, Yao J, Lv Y, Zhang X, Han C, Chen L, et al. Abnormalities of regional homogeneity and its correlation with clinical symptoms in Naïve patients with first-episode schizophrenia. Brain Imaging Behav. (2019) 13:503–13. doi: 10.1007/s11682-018-9882-4
29. Jiang L, Xu T, He Y, Hou XH, Wang J, Cao XY, et al. Toward neurobiological characterization of functional homogeneity in the human cortex: regional variation, morphological association and functional covariance network organization. Brain Struct Funct. (2015) 220:2485–507. doi: 10.1007/s00429-014-0795-8
30. Jiang L, Zuo XN. Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. Neuroscientist. (2016) 22:486–505. doi: 10.1177/1073858415595004
31. Lan Z, Xu S, Wu Y, Xia L, Hua K, Li M, et al. Alterations of regional homogeneity in preschool boys with autism spectrum disorders. Front Neurosci. (2021) 15:644543. doi: 10.3389/fnins.2021.644543
32. Sun F, Liu Z, Yang J, Fan Z, Yang J. Differential dynamical pattern of regional homogeneity in bipolar and unipolar depression: a preliminary resting-state fMRI study. Front Psychiatry. (2021) 12:764932. doi: 10.3389/fpsyt.2021.764932
33. Yan M, Chen J, Liu F, Li H, Huang R, Tang Y, et al. Disrupted regional homogeneity in major depressive disorder with gastrointestinal symptoms at rest. Front Psychiatry. (2021) 12:636820. doi: 10.3389/fpsyt.2021.636820
34. Gao S, Ming Y, Wang J, Gu Y, Ni S, Lu S, et al. Enhanced prefrontal regional homogeneity and its correlations with cognitive dysfunction/psychopathology in patients with first-diagnosed and drug-naive schizophrenia. Front Psychiatry. (2020) 11:580570. doi: 10.3389/fpsyt.2020.580570
35. Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. (2016) 61:108–20. doi: 10.1016/j.neubiorev.2015.12.007
36. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. (2008) 1124:1–38. doi: 10.1196/annals.1440.011
37. Gao J, Tang X, Wang C, Yu M, Sha W, Wang X, et al. Aberrant cerebellar neural activity and cerebro-cerebellar functional connectivity involving executive dysfunction in schizophrenia with primary negative symptoms. Brain Imaging Behav. (2020) 14:869–80. doi: 10.1007/s11682-018-0032-9
38. Shukla DK, Chiappelli JJ, Sampath H, Kochunov P, Hare SM, Wisner K, et al. Aberrant frontostriatal connectivity in negative symptoms of schizophrenia. Schizophr Bull. (2019) 45:1051–9. doi: 10.1093/schbul/sby165
39. Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. (2011) 127:46–57. doi: 10.1016/j.schres.2010.12.020
40. Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. (2008) 64:774–81. doi: 10.1016/j.biopsych.2008.03.031
41. Modinos G, Costafreda SG, van Tol MJ, McGuire PK, Aleman A, Allen P. Neuroanatomy of auditory verbal hallucinations in schizophrenia: a quantitative meta-analysis of voxel-based morphometry studies. Cortex. (2013) 49:1046–55. doi: 10.1016/j.cortex.2012.01.009
42. Palaniyappan L, Balain V, Radua J, Liddle PF. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr Res. (2012) 137:169–73. doi: 10.1016/j.schres.2012.01.038
43. Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. (2018) 44:168–81. doi: 10.1093/schbul/sbx034
44. Gong J, Wang J, Luo X, Chen G, Huang H, Huang R, et al. Abnormalities of intrinsic regional brain activity in first-episode and chronic schizophrenia: a meta-analysis of resting-state functional MRI. J Psychiatry Neurosci. (2020) 45:55–68. doi: 10.1503/jpn.180245
45. Li S, Hu N, Zhang W, Tao B, Dai J, Gong Y, et al. Dysconnectivity of multiple brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Front Psychiatry. (2019) 10:482. doi: 10.3389/fpsyt.2019.00482
46. Li T, Wang Q, Zhang J, Rolls ET, Yang W, Palaniyappan L, et al. Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. (2017) 43:436–48. doi: 10.1093/schbul/sbw099
47. Xiao B, Wang S, Liu J, Meng T, He Y, Luo X. Abnormalities of localized connectivity in schizophrenia patients and their unaffected relatives: a meta-analysis of resting-state functional magnetic resonance imaging studies. Neuropsychiatr Dis Treat. (2017) 13:467–75. doi: 10.2147/ndt.S126678
48. Li Y, Li WX, Xie DJ, Wang Y, Cheung EFC, Chan RCK. Grey matter reduction in the caudate nucleus in patients with persistent negative symptoms: an ALE meta-analysis. Schizophr Res. (2018) 192:9–15. doi: 10.1016/j.schres.2017.04.005
49. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. (2011) 39:91–2. doi: 10.1016/j.jcms.2010.11.001
50. Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. (2003) 61:557–69. doi: 10.1016/j.brainresbull.2003.06.001
51. Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. (2009) 30:2907–26. doi: 10.1002/hbm.20718
52. Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. (2012) 59:2349–61. doi: 10.1016/j.neuroimage.2011.09.017
53. Bergé D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. (2011) 123:431–9. doi: 10.1111/j.1600-0447.2010.01635.x
54. Jiang Y, Duan M, Chen X, Zhang X, Gong J, Dong D, et al. Aberrant prefrontal-thalamic-cerebellar circuit in schizophrenia and depression: evidence from a possible causal connectivity. Int J Neural Syst. (2019) 29:1850032. doi: 10.1142/s0129065718500326
55. Zhang X, Zhang Y, Liao J, Jiang S, Yan J, Yue W, et al. Progressive grey matter volume changes in patients with schizophrenia over 6 weeks of antipsychotic treatment and their relationship to clinical improvement. Neurosci Bull. (2018) 34:816–26. doi: 10.1007/s12264-018-0234-6
56. Kim G-W, Kim Y-H, Jeong G-W. Whole brain volume changes and its correlation with clinical symptom severity in patients with schizophrenia: a DARTEL-based VBM study. PLoS One. (2017) 12:e0177251. doi: 10.1371/journal.pone.0177251
57. Preuss UW, Zetzsche T, Pogarell O, Mulert C, Frodl T, Müller D, et al. Anterior cingulum volumetry, auditory P300 in schizophrenia with negative symptoms. Psychiatry Res. (2010) 183:133–9. doi: 10.1016/j.pscychresns.2010.05.008
58. Papez JW. A proposed mechanism of emotion. 1937. J Neuropsychiatry Clin Neurosci. (1995) 7:103–12. doi: 10.1176/jnp.7.1.103
59. Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. (2006) 7:268–77. doi: 10.1038/nrn1884
60. Lee YM, Chung YI, Park JM, Lee BD, Moon E, Jeong HJ, et al. Decreased gray matter volume is associated with the subtypes of psychotic symptoms in patients with antipsychotic-naïve mild or moderate Alzheimer’s disease: a voxel-based morphometry study. Psychiatry Res Neuroimaging. (2016) 249:45–51. doi: 10.1016/j.pscychresns.2015.12.002
61. Rollins CPE, Garrison JR, Simons JS, Rowe JB, O’Callaghan C, Murray GK, et al. Meta-analytic evidence for the plurality of mechanisms in transdiagnostic structural mri studies of hallucination status. EClin Med. (2019) 8:57–71. doi: 10.1016/j.eclinm.2019.01.012
62. Schienle A, Stark R, Walter B, Blecker C, Ott U, Kirsch P, et al. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. (2002) 13:2023–6. doi: 10.1097/00001756-200211150-00006
63. Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry (2008) 63:577–86. doi: 10.1016/j.biopsych.2007.05.031
64. Namkung H, Kim SH, Sawa A. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci. (2017) 40:200–7. doi: 10.1016/j.tins.2017.02.002
65. Li H, Ou Y, Liu F, Su Q, Zhang Z, Chen J, et al. Region-specific insular volumetric decreases in drug-naive, first-episode schizophrenia and their unaffected siblings. Am J Med Genet B Neuropsychiatr Genet. (2020) 183:106–12. doi: 10.1002/ajmg.b.32765
66. Szendi I, Szabó N, Domján N, Kincses ZT, Palkó A, Vécsei L, et al. A new division of schizophrenia revealed expanded bilateral brain structural abnormalities of the association cortices. Front Psychiatry. (2017) 8:127. doi: 10.3389/fpsyt.2017.00127
67. Virupaksha HS, Kalmady SV, Shivakumar V, Arasappa R, Venkatasubramanian G, Gangadhar BN. Volume and asymmetry abnormalities of insula in antipsychotic-naive schizophrenia: a 3-tesla magnetic resonance imaging study. Indian J Psychol Med. (2012) 34:133–9. doi: 10.4103/0253-7176.101778
68. Yoshida T, McCarley RW, Nakamura M, Lee K, Koo MS, Bouix S, et al. A prospective longitudinal volumetric MRI study of superior temporal gyrus gray matter and amygdala-hippocampal complex in chronic schizophrenia. Schizophr Res. (2009) 113:84–94. doi: 10.1016/j.schres.2009.05.004
69. Makowski C, Bodnar M, Shenker JJ, Malla AK, Joober R, Chakravarty MM, et al. Linking persistent negative symptoms to amygdala-hippocampus structure in first-episode psychosis. Transl Psychiatry. (2017) 7:e1195. doi: 10.1038/tp.2017.168
70. Rajarethinam R, DeQuardo JR, Miedler J, Arndt S, Kirbat R, Brunberg JA, et al. Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psychiatry Res. (2001) 108:79–87. doi: 10.1016/s0925-4927(01)00120-2
71. Shan X, Zhang H, Dong Z, Chen J, Liu F, Zhao J, et al. Increased subcortical region volume induced by electroconvulsive therapy in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. (2021) 271:1285–95. doi: 10.1007/s00406-021-01303-6
72. Fernando AB, Murray JE, Milton AL. The amygdala: securing pleasure and avoiding pain. Front Behav Neurosci. (2013) 7:190. doi: 10.3389/fnbeh.2013.00190
73. Kumfor F, Irish M, Hodges JR, Piguet O. Discrete neural correlates for the recognition of negative emotions: insights from frontotemporal dementia. PLoS One. (2013) 8:e67457. doi: 10.1371/journal.pone.0067457
74. Radua J, Phillips ML, Russell T, Lawrence N, Marshall N, Kalidindi S, et al. Neural response to specific components of fearful faces in healthy and schizophrenic adults. Neuroimage. (2010) 49:939–46. doi: 10.1016/j.neuroimage.2009.08.030
75. Rajarethinam RP, DeQuardo JR, Nalepa R, Tandon R. Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res. (2000) 41:303–12. doi: 10.1016/s0920-9964(99)00083-3
76. Kaur A, Basavanagowda DM, Rathod B, Mishra N, Fuad S, Nosher S, et al. Structural and functional alterations of the temporal lobe in schizophrenia: a literature review. Cureus. (2020) 12:e11177. doi: 10.7759/cureus.11177
77. Ohi K, Matsuda Y, Shimada T, Yasuyama T, Oshima K, Sawai K, et al. Structural alterations of the superior temporal gyrus in schizophrenia: detailed subregional differences. Eur Psychiatry. (2016) 35:25–31. doi: 10.1016/j.eurpsy.2016.02.002
78. Nenadic I, Sauer H, Smesny S, Gaser C. Aging effects on regional brain structural changes in schizophrenia. Schizophr Bull. (2012) 38:838–44. doi: 10.1093/schbul/sbq140
79. Benoit A, Bodnar M, Malla AK, Joober R, Lepage M. The structural neural substrates of persistent negative symptoms in first-episode of non-affective psychosis: a voxel-based morphometry study. Front Psychiatry. (2012) 3:42. doi: 10.3389/fpsyt.2012.00042
80. Asmal L, du Plessis S, Vink M, Fouche JP, Chiliza B, Emsley R. Insight and white matter fractional anisotropy in first-episode schizophrenia. Schizophr Res. (2017) 183:88–94. doi: 10.1016/j.schres.2016.11.005
81. Joo SW, Kim H, Jo YT, Yoon W, Kim Y, Lee J. Shared and distinct white matter abnormalities in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 108:110175. doi: 10.1016/j.pnpbp.2020.110175
82. Abdolalizadeh A, Ostadrahimi H, Ohadi MAD, Saneei SA, Bayani Ershadi AS. White matter microstructural associates of apathy-avolition in schizophrenia. J Psychiatr Res. (2021) 142:110–6. doi: 10.1016/j.jpsychires.2021.07.042
83. Amodio A, Quarantelli M, Mucci A, Prinster A, Soricelli A, Vignapiano A, et al. Avolition-Apathy and white matter connectivity in schizophrenia: reduced fractional anisotropy between amygdala and insular cortex. Clin EEG Neurosci. (2018) 49:55–65. doi: 10.1177/1550059417745934
84. Tan AS, Chew QH, Sim K. Cerebral white matter changes in deficit and non-deficit subtypes of schizophrenia. J Neural Transm. (2020) 127:1073–9. doi: 10.1007/s00702-020-02207-w
85. McGovern RA, Sheth SA. Role of the dorsal anterior cingulate cortex in obsessive-compulsive disorder: converging evidence from cognitive neuroscience and psychiatric neurosurgery. J Neurosurg. (2017) 126:132–47. doi: 10.3171/2016.1.Jns15601
86. Lee JS, Han K, Lee SK, Seok JH, Kim JJ. Altered structural connectivity and trait anhedonia in patients with schizophrenia. Neurosci Lett. (2014) 579:7–11. doi: 10.1016/j.neulet.2014.07.001
87. Ohtani T, Bouix S, Hosokawa T, Saito Y, Eckbo R, Ballinger T, et al. Abnormalities in white matter connections between orbitofrontal cortex and anterior cingulate cortex and their associations with negative symptoms in schizophrenia: a DTI study. Schizophr Res. (2014) 157:190–7. doi: 10.1016/j.schres.2014.05.016
88. White T, Cullen K, Rohrer LM, Karatekin C, Luciana M, Schmidt M, et al. Limbic structures and networks in children and adolescents with schizophrenia. Schizophr Bull. (2008) 34:18–29. doi: 10.1093/schbul/sbm110
89. Gu C, Zhang Y, Wei F, Cheng Y, Cao Y, Hou H. Magnetic resonance imaging DTI-FT study on schizophrenic patients with typical negative first symptoms. Exp Ther Med. (2016) 12:1450–4. doi: 10.3892/etm.2016.3469
90. Hovington CL, Bodnar M, Chakravarty MM, Joober R, Malla AK, Lepage M. Investigation of white matter abnormalities in first episode psychosis patients with persistent negative symptoms. Psychiatry Res. (2015) 233:402–8. doi: 10.1016/j.pscychresns.2015.06.017
91. Li Z, Lei W, Deng W, Zheng Z, Li M, Ma X, et al. Aberrant spontaneous neural activity and correlation with evoked-brain potentials in first-episode, treatment-naïve patients with deficit and non-deficit schizophrenia. Psychiatry Res Neuroimaging. (2017) 261:9–19. doi: 10.1016/j.pscychresns.2017.01.001
92. Wang X, Li F, Zheng H, Wang W, Zhang W, Liu Z, et al. Breakdown of the striatal-default mode network loop in schizophrenia. Schizophr Res. (2015) 168:366–72. doi: 10.1016/j.schres.2015.07.027
93. De Barros A, Arribarat G, Lotterie JA, Dominguez G, Chaynes P, Péran P. Iron distribution in the lentiform nucleus: a post-mortem MRI and histology study. Brain Struct Funct. (2021) 226:351–64. doi: 10.1007/s00429-020-02175-7
94. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U.S.A. (2001) 98:676–82. doi: 10.1073/pnas.98.2.676
95. Jang JH, Jung WH, Choi JS, Choi CH, Kang DH, Shin NY, et al. Reduced prefrontal functional connectivity in the default mode network is related to greater psychopathology in subjects with high genetic loading for schizophrenia. Schizophr Res. (2011) 127:58–65. doi: 10.1016/j.schres.2010.12.022
96. Saris IMJ, Aghajani M, Reus LM, Visser PJ, Pijnenburg Y, van der Wee NJA, et al. Social dysfunction is transdiagnostically associated with default mode network dysconnectivity in schizophrenia and Alzheimer’s disease. World J Biol Psychiatry. (2021) 17:1–14. doi: 10.1080/15622975.2021.1966714
97. Camchong J, MacDonald AW III, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. (2011) 37:640–50. doi: 10.1093/schbul/sbp131
98. Zhao J, Zhang Y, Liu F, Chen J, Zhao J, Guo W. Abnormal global-brain functional connectivity and its relationship with cognitive deficits in drug-naive first-episode adolescent-onset schizophrenia. Brain Imaging Behav. (2022) 16:1303–13. doi: 10.1007/s11682-021-00597-3
99. Lee H, Lee DK, Park K, Kim CE, Ryu S. Default mode network connectivity is associated with long-term clinical outcome in patients with schizophrenia. Neuroimage Clin. (2019) 22:101805. doi: 10.1016/j.nicl.2019.101805
100. Fox JM, Abram SV, Reilly JL, Eack S, Goldman MB, Csernansky JG, et al. Default mode functional connectivity is associated with social functioning in schizophrenia. J Abnorm Psychol. (2017) 126:392–405. doi: 10.1037/abn0000253
101. Brakowski J, Manoliu A, Homan P, Bosch OG, Herdener M, Seifritz E, et al. Aberrant striatal coupling with default mode and central executive network relates to self-reported avolition and anhedonia in schizophrenia. J Psychiatr Res. (2022) 145:263–75. doi: 10.1016/j.jpsychires.2020.10.047
102. Zhou C, Yu M, Tang XW, Wang X, Zhang XB, Zhang XR, et al. Convergent and divergent altered patterns of default mode network in deficit and non-deficit schizophrenia. Prog Neuro Psychopharmacol Biol Psychiatry. (2019) 89:427–34. doi: 10.1016/j.pnpbp.2018.10.012
103. Sendi MSE, Zendehrouh E, Ellis CA, Liang Z, Fu Z, Mathalon DH, et al. Aberrant dynamic functional connectivity of default mode network in schizophrenia and links to symptom severity. Front Neural Circuits. (2021) 15:649417. doi: 10.3389/fncir.2021.649417
104. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. (2015) 38:433–47. doi: 10.1146/annurev-neuro-071013-014030
105. Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol. (2012) 8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049
106. Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. (2006) 66:1817–23. doi: 10.1212/01.wnl.0000219679.95223.4c
107. Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. (1993) 50:873–80. doi: 10.1001/archneur.1993.00540080076020
108. Marchand WR, Lee JN, Suchy Y, Johnson S, Thatcher J, Gale P. Aberrant functional connectivity of cortico-basal ganglia circuits in major depression. Neurosci Lett. (2012) 514:86–90. doi: 10.1016/j.neulet.2012.02.063
109. Schmahmann JD. Vascular syndromes of the thalamus. Stroke. (2003) 34:2264–78. doi: 10.1161/01.Str.0000087786.38997.9e
110. Bernard JA, Orr JM, Mittal VA. Cerebello-thalamo-cortical networks predict positive symptom progression in individuals at ultra-high risk for psychosis. Neuroimage Clin. (2017) 14:622–8. doi: 10.1016/j.nicl.2017.03.001
111. Cao H, Chén OY, Chung Y, Forsyth JK, McEwen SC, Gee DG, et al. Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun. (2018) 9:3836. doi: 10.1038/s41467-018-06350-7
112. Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. (2014) 24:3116–30. doi: 10.1093/cercor/bht165
113. Cao H, Wei X, Hu N, Zhang W, Xiao Y, Zeng J, et al. Cerebello-Thalamo-Cortical hyperconnectivity classifies patients and predicts long-term treatment outcome in first-episode schizophrenia. Schizophr Bull. (2022) 48:505–13. doi: 10.1093/schbul/sbab112
114. Martino M, Magioncalda P, Yu H, Li X, Wang Q, Meng Y, et al. Abnormal resting-state connectivity in a substantia nigra-related striato-thalamo-cortical network in a large sample of first-episode drug-naïve patients with schizophrenia. Schizophr Bull. (2018) 44:419–31. doi: 10.1093/schbul/sbx067
115. Skåtun KC, Kaufmann T, Brandt CL, Doan NT, Alnæs D, Tønnesen S, et al. Thalamo-cortical functional connectivity in schizophrenia and bipolar disorder. Brain Imaging Behav. (2018) 12:640–52. doi: 10.1007/s11682-017-9714-y
116. Xi C, Liu ZN, Yang J, Zhang W, Deng MJ, Pan YZ, et al. Schizophrenia patients and their healthy siblings share decreased prefronto-thalamic connectivity but not increased sensorimotor-thalamic connectivity. Schizophr Res. (2020) 222:354–61. doi: 10.1016/j.schres.2020.04.033
117. Paillère-Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res. (2001) 50:19–26. doi: 10.1016/s0920-9964(00)00137-7
118. Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. (2001) 158:234–43. doi: 10.1176/appi.ajp.158.2.234
119. Kawasaki Y, Suzuki M, Nohara S, Hagino H, Takahashi T, Matsui M, et al. Structural brain differences in patients with schizophrenia and schizotypal disorder demonstrated by voxel-based morphometry. Eur Arch Psychiatry Clin Neurosci. (2004) 254:406–14. doi: 10.1007/s00406-004-0522-1
120. Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS. Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. (2005) 29:587–91. doi: 10.1016/j.pnpbp.2005.01.020
121. Bassitt DP, Neto MR, de Castro CC, Busatto GF. Insight and regional brain volumes in schizophrenia. Eur Arch Psychiatry Clin Neurosci. (2007) 257:58–62. doi: 10.1007/s00406-006-0685-z
122. Meisenzahl EM, Koutsouleris N, Bottlender R, Scheucrecker J, Jaeger A, Teipel SJ, et al. Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophr Res. (2008) 104:44–60. doi: 10.1016/j.schres.2008.06.023
123. Herold R, Feldmann A, Simon M, Tényi T, Kövér F, Nagy F, et al. Regional gray matter reduction and theory of mind deficit in the early phase of schizophrenia: a voxel-based morphometric study. Acta Psychiatr Scand. (2009) 119:199–208. doi: 10.1111/j.1600-0447.2008.01297.x
124. Whitford TJ, Farrow TFD, Williams LM, Gomes L, Brennan J, Harris AWF. Delusions and dorso-medial frontal cortex volume in first-episode schizophrenia: a voxel-based morphometry study. Psychiatry Res Neuroimaging. (2009) 172:175–9. doi: 10.1016/j.pscychresns.2008.07.011
125. Cascella NG, Fieldstone SC, Rao VA, Pearlson GD, Sawa A, Schretlen DJ. Gray-matter abnormalities in deficit schizophrenia. Schizophr Res. (2010) 120:63–70. doi: 10.1016/j.schres.2010.03.039
126. Anderson V, Goldstein M, Kydd R, Russell B Extensive gray matter volume reduction in treatment-resistant schizophrenia. Int J Neuropsychopharmacol. (2015) 18:pyv016. doi: 10.1093/ijnp/pyv016
127. Huang P, Xi Y, Lu ZL, Chen Y, Li X, Li W, et al. Decreased bilateral thalamic gray matter volume in first-episode schizophrenia with prominent hallucinatory symptoms: a volumetric MRI study. Sci Rep. (2015) 5:14505. doi: 10.1038/srep14505
128. Poletti S, Vai B, Smeraldi E, Cavallaro R, Colombo C, Benedetti F. Adverse childhood experiences influence the detrimental effect of bipolar disorder and schizophrenia on cortico-limbic grey matter volumes. J Affect Disord. (2016) 189:290–7. doi: 10.1016/j.jad.2015.09.049
129. Huang X, Pu W, Li X, Greenshaw AJ, Dursun SM, Xue Z, et al. Decreased left putamen and thalamus volume correlates with delusions in first-episode schizophrenia patients. Front Psychiatry. (2017) 8:245. doi: 10.3389/fpsyt.2017.00245
130. Kuroki N, Kashiwagi H, Ota M, Ishikawa M, Kunugi H, Sato N, et al. Brain structure differences among male schizophrenic patients with history of serious violent acts: an MRI voxel-based morphometric study. BMC Psychiatry. (2017) 17:105. doi: 10.1186/s12888-017-1263-9
131. Spalthoff R, Gaser C, Nenadić I. Altered gyrification in schizophrenia and its relation to other morphometric markers. Schizophr Res. (2018) 202:195–202. doi: 10.1016/j.schres.2018.07.014
132. Zhao CA, Zhu JB, Liu XA, Pu CC, Lai YA, Chen LA, et al. Structural and functional brain abnormalities in schizophrenia: a cross-sectional study at different stages of the disease. Prog Neuro Psychopharmacol Biol Psychiatry. (2018) 83:27–32. doi: 10.1016/j.pnpbp.2017.12.017
133. Lei W, Kirkpatrick B, Wang Q, Deng W, Li M, Guo W, et al. Progressive brain structural changes after the first year of treatment in first-episode treatment-naive patients with deficit or nondeficit schizophrenia. Psychiatry Res Neuroimaging. (2019) 288:12–20. doi: 10.1016/j.pscychresns.2019.04.009
134. Neugebauer K, Hammans C, Wensing T, Kumar V, Grodd W, Mevissen L, et al. Nerve growth factor serum levels are associated with regional gray matter volume differences in schizophrenia patients. Front Psychiatry. (2019) 10:275. doi: 10.3389/fpsyt.2019.00275
135. Rametti G, Junqué C, Falcón C, Bargalló N, Catalán R, Penadés R, et al. A voxel-based diffusion tensor imaging study of temporal white matter in patients with schizophrenia. Psychiatry Res. (2009) 171:166–76. doi: 10.1016/j.pscychresns.2008.05.003
136. Spalletta G, De Rossi P, Piras F, Iorio M, Dacquino C, Scanu F, et al. Brain white matter microstructure in deficit and non-deficit subtypes of schizophrenia. Psychiatry Res. (2015) 231:252–61. doi: 10.1016/j.pscychresns.2014.12.006
137. Ebdrup BH, Raghava JM, Nielsen M, Rostrup E, Glenthøj B. Frontal fasciculi and psychotic symptoms in antipsychotic-naive patients with schizophrenia before and after 6 weeks of selective dopamine D2/3 receptor blockade. J Psychiatry Neurosci. (2016) 41:133–41. doi: 10.1503/jpn.150030
138. Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D’Angelo D, Mauro CJ, et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. (2010) 117:13–20. doi: 10.1016/j.schres.2009.09.030
139. Cui LB, Liu K, Li C, Wang LX, Guo F, Tian P, et al. Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Res. (2016) 173:13–22. doi: 10.1016/j.schres.2016.02.039
140. Alonso-Solís A, Vives-Gilabert Y, Portella MJ, Rabella M, Grasa EM, Roldán A, et al. Altered amplitude of low frequency fluctuations in schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res. (2017) 189:97–103. doi: 10.1016/j.schres.2017.01.042
141. Salvador R, Landin-Romero R, Anguera M, Canales-Rodriguez EJ, Radua J, Guerrero-Pedraza A, et al. Non redundant functional brain connectivity in schizophrenia. Brain Imaging Behav. (2017) 11:552–64. doi: 10.1007/s11682-016-9535-4
142. Lian N, Lv H, Guo W, Shen Y, Wu R, Liu Y, et al. A comparative study of magnetic resonance imaging on the gray matter and resting-state function in prodromal and first-episode schizophrenia. Am J Med Genet B Neuropsychiatr Genet. (2018) 177:537–45. doi: 10.1002/ajmg.b.32644
143. Wu R, Ou Y, Liu F, Chen J, Li H, Zhao J, et al. Reduced brain activity in the right putamen as an early predictor for treatment response in drug-naive, first-episode schizophrenia. Front Psychiatry. (2019) 10:741. doi: 10.3389/fpsyt.2019.00741
144. Gao B, Wang Y, Liu W, Chen Z, Zhou H, Yang J, et al. Spontaneous activity associated with delusions of schizophrenia in the left medial superior frontal gyrus: a resting-state fMRI study. PLoS One. (2015) 10:e0133766. doi: 10.1371/journal.pone.0133766
145. Gou N, Liu Z, Palaniyappan L, Li M, Pan Y, Chen X, et al. Effects of DISC1 polymorphisms on resting-state spontaneous neuronal activity in the early-stage of schizophrenia. Front Psychiatry. (2018) 9:137. doi: 10.3389/fpsyt.2018.00137
146. Yang F, Ma H, Yuan J, Wei Y, Xu L, Zhang Y, et al. Correlation of abnormalities in resting state fMRI with executive functioning in chronic schizophrenia. Psychiatry Res. (2021) 299:113862. doi: 10.1016/j.psychres.2021.113862
147. Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. (2007) 33:1004–12. doi: 10.1093/schbul/sbm052
148. Fan FM, Tan SP, Yang FD, Tan YL, Zhao YL, Chen N, et al. Ventral medial prefrontal functional connectivity and emotion regulation in chronic schizophrenia: a pilot study. Neurosci Bull. (2013) 29:59–74. doi: 10.1007/s12264-013-1300-8
149. Chang X, Shen H, Wang L, Liu Z, Xin W, Hu D, et al. Altered default mode and fronto-parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Res. (2014) 1562:87–99. doi: 10.1016/j.brainres.2014.03.024
150. Manoliu A, Riedl V, Zherdin A, Mühlau M, Schwerthöffer D, Scherr M, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. (2014) 40:428–37. doi: 10.1093/schbul/sbt037
151. Zhuo C, Zhu J, Qin W, Qu H, Ma X, Tian H, et al. Functional connectivity density alterations in schizophrenia. Front Behav Neurosci. (2014) 8:404. doi: 10.3389/fnbeh.2014.00404
152. Alonso-Solís A, Vives-Gilabert Y, Grasa E, Portella MJ, Rabella M, Sauras RB, et al. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res. (2015) 161:261–8. doi: 10.1016/j.schres.2014.10.047
153. Chang X, Xi YB, Cui LB, Wang HN, Sun JB, Zhu YQ, et al. Distinct inter-hemispheric dysconnectivity in schizophrenia patients with and without auditory verbal hallucinations. Sci Rep. (2015) 5:11218. doi: 10.1038/srep11218
154. Duan M, Chen X, He H, Jiang Y, Jiang S, Xie Q, et al. Altered basal ganglia network integration in schizophrenia. Front Hum Neurosci. (2015) 9:561. doi: 10.3389/fnhum.2015.00561
155. Wang D, Zhou Y, Zhuo C, Qin W, Zhu J, Liu H, et al. Altered functional connectivity of the cingulate subregions in schizophrenia. Transl Psychiatry. (2015) 5:e575. doi: 10.1038/tp.2015.69
156. Xu L, Qin W, Zhuo C, Zhu J, Liu H, Liu X, et al. Selective functional disconnection of the dorsal subregion of the temporal pole in schizophrenia. Sci Rep. (2015) 5:11258. doi: 10.1038/srep11258
157. Zhou Y, Ma X, Wang D, Qin W, Zhu J, Zhuo C, et al. The selective impairment of resting-state functional connectivity of the lateral subregion of the frontal pole in schizophrenia. PLoS One. (2015) 10:e0119176. doi: 10.1371/journal.pone.0119176
158. Chen X, Duan M, He H, Yang M, Klugah-Brown B, Xu H, et al. Functional abnormalities of the right posterior insula are related to the altered self-experience in schizophrenia. Psychiatry Res Neuroimaging. (2016) 256:26–32. doi: 10.1016/j.pscychresns.2016.09.006
159. Liu X, Zhuo C, Qin W, Zhu J, Xu L, Xu Y, et al. Selective functional connectivity abnormality of the transition zone of the inferior parietal lobule in schizophrenia. Neuroimage Clin. (2016) 11:789–95. doi: 10.1016/j.nicl.2016.05.021
160. Penner J, Ford KA, Taylor R, Schaefer B, Théberge J, Neufeld RW, et al. Medial prefrontal and anterior insular connectivity in early schizophrenia and major depressive disorder: a resting functional MRI evaluation of large-scale brain network models. Front Hum Neurosci. (2016) 10:132. doi: 10.3389/fnhum.2016.00132
161. Peters H, Riedl V, Manoliu A, Scherr M, Schwerthoffer D, Zimmer C, et al. Changes in extra-striatal functional connectivity in patients with schizophrenia in a psychotic episode. Br J Psychiatry. (2017) 210:75–82. doi: 10.1192/bjp.bp.114.151928
162. Zhuo C, Zhu J, Wang C, Qu H, Ma X, Tian H, et al. Brain structural and functional dissociated patterns in schizophrenia. BMC Psychiatry. (2017) 17:45. doi: 10.1186/s12888-017-1194-5
163. Ferri J, Ford JM, Roach BJ, Turner JA, van Erp TG, Voyvodic J, et al. Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol Med. (2018) 48:2492–9. doi: 10.1017/s003329171800003x
164. Penner J, Osuch EA, Schaefer B, Théberge J, Neufeld RWJ, Menon RS, et al. Higher order thalamic nuclei resting network connectivity in early schizophrenia and major depressive disorder. Psychiatry Res Neuroimaging. (2018) 272:7–16. doi: 10.1016/j.pscychresns.2017.12.002
165. Penner J, Osuch EA, Schaefer B, Théberge J, Neufeld RWJ, Menon RS, et al. Temporoparietal junction functional connectivity in early schizophrenia and major depressive disorder. Chronic Stress. (2018) 2:2470547018815232. doi: 10.1177/2470547018815232
166. Sharma A, Kumar A, Singh S, Bhatia T, Beniwal RP, Khushu S, et al. Altered resting state functional connectivity in early course schizophrenia. Psychiatry Res Neuroimaging. (2018) 271:17–23. doi: 10.1016/j.pscychresns.2017.11.013
167. Dong DB, Duan MJ, Wang YL, Zhang XX, Jia XY, Li YJ, et al. Reconfiguration of dynamic functional connectivity in sensory and perceptual system in schizophrenia. Cereb Cortex. (2019) 29:3577–89. doi: 10.1093/cercor/bhy232
Keywords: persistent negative symptoms, schizophrenia, structural MRI, functional MRI, meta-analysis
Citation: Zhu T, Wang Z, Zhou C, Fang X, Huang C, Xie C, Ge H, Yan Z, Zhang X and Chen J (2022) Meta-analysis of structural and functional brain abnormalities in schizophrenia with persistent negative symptoms using activation likelihood estimation. Front. Psychiatry 13:957685. doi: 10.3389/fpsyt.2022.957685
Received: 31 May 2022; Accepted: 05 September 2022;
Published: 27 September 2022.
Edited by:
Seok Jun Hong, Sungkyunkwan University, South KoreaReviewed by:
Wei Deng, Affiliated Mental Health Center & Hangzhou Seventh People’s Hospital, ChinaDrozdstoy Stoyanov Stoyanov, Plovdiv Medical University, Bulgaria
Copyright © 2022 Zhu, Wang, Zhou, Fang, Huang, Xie, Ge, Yan, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangrong Zhang, drxrz@hotmail.com; Jiu Chen, ericcst@aliyun.com
†These authors have contributed equally to this work and share first authorship
 Tingting Zhu1†
Tingting Zhu1† Zixu Wang
Zixu Wang Chao Zhou
Chao Zhou Xinyu Fang
Xinyu Fang Chengbing Huang
Chengbing Huang Chunming Xie
Chunming Xie Honglin Ge
Honglin Ge Xiangrong Zhang
Xiangrong Zhang Jiu Chen
Jiu Chen